Abstract
Objective
To determine which clinical measure of childhood obesity should be monitored to best reflect change in adiposity in a weight management programme and estimate the degree of change needed to be relatively certain of fat reduction.
Subjects
92 obese children with a mean (range) age of 12.8 (6.9–18.9) years and a mean body mass index standard deviation score (BMI SDS) of +3.38 (+2.27 to +4.47) attending a hospital‐based clinic on a regular, 3 monthly basis.
Measurements
Pairs of weight and height measured up to 2.41 years apart used to derive BMI as kg/m2, and adjusted for age and gender to give weight and BMI SDS (BMI‐z score) using British 1990 Growth Reference Data. Contemporaneous adiposity estimated by fatness measured by a bioimpedance segmental body composition analyser.
Results
Changes in BMI‐z scores, compared to BMI, weight and weight SDS, most accurately reflected loss of fat. Reductions of 0.25, 0.5, 0.75, and 1 BMI SDS equate to expected mean falls in total body fat percentage of 2.9%, 5.8%, 8.7% and 11.6%. Approximate 95% prediction intervals indicated that a fall in BMI SDS of at least 0.6 over 6–12 months (or 0.5 over 0–6 months) is consistent with actual fat loss.
Conclusion
Change in BMI‐z score best reflects percentage fat loss compared to BMI, weight and weight SDS. The wide variation in likely percentage fat loss for a given BMI SDS reduction means a loss of 0.5–0.6 is required to be relatively certain of definite percentage fat reduction.
Keywords: BMI‐z scores, adiposity, bio‐impedance, obesity
Childhood obesity is now a significant global problem. The increasing prevalence of childhood obesity in the United Kingdom, together with the stated government aim to reduce the year on year increase of obesity in under 11 year olds1 by 2010, will inevitably focus attention in this country on the development of community, primary care and school‐based weight management programmes for treating childhood obesity. The paucity of randomised trials in this area and the absence of sufficiently powered interventions so far reported2 will inevitably necessitate a thorough evaluation of future interventions to identify which have the greatest efficacy at least economic cost. Many of these interventions are likely to be conducted in areas with very basic clinical evaluation tools, allowing simply an estimate of height and weight rather than more sophisticated measures such as skinfold thickness. We have used our data from a hospital‐based clinic, which experiences a wide spectrum of success in weight management, to explore the best measure of body composition to represent actual loss of adiposity as percentage fat mass reduction, as determined by bioimpedance. We sought a simple, empirical relationship that could be used to indicate the likely reduction in percentage fat. When evaluating success in weight management for obesity, the evidence points to the need to reduce adiposity (fat mass as a percentage of whole body weight) rather than any other measures such as improved fitness.3,4 Adiposity or fat mass is intimately linked to both blood pressure and insulin sensitivity in childhood populations, whereas fitness is probably associated with any improvements through its modulating effect on adiposity. For this reason, it is likely that a reduction in the level of adiposity is required to improve morbidity and long‐term health, while still acknowledging the importance and positive benefits of regular exercise through improved skeletal muscle function.5,6
The Care of Childhood Obesity Clinic is a hospital‐based intervention aimed solely at children with International Obesity Task Force (IOTF) defined obesity. Through a diet and exercise lifestyle modulation scheme, 83% of our patients attending the clinic for a year or more reduce body mass index (BMI)‐z scores, with 28% achieving a loss of greater than 0.5 BMI standard deviation score (SDS).7
Methods
Weight was measured on a digital scales (Seca, Hamburg, Germany) in light clothing with shoes removed and height was measured using a Harpenden stadiometer (Holtain, Crymych, UK).8 BMI was calculated as kg/m2 and adjusted for age and gender to give a BMI standard deviation score (SDS) using British 1990 Growth Reference Data from the Child Growth Foundation.9 A relatively recent addition to our evaluation of obese children over 7 years of age has been an estimate of adiposity using the Tanita bioimpedance segmental body composition analyser (model BC‐418MA; Tanita, Yiewsley, UK). This model has been validated against more complex and expensive investigations to assess adiposity.10,11 More recently, it has been further validated across the pubertal age range (11±3.6 (males) and 11±3.0 (females) years) against DXA and air‐displacement plethysmography (BOD POD).12 At each 3 monthly standard clinic visit, bioimpedance is therefore estimated as a measure of adiposity and we used this as our “gold standard” measure.
Results
Repeated Tanita data over time were available on 92 obese children and young people (41 male). At first assessment the mean (range) age was 12.8 (6.9–18.9) years. All patients were obese by IOTF guidelines (BMI SDS >2.25 for females and >2.37 for males)13 with a mean BMI SDS of +3.38 (+2.27 to +4.47) and a mean percentage total body fat of 44.7% (27.7% to 62.4%). Eighty six (93%) patients were of white ethnic origin (two Black, two South‐Asian, two mixed race) and 19% were pre‐pubertal. The first and most recent Tanita measurements were used, with a median interval between them of 0.83 years (range 0.01–2.41 years).
Which measure of adiposity best predicts reduction in percentage fat mass over this period?
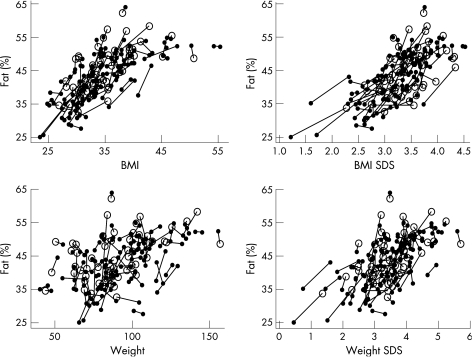
Exploratory analyses were carried out to investigate the relationships between percentage fat and BMI, BMI SDS, weight and weight SDS (see fig 1 for the raw data), firstly using one result per child, then using both results but allowing for correlated results for each child (data not shown). The relationships between percentage fat and both BMI SDS and weight SDS were approximately linear, with a slight improvement in the model for BMI SDS if adjustment was made for age. The relationships between percentage fat and BMI and weight were more complex; the former was approximately quadratic in BMI, requiring adjustment for age, while the latter required square root transformation of weight and adjustment for both age and sex. In general, as better fits were obtained (in terms of the residual variation) for BMI and BMI SDS than for weight or weight SDS, only the former were explored in the following analysis.
Figure 1 %Fat in relation to (a) BMI, (b) BMI SDS, (c) weight (kg) and (d) weight SDS. Pairs of results for the same child are connected with a line; open circles denote measurements made at the very first clinic visit and closed circles represent measurements made at subsequent visits.
Estimation of the fall in percentage fat from the fall in BMI SDS
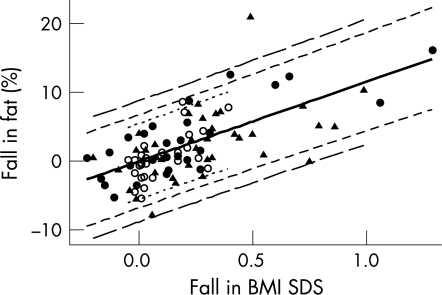
The linear relationship between percentage fat and BMI SDS suggested to us that the change in percentage fat (defining ”change” as ”initial minus final”) should be linearly related to the corresponding change in BMI SDS, with a possible adjustment for the increase in age, that is, the time interval (in decimal years) between the two measurements, as follows: change in %fat = b1×change in BMI SDS+b2×time interval+e, where b1 and b2 were coefficients to be estimated and e was the residual for the individual, assumed to be approximately normally distributed with mean 0 and variance σ2. The variance σ2 would be expected to vary according to the time interval between the two measurements; for a given individual, pairs of percentage fat results closer in time would be expected to be more highly correlated than those further apart in time, and therefore the variance of the difference would be smaller. The children were divided arbitrarily into three subgroups according to the time interval between each child's pair of measurements: up to 0.5 year (median 0.23 years; n = 27), from 0.5 to 1 year (median 0.71 year; n = 28) and over 1 year (median 1.22 year; n = 37). The model above was fitted using REML in the SAS PROC MIXED procedure (SAS release 8.2; SAS, Cary, NC, USA), with the same regression coefficients (b1 and b2) for the three subgroups but different variances. The time interval coefficient (b2) was not statistically significant (p = 0.400) and this term was dropped from the model. The coefficient for the model using change in BMI SDS alone (b1) was estimated to be 11.60 (SE 1.155) and the variances for the three subgroups were 7.68, 11.69 and 19.80, respectively. The mean predicted change in percentage fat, for a given fall in BMD SDS, thus could be estimated from 11.60×change in BMI SDS and the standard error (SE) of this estimate was √{(1.155×change in BMI SDS)2}. Figure 2 shows the data for the three subgroups, the predicted changes and approximate 95% prediction intervals (PIs).
Figure 2 Relationship between simple fall in %fat and fall in BMI SDS. Pairs of results which were less than 0.5 years apart, between 0.5 and 1 year apart and more than 1 year apart are shown, respectively, as open circles, closed circles and triangles. The solid black line indicates the predicted values and approximate 95% prediction intervals (PIs)* for the three respective subgroups are shown with dotted, short‐dashed and long‐dashed lines. *The 95% prediction intervals were estimated from the predicted mean fall in %fat±1.99×√{(1.155×fall in BMI SDS)2+V}, where V is the variance estimated for the relevant subgroup (see text).
Values for clinical usefulness
In the above model, reductions of 0.25, 0.5, 0.75, and 1 BMI SDS equate to expected mean (SE) falls in total body fat percentage of 2.9% (0.3%), 5.8% (0.6%), 8.7% (0.9%) and 11.6% (1.2%). If these reductions are observed over an interval of time from 6 months to 1 year, then the approximate 95% PIs are −3.9% to 9.7%, −1.1% to 12.7%, 1.7% to 15.7% and 4.4% to 18.8%, respectively. One can calculate that the minimum BMI SDS change to be consistent with a fat reduction (ie, with the lower limit of the 95% PI >0) over this period is 0.60–7% (95% PI 0.0% to 13.9%). Over a shorter interval (less than 6 months), the equivalent value BMI SDS change would be 0.49–mean percentage fat reduction 5.7% (95% PI 0.1% to 11.3%)
Estimation of the fall in percentage fat from the fall in BMI
If only raw BMI data, and not BMI SDS data, are available, preliminary work (above) suggested that fall in percentage fat might also be predicted from changes in BMI, the square of the BMI and increase in age, that is, the time interval, as follows:
Change in %fat = b1×change in BMI+b2×change in BMI2+b3 time interval+e
The model fitted using REML, with the same three variance subgroups as before (ie, time intervals <0.5, 0.5–1 and >1 year). Although the coefficient for the time interval was not significant in this case (p = 0.070), the term was retained because of its impact on the Akaike information criterion (AIC; ie, 494.8 compared with 498.2), which assessed the goodness of fit of the model while taking into account the number of parameters. This model fitted slightly better than the model using BMI SDS above (AIC = 498.3). The estimates of the coefficients b1, b2 and b3 were 3.90 (SE 0.995), −0.0339 (SE 0.0145) and 0.818 (SE 0.446), respectively, and the variances of the three subgroups were 6.61, 10.44 and 17.43.
Table 1 gives examples of the fall in percentage fat estimated from the fall in BMI and BMI2, for time intervals of 0.75 years and 1.25 years, respectively. Falls in BMI of between 1 and 5 units are used for illustration and are shown separately for initial BMI values of 30, 35, 40 and 45 units, since the change in BMI2 depends on the initial BMI.
Table 1 Predicted mean (SE)* loss in percentage fat using change in BMI and BMI2 over time intervals of (a) 0.75 years and (b) 1.25 years.
| Initial BMI | Fall in BMI | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| (a) Over 0.75 years | |||||
| 30 | 2.5% (SE 0.3%) (PI±6.5%) | 4.5% (SE 0.5%) (PI±6.5%) | 6.5% (SE 0.7%) (PI±6.6%) | 8.6% (SE 0.9%) (PI±6.7%) | 10.8% (SE 1.2%) (PI±6.8%) |
| 35 | 2.2% (SE 0.3%) (PI±6.5%) | 3.8% (SE 0.4%) (PI±6.5%) | 5.5% (SE 0.5%) (PI±6.5%) | 7.3% (SE 0.6%) (PI±6.5%) | 9.1% (SE 0.8%) (PI±6.6%) |
| 40 | 1.8% (SE 0.4%) (PI±6.5%) | 3.1% (SE 0.5%) (PI±6.5%) | 4.5% (SE 0.6%) (PI±6.5%) | 5.9% (SE 0.8%) (PI±6.6%) | 7.4% (SE 0.9%) (PI±6.7%) |
| 45 | 1.5% (SE 0.4%) (PI±6.5%) | 2.4% (SE 0.7%) (PI±6.6%) | 3.5% (SE 1.0%) (PI±6.7%) | 4.6% (SE 1.2%) (PI±6.9%) | 5.7% (SE 1.5%) (PI±7.0%) |
| (b) Over 1.25 years | |||||
| 30 | 2.9% (SE 0.5%) (PI±8.4%) | 4.9% (SE 0.6%) (PI±8.4%) | 6.9% (SE 0.7%) (PI±8.4%) | 9.0% (SE 1.0%) (PI±8.5%) | 11.2% (SE 1.2%) (PI±8.6%) |
| 35 | 2.6% (SE 0.5%) (PI±8.4%) | 4.2% (SE 0.5%) (PI±8.4%) | 5.9% (SE 0.6%) (PI±8.4%) | 7.7% (SE 0.7%) (PI±8.4%) | 9.5% (SE 0.8%) (PI±8.5%) |
| 40 | 2.2% (SE 0.5%) (PI±8.4%) | 3.5% (SE 0.6%) (PI±8.4%) | 4.9% (SE 0.7%) (PI±8.4%) | 6.3% (SE 0.8%) (PI±8.5%) | 7.8% (SE 1.0%) (PI±8.5%) |
| 45 | 1.9% (SE 0.6%) (PI±8.4%) | 2.9% (SE 0.8%) (PI±8.4%) | 3.9% (SE 1.0%) (PI±8.5%) | 5.0% (SE 1.3%) (PI±8.7%) | 6.1% (SE 1.5%) (PI±8.8%) |
Approximate 95% PIs are shown in parentheses.
*Predicted mean loss in percentage fat calculated from 3.90×fall in BMI−0.0339×fall in BMI2+0.818×time interval.
Standard error (SE) calculated from √{(0.990×(fall in BMI)2)+(0.000209×(fall in BMI2)2)+(0.199×(time interval)2)−(0.02836×fall in BMI×fall in BMI2−(0.07752×fall in BMI×time interval)+(0.000312×fall in BMI2×time interval)}.
Discussion
We believe this study makes some important observations regarding measuring changes in adiposity in the clinical setting and how much we should aim for to be relatively certain of beneficial effects.
In clinical terms the change in BMI‐z score appears to give the simplest surrogate measure of percentage loss in fat mass or adiposity. In a setting where BMI SDS scores are not available, a model derived from changes in BMI and BMI2 could be used. This model fitted our data slightly better but was more cumbersome to use than the change in BMI‐z score.
In 2005, Cole et al presented data suggesting that BMI might be a slightly better measure of adiposity change over time than BMI‐z scores.14 However, there are a number of important differences between our study and that of Cole et al. Their study was observational and examined reproducibility of measures over 9 months, thereby not assuming a downward trend over time, while our children were mainly “improving” in a weight management programme. Furthermore, we have been able to study changes in BMI/BMI SDS in relation to a validated measure of actual percentage fat loss. Although we acknowledge that bioimpedance is slightly less accurate than more sophisticated research tools such as DXA or MRI scans, and is incapable of differentiating visceral (central) from subcutaneous fat which is of great relevance to obesity co‐morbidities,15 its ease of use and cost made serial measurements affordable and simple for regular clinical use.
We have developed equations that could be used prospectively. While the variability in percentage fat loss for a given BMI or BMI SDS reduction is wide, our data do suggest that any intervention in childhood obesity needs to be able to demonstrate a fall of at least 0.6 BMI SDS to be more or less certain of reducing adiposity. This is interesting because other researchers have identified a minimum reduction of 0.5 as being required to produce significant improvements in indices of blood pressure, lipid profile and measures of insulin resistance.16,17 Furthermore, others have identified that a rise in BMI SDS around the 0.5 level increases the risk of developing metabolic syndrome.18 We believe it likely that the almost certain improvement in adiposity associated with this level of BMI SDS reduction leads to the documented improvement in cardiovascular and endocrine outcome measures. When planning intervention studies for treating childhood obesity, we suggest that this should be the level at which success is defined as the basis for working out statistical power.
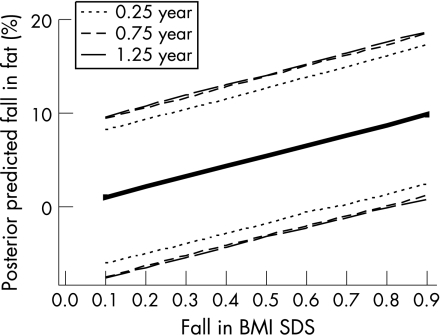
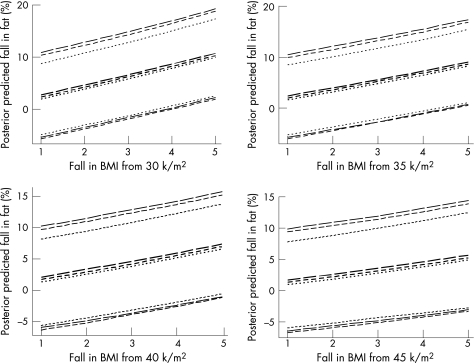
In deriving our equations we used arbitrary subgrouping of the time intervals. We further explored a Bayesian approach that allowed us to relate the variance with the actual time interval between the two measurements. Although the variance model was not easily validated, this approach led to similar findings. Details of this approach are given in the Appendix and figs 3 and 4.
Figure 3 Alternative Bayesian MCMC estimation of the relationship between fall in %fat and fall in BMI SDS. Median posterior predicted values are shown together with 95% credible regions, shown as dotted, short‐dashed and long‐dashed lines, respectively, for pairs of results which were 0.25, 0.75 and 1.25 years apart.
Figure 4 Alternative Bayesian MCMC estimation of the relationship between fall in %fat and fall in BMI and increase in age. Separate plots are shown for BMI changes from 30, 35, 40 and 45 k/m2. Median posterior predicted values (in darker type) are shown together with 95% credible regions (in lighter type). The dotted, short‐dashed and long‐dashed lines, respectively, connect results for time intervals of 0.25, 0.75 and 1.25 years apart.
In essence, we believe that we have shown that it is possible to use either measurement of BMI or BMI‐z scores to predict actual changes in percentage fat. In purely clinical terms, however, it is likely that staff may have to rely on BMI estimates to evaluate success. We believe this provides a useful, additional tool to evaluate clinical success, although our equations will need validation for different ages, racial groups and longer time intervals than those used in this study.
What is already known on this topic
Childhood obesity prevalence continues to increase in the UK.
Effective management strategies are urgently needed.
What this study adds
For a given reduction in BMI SDS or BMI, the range of percentage fat loss is wide.
For BMI SDS, a reduction of between 0.5–0.6 is required to be relatively certain of actually reducing adiposity.
Acknowledgements
We would like to thank Nicky Welton for helpful discussions about MCMC posterior predicted values. Anna Ford is supported by a grant from The BUPA Foundation.
Abbreviations
AIC - Akaike information criterion
BMI - body mass index
IOTF - International Obesity Task Force
MCMC - Markov chain Monte Carlo
PI - prediction interval
SDS - standard deviation score
Appendix
ALTERNATIVE BAYESIAN APPROACH
In the methods described in this paper, uncertainty in the estimation of the three variances was not taken into account in the prediction. This was achievable using a Bayesian approach, and as an alternative approach we explored the use of Markov chain Monte Carlo (MCMC) methods, using WinBUGS V.1.4.1 (Imperial College and MRC, London, UK). The variance σ2 was assumed to be related to the time interval between a child's pairs of measurements (t) in the following way: σ2 = 1/α1×(1−α2t), but limited exploratory analysis did seem to support this. Posterior distributions were obtained for α1 and α2, as well as for the regression coefficients (the b's.). Wide normal priors were used for the regression coefficients, a gamma prior for α1, a uniform prior for α2, and a long burn‐in of 10 000 iterations (despite quite rapid convergence).
For the first model, to estimate the fall in percentage fat from the fall in BMI SDS, median posteriors after a further 40 000 iterations were calculated to be 11.03 (95% credible interval 8.46–13.58), 0.053 (0.035–0.074) and 0.0082 (0.0002–0.0912) for b1, α1 and α2, respectively. Posterior predicted values were obtained for the changes in percentage fat associated with incremental changes of 0.1–0.9 in BMI SDS, over intervals of 0.25 and 0.75 years, and the median posteriors are plotted in fig 3. The 95% credible intervals are expected to be wider than the PIs in fig 2, because in the Bayesian analysis the predicted values incorporated uncertainty in all three coefficients. Figure 3 suggested a fall in BMI SDS of 0.7 or more over 9+ months would be consistent with percentage fat reduction, and a smaller change (0.6) over 3 months.
For the second model, which used change in BMI, BMI2 and the time interval, median posteriors (and 95% credible region) for the coefficients b1, b2 and b3 were 3.83 (1.56 to 6.05), −0.0339 (−0.0663 to −0.0010) and 0.796 (−0.134 to 1.726), respectively, and for the coefficients α1 and α2 were 0.060 (0.041 to 0.084) and 0.0048 (0.0001 to 0.0640). Median posterior predicted values (and 95% credible regions) are shown in fig 4 for integral changes in BMI from 1 to 5, and over time intervals of 0.25, 0.75 and 1.25 years.
Footnotes
Competing interests: None.
References
- 1.Department of Health National standards, local action. health and social care standards and planning framework, 2005/06–2007/08. London: Stationery Office,
- 2.Summerbell C D, Ashton V, Campbell K J.et al Interventions for treating obesity in children. Cochrane Database Syst Rev 2003(3)CD001872. [DOI] [PubMed]
- 3.Ball G D, Shaibi G Q, Cruz M L.et al Insulin sensitivity, cardiorespiratory fitness, and physical activity in overweight Hispanic youth. Obes Res 200412(1)77–85. [DOI] [PubMed] [Google Scholar]
- 4.Boreham C, Twisk J, Murray L.et al Fitness, fatness, and coronary heart disease risk in adolescents: the Northern Ireland Young Hearts Project. Med Sci Sports Exerc 200133(2)270–274. [DOI] [PubMed] [Google Scholar]
- 5.Winder W W, Hardie D G. Inactivation of acetyl‐CoA carboxylase and activation of AMP‐activated protein kinase in muscle during exercise. Am J Physiol 1996270(2 Pt 1)E299–E304. [DOI] [PubMed] [Google Scholar]
- 6.Hood D A. Invited review: contractile activity‐induced mitochondrial biogenesis in skeletal muscle. J Appl Physiol 200190(3)1137–1157. [DOI] [PubMed] [Google Scholar]
- 7.Sabin M A, Ford A L, Hunt L P.et al Which factors are associated with a successful outcome in a weight management programme for obese children? J Eval Clin Pract. In press [DOI] [PubMed]
- 8.Gibson P, Edmunds L, Haslam D W.et alAn approach to weight management in children and adolescents (2–18 years) in primary care. Royal College of Paediatrics and Child Health and National Obesity Forum joint information leaflet. London: RCPCH, 2002 [PubMed]
- 9.Cole T J, Freeman J V, Preece M A. Body mass index reference curves for the UK, 1990. Arch Dis Child 199573(1)25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pietrobelli A, Andreoli A, Cervelli V.et al Predicting fat‐free mass in children using bioimpedance analysis. Acta Diabetol 200340(Suppl 1)S212–S215. [DOI] [PubMed] [Google Scholar]
- 11.Pietrobelli A, Rubiano F, St‐Onge M P.et al New bioimpedance analysis system: improved phenotyping with whole‐body analysis. Eur J Clin Nutr 200458(11)1479–1484. [DOI] [PubMed] [Google Scholar]
- 12.McCarthy H D, Cole T J, Fry T.et al Body fat reference curves for children. Int J Obes (Lond) 200630(4)598–602. [DOI] [PubMed] [Google Scholar]
- 13.Cole T J, Bellizzi M C, Flegal K M.et al Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 2000320(7244)1240–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cole T J, Faith M S, Pietrobelli A.et al What is the best measure of adiposity change in growing children: BMI, BMI%, BMI z‐score or BMI centile? Eur J Clin Nutr 200559(3)419–425. [DOI] [PubMed] [Google Scholar]
- 15.Montague C T, O'Rahilly S. The perils of portliness: causes and consequences of visceral adiposity. Diabetes 200049(6)883–888. [DOI] [PubMed] [Google Scholar]
- 16.Reinehr T, Kiess W, Kapellen T.et al Insulin sensitivity among obese children and adolescents, according to degree of weight loss. Pediatrics 2004114(6)1569–1573. [DOI] [PubMed] [Google Scholar]
- 17.Reinehr T, Andler W. Changes in the atherogenic risk factor profile according to degree of weight loss. Arch Dis Child 200489(5)419–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weiss R, Dziura J, Burgert T S.et al Obesity and the metabolic syndrome in children and adolescents. N Engl J Med 2004350(23)2362–2374. [DOI] [PubMed] [Google Scholar]