Abstract
There is evidence that sleep enhances memory and learning. Childhood is a critical period for neurodevelopment, and minor but persistent disruption of sleep may have long‐term implications for cognitive performance. Sleep is critical for health and is undervalued both in our 24 h society and in paediatric clinical practice. Paediatricians need to understand the neurodevelopmental consequences of poor quality sleep in children.
Keywords: sleep, memory, learning, sleep disordered breathing
If sleep does not serve an absolutely vital function, then it is the biggest mistake the evolutionary process ever made. Dr Alan Rechtshaffen, sleep research pioneer
What is sleep?
Sleep, a reversible state of perceptual disengagement, is a universal behaviour across the animal kingdom. Despite the inherent vulnerability of the sleeping child, sleep behaviour is the primary brain activity of early development, occupying on average 13 months of the first 2 years of human life. Sleep subserves complex inter‐related physiological processes, is conspicuously important to the developing child and yet remains a blind spot in the undergraduate and post‐graduate medical curriculum.1 Most paediatricians will be familiar with the importance of sleep to endocrine and respiratory physiology, for example the association of growth hormone secretion with sleep in pre‐pubertal children or the impact of reduced nocturnal respiratory function in chronic respiratory and neuromuscular disease. However, many would struggle to describe the primary importance of sleep to the developing brain.
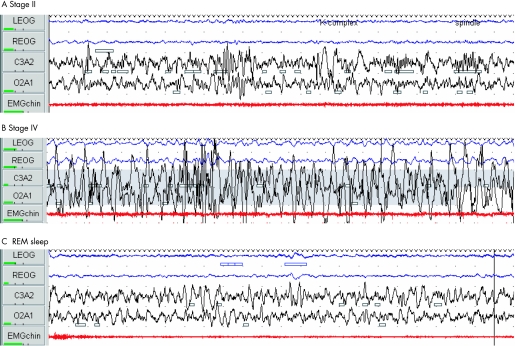
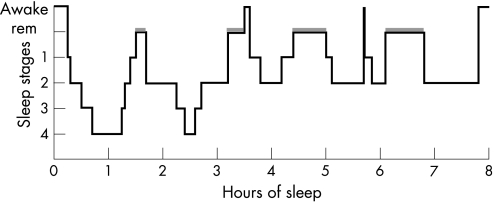
Sleep physicians traditionally describe sleep in terms of its neurophysiology, as EEG recordings provided the earliest window on the activity of the brain in sleep. The discovery of REM (rapid eye movement) sleep by Aserinsky and Kleitman in 1953 revealed that sleep is an active rather than a passive brain process.2 We now recognise discrete patterns of EEG, muscle activity and eye movements throughout REM sleep (dream sleep) and the four stages of non‐REM sleep (fig 1). Humans, primates and cats cycle through the stages of sleep in a predictable order referred to as “sleep architecture”. For example, adults normally pass through sleep cycles of non‐REM sleep and REM sleep, which typically last about 90–110 min (fig 2). Sleep cycles in children are typically shorter, around 50 min in infants, gradually extending through childhood. Non‐REM deep sleep is sub‐divided into four stages (I to IV), corresponding to increasing depths of sleep. Sleep is entered through stage I, discernable through a slowing of the EEG as the fast alpha waves typical of the drowsy wake state are replaced by lower frequency theta waves. This stage is also accompanied by slow rolling eye movements; awareness of this stage may be a familiar sensation to the reader. Stage I, however, is a brief transition phase which rapidly progresses to stage II sleep when sleep onset is formally defined. Stage II sleep is the most prevalent state of sleep (table 1).
Figure 1 Characteristic 30 s epoch polysomnographic traces of REM and non‐REM sleep stages II and IV in a 4 year old child. Note EEG (black), EOG (blue) and EMG (red). (A) Stage II sleep: low voltage mixed frequency EEG and characteristic K complexes (large positive deflection followed by negative deflection for >0.5 s) and 12–14 Hz spindles. (B) Stage IV sleep: EEG high amplitude slow wave (⩽4 Hz) for >50% of the epoch. EOG reflects the high frequency activity of EEG. (C) REM sleep: low voltage mixed frequency EEG with characteristic saw‐tooth waves. There was conjugate eye movement on EOG in this epoch and marked suppression of tone on sub‐mental EMG.
Figure 2 Hypnogram graphically illustrating typical sleep architecture with the slow wave sleep of non‐REM stages III and IV predominantly located in the first half of the night and REM sleep cycles (shaded boxes) featuring more prominently during the latter half of sleep.
Table 1 Sleep stages in three age groups of preadolescent children3.
| Aged 6–7 years | Aged 8–9 years | Aged 10–11 years | |
|---|---|---|---|
| Stage I, % | 5 | 5 | 6 |
| Stage II, % | 49 | 53 | 52 |
| Stage III and IV, % | 21 | 20 | 20 |
| REM, % | 25 | 22 | 22 |
The electrophysiological markers of stage II sleep may have particular relevance to memory consolidation; bursts of fast theta rhythms, or “sleep spindles” generated from thalamic nuclei4 and intermittent high amplitude K complexes (large positive and immediate negative deflection of the EEG) have both been associated with learning. The theoretical significance of these EEG events will be discussed later.
Stages III and IV sleep are most easily recognised by the untrained eye and are characterised by the slowest electrical waves in the delta frequency (<4 Hz), which reflect synchronised depolarisation and hyperpolarisation in large populations of neurons (also termed slow wave sleep). Slow wave sleep is characterised by a predominance of vagal activity with slowing and regularising of cardio‐respiratory rates. Children in this sleep stage are difficult to rouse, hence the alternate term ”deep sleep”. Even though the child is deeply asleep, EMG monitoring shows that tonic activity of the postural muscles is preserved, although in practice whole body movement is almost entirely absent. It is, however, in this stage of sleep that parasomnias (behaviours in sleep) such as sleep walking are possible. In contrast, REM sleep, with its desynchronised cortical depolarisation and hippocampal rhythmic theta waves, is characterised by profound muscular hypotonia and increased sympathetic nervous system activation with irregularity of cardiac and respiratory rates. The muscular hypotonia of REM sleep is functionally important as it prevents the sleeper from physically acting out their dreams. It also has potentially adverse consequences for vulnerable populations, engendering risk of upper airway obstruction and hypoventilation. A distinctive feature of REM sleep is activation of cholinergic neurons to levels seen in the waking state.5 This contrasts with non‐REM sleep where acetylcholine release is suppressed. This unique feature of REM sleep will be alluded to later in the discussion.
As the brain journeys through the varied electrophysiological landscape of sleep, different areas of the brain are engaged or disengaged. Functional brain imaging demonstrates that REM sleep is characterised by activation of hippocampal and limbic structures as well as the medial pre‐frontal cortex. In contrast, slow wave sleep orchestrates wider cortical involvement in the rhythmic activity characteristic of this stage with activation of thalamo‐cortical, hippocampo‐cortical and cortico‐cortical networks.6,7
The development of sleep
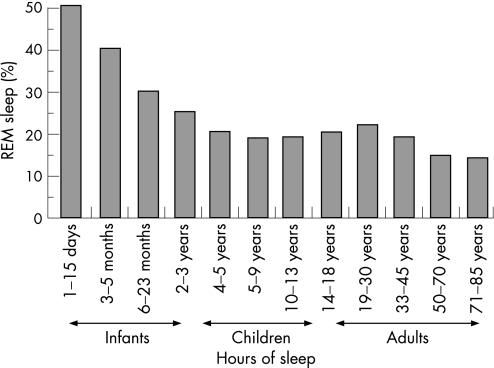
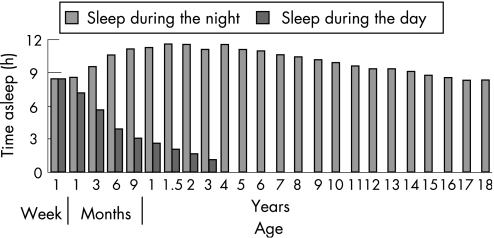
Sleep requirements alter through life as a function of age, and there are distinct differences between adult and infant sleep patterns. Even before birth, sleep is crucial for the foetus. Ultrasound research on foetal behaviour shows that it is only when the foetus is sleeping that it practises respiratory‐like movements, a vital preparation for breathing in the outside world.8 At birth infant sleep patterns are distinct from those of adults; unlike older infants, children and adults, very young infants fall directly into REM sleep and frequently transit from one sleep state to another, with sleep cycles lasting only about 60 min. Newborns spend up to 20 of every 24 h asleep, reducing to 14 h by 4 months, 11 h by 6 months and to around 10 h for the rest of the first 2 years. Around 50% of newborn sleep is REM‐like, so called ”active sleep”. REM sleep decreases steadily throughout childhood (fig 3) to reach an adult level of around 20–25% of total sleep time. Slow wave sleep peaks in early childhood and progressively wanes over life. Unlike adults, infants fall asleep directly in REM‐like active sleep, often awakening momentarily during the transition between stages. As infants mature, the quiet sleep stages lengthen and by 6 months the sleep cycle starts with non‐REM deep sleep. The infants have to learn to pass from one phase to the next without fully waking. The division of sleep between day and night also changes over the first 3 years of life (fig 4). By the age of 2, infants will have spent on average some 10 000 h asleep and some 7500 h awake, although of course there are many individual differences in sleep patterns.
Figure 3 Ontogenic development of the human sleep‐dream cycle. Data reproduced with permission from Dr Eric H Chudler, http://faculty.washington.edu/chudler/neurok.html
Figure 4 Changes in night‐time and daytime sleep with age. Data reproduced with permission from Dr Eric H Chudler, http://faculty.washington.edu/chudler/neurok.html
Sleep, memory and learning
The role of sleep in human learning is an exciting frontier of neuroscience research. Learning is a complex process requiring the brain to store and retain memory that can be called upon for future purposes. Before looking at the role of sleep in learning, it is important to briefly touch upon the nature of these dimensions.
Firstly, there are several steps in the formation of memory, which is not a static process (table 2). Secondly, memory is not a single entity (table 3). Broadly it can be categorised as declarative memory or “knowing that” (typified by factual recall, for example learning the alphabet) and as non‐declarative or procedural memory “knowing how” to do something (for example, riding a bike; table 2).
Table 2 The stages of memory formation.
| Acquisition and encoding | The process of getting information into memory. Initially memory traces are fragile and unstable |
| Consolidation/stabilisation | Processes responsible for putting information into long‐term storage, which, if disrupted, lead to forgetting |
| Association/ integration | Linking and combining new memories with those already in long‐term storage |
| Recall/recognition | Retrieval of information from storage. Recall involves calling something to mind without the help of an external stimulus reminder. Recognition involves remembering, triggered by the presence of an external stimulus |
| Reconsolidation | The re‐storage of information into permanent store after it has been recalled |
| Forgetting | The inability to access information that is represented in memory |
Table 3 The nature of memory.
| Declarative memory (“Knowing that”) | |
| Episodic | Place and time dependant, eg, I found ten pence at the train station this morning |
| Semantic | Knowledge independent of time, eg, blue is a colour or that Paris is the capital of France |
| Autobiographical | Memories for events and issues relating to oneself. Can be semantic, eg, knowing you own a dog, or episodic, eg, remembering receiving the dog as a present |
| Non‐declarative memory (“Knowing how”) | |
| Procedural | Skills such as knowing how to play the piano |
| Priming | Enhanced response to something because of recent exposure to it (eg, after buying a new car, suddenly noticing many similar ones on the road) |
| Non‐associative | A behavioural change brought by repeated presentation (eg, a loud crash that is initially startling and gradually becomes less so if it is repeated again and again) |
| Classical conditioning | A response originally elicited by one stimulus, can now be elicited by another one that originally had no effect (eg, Pavlov turning the light on before feeding the dogs resulted in the dogs salivating to the light alone) |
Even this simple differentiation highlights the very diverse brain regions likely to be recruited in learning and the intrinsic complexity of mapping the process. Furthermore, when considering the role of sleep in learning, we need to understand these dimensions in relation to the different sleep stages already described. A comprehensive review of all existing literature is beyond the scope of this article and the reader may wish to refer to more detailed reviews.9,10 As current research builds up a picture of sleep and learning, inevitably individual examples only provide one piece of a complex jigsaw. Much of the evidence comes from animal and adult human research and application to infants and children is inferred. This is partly explained by the cost and ethical limitations of research techniques, such as functional imaging in young children. Nonetheless, future paediatric research will be important to study the function of sleep in the context of normal brain and behavioural development.
Three categories of evidence for a role of sleep in memory consolidation will be considered:
how sleep deprivation affects learning;
improvements in learning achieved following a period of sleep; and
neural activation in sleep following learning.
The impact of sleep deprivation
Much of the earliest research on sleep and memory consolidation focused on the role of REM sleep. This was a logical starting point given that REM sleep predominates during the intensive early period of infant brain maturation and is known to be associated with dreaming. The earliest experimental data used animal models. It was already established that newborn rats exposed to environmental enrichment develop a larger cerebral cortex, more synaptic connections and better problem solving abilities than controls. In 1983 Mirmiran and Uylings reported the effects of pharmacological REM deprivation in female rats using clonidine.11 Clonidine acts through inhibition of cholinergic activation which, as already described, is required for normal REM sleep. Controlling for a possible drug‐related effect, the authors noted that REM deprivation removed the benefits of environmental enrichment noted earlier.
More recent use of functional imaging techniques provides illuminating evidence of dysfunctional brain activity after sleep deprivation. Functional MRI in sleep‐deprived healthy young adults using a verbal learning task revealed increased pre‐frontal and parietal activity and decreased hippocampal activity compared to controls who experienced normal sleep.12 The sleep deprived adults performed significantly worse in the task. The researchers suggested that the hippocampus (as the brain's memory encoding centre) failed to engage normally in the sleep‐deprived state, leading to compensatory activity in the cortical areas associated with high working memory and cognitive load. Literally the brain had to work harder to complete the task. More recent research has used a similar experimental model in the context of visual memory.12a Adult volunteers were tested on recall of images with negative, positive and neutral emotional valence. Sleep deprivation negatively impacted on the accurate recall of all images and emotional valence of images reinforced recall in both sleep refreshed and sleep deprived subjects. Functional imaging again indicated a hippocampal deficit in sleep deprived subjects suggesting off‐line processing of emotional memories during sleep. This latter example also highlights the importance of emotional and situational context for memory and the inherent complexity of memory research.
Learning enhancement following sleep
If absence of sleep is negatively associated with learning and memory, is there evidence for sleep making a positive contribution to these processes? A fascinating animal experiment of sleep‐dependant learning enhancement focused on birds that learn their song (zebra finches, canaries). The baby chicks were divided into two groups. Both groups heard the mother's song for a given period, followed by a period of rest. The first group of birds simply rested in an awake state, whereas for the second group of chicks sleep was induced during the same resting period. Subsequently it was shown that the group which slept during the resting period learnt the mother song faster and more accurately than the group that merely rested. Moreover, whereas in the resting group brain activity reduced considerably, in the sleeping group brain activity was as great as during the initial awake period, suggesting that the sleeping chicks were practising or re‐running the sounds of the mother song to commit them to memory.13
In a similar study of cats, researchers compared doubling the amount of training in one group with half the training time plus sleep in the other. Both groups of cats initially received the same amount of environmental stimulation. Then one group slept for 6 h while the other was kept awake and continued to receive environmental stimulation. Comparisons of brain connectivity between the two groups revealed twice the amount of neuronal connectivity in the sleeping cats' brains compared to those which received twice the amount of training. Brain activity in the sleeping cats turned out to be greatest during non‐REM sleep.14
The studies on birds and cats suggest that sleep plays a critical role in learning and memory, perhaps associated with the development and more efficient working of neural assemblies. Few studies of human infants have investigated brain function associated with learning during sleep. However, one study of evoked potentials is of interest. Newborns exposed to vowel sounds during sleep were shown to have enhanced discrimination of similar‐sounding sounds (eg, /y/ and /i/) compared to controls who were not exposed during sleep.15 While of experimental interest, the exposure of children to nocturnal stimuli is unlikely to become a clinical recommendation!
Studies in adult humans have illustrated that declarative memory tasks such as recall of word pairs are preferentially enhanced by slow wave sleep, while procedural learning tasks (such as mirror tracing) are preferentially enhanced by REM sleep. In an elegant experiment, adult volunteers were taught both a mirror tracing skill and word pair task.16 One group learned the tasks at 22:15 h and were then allowed to sleep (experiencing predominantly slow wave sleep; see fig 2) before being retested at 02:15 h. A second group were allowed to sleep for the first half of the night, learned their task at 02:15 h and then were retested after sleep (predominantly REM) at 06:15 h. Both groups were tested alongside controls who had had no sleep. There was a significant improvement in declarative task performance in the early slow wave sleep exposed group compared to the late taught group and controls. In the non‐declarative task, late taught groups (exposed to predominantly REM sleep) showed significant improvement compared to early taught groups and controls. It may not surprise the reader to learn that the association between slow wave sleep and declarative memory consolidation, and REM sleep and non‐declarative memory consolidation is not quite as straightforward as this research suggests, not least because learning tasks rarely conform to the rather simplistic declarative/non‐declarative differentiation. Nonetheless, such studies generate important hypotheses for testing pathways to memory consolidation in relation to sleep neurophysiology in children in whom memory functions are still developing.
Cholinergic activity in REM sleep and wakefulness is thought to facilitate neural connectivity between the cortex and the hippocampus. Thus new tasks learnt when awake are transferred from the cortex and encoded in the hippocampus. In slow wave sleep, reduced cholinergic activity suppresses this direction of information flow but conversely promotes the reactivation of hippocampally located memory and transfer to cortical structures, thus promoting memory consolidation.10 This theory is supported by the results of experimental pharmacological cholinergic activation in healthy adults which has been demonstrated to block slow wave sleep‐related consolidation of declarative memory.17 Acetylcholine may therefore act as a directional switch enabling communication between the cortex and hippocampus to facilitate either memory encoding or consolidation.
A further interesting discovery is the relevance of neocortical slow oscillations. These at <1 Hz are of lower frequency than classical slow waves. Emerging during the transition into slow wave sleep, these slow oscillations appear to originate in the prefrontal cortex and recruit the entire neocortex. Their appearance in sleep correlates with daytime learning tasks.18 Evidence reviewed by Born et al10 suggests an intriguing hypothesis, namely that during slow depolarisation, efferents to the thalamus trigger reciprocal thalamo‐cortical spindle discharges and activation of hippocampal memories prompting hippocampo‐neocortical discharge. Thus the slow oscillations are thought to encourage a synchronisation of memory activation enhancing connections between vital memory structures in the brain.
While many of the studies of sleep and memory have focused on slow wave and REM sleep, more recent research has suggested a role for the sleep spindle, characteristic of stage II sleep, in triggering cellular mechanisms that enhance lasting structural or functional neural change.19,20 One research group found that the number of sleep spindles over the frontal cortex correlated with the retention of verbal memory,21 whereas the number of sleep spindles over the parietal cortex correlated with visuospatial memory retention.22 The authors concluded that the beneficial effects of sleep on learning are differentiated according to brain region and memory task.
Reactivation of neural networks associated with learning during sleep
Perhaps some of the most persuasive and direct evidence of a role for sleep in learning comes from imaging studies indicating that neural networks associated with new learning are reactivated in sleep. For example, PET studies of the brain following a spatial learning task (navigating through a three‐dimensional virtual town) showed the right hippocampus and parahippocampal gyrus to be activated both during the task learning exercise and then again in subsequent slow wave sleep.23 Furthermore, there was a significant correlation between regional cerebral blood flow and subsequent performance when subjects were re‐tested after sleep. Interestingly, there are some circumstances where the beneficial effect of sleep on memory may not always be adaptive. A recent study suggested that sleep may promote the maintenance of potentially negative emotional memories in adults with posttraumatic stress disorder24; no such studies have been conducted in children.
Convergent evidence suggests a role for sleep in memory processing and neural plasticity. This area is not without controversy and debate in the sleep science community,25 but there is a growing body of evidence to support such a relationship. As Stickgold and Walker noted in their review of 46 recently published research articles, 83% supported a relationship between sleep and memory.26 We have presented a sample of those studies that provide evidence of the role of both REM and non‐REM sleep stages in the consolidation of memory. Furthermore, we have indicated the relationship between the electrophysiological and neuroanatomical characteristics of these stages and memory consolidation. Future research will confirm the cellular and molecular mechanisms linking these features of sleep to long‐term potentiation of neural pathways responsible for memory and learning. A major challenge for sleep science is to now translate these approaches to the study of children to better understand the developmental aspects of the relationship between sleep, memory and learning. The need for such evidence is becoming increasingly important as we understand more about the effect of sleep disorders in children on their behaviour and cognitive function.
Evidence for learning and memory impairment in sleep disordered breathing
Sleep disordered breathing provides an important clinical model to study the impact of sleep disruption on learning and memory function, as it commonly occurs in otherwise healthy children. The term describes a spectrum of upper airway obstruction in sleep with a common symptom of snoring. The most severe form, obstructive sleep apnoea, has prevalence rates of 0.9% to 4.3%.27,28 Primary snoring occurs in around 10% of pre‐school children29 and, unlike obstructive sleep apnoea, it is not characterised by perceptible repetitive arousals in sleep or intermittent hypoxaemia. Aetiological factors in childhood include adenotonsillar hypertrophy and upper airway collapsibility,30 although obesity with associated neck adiposity is an increasing contributor31 as the population prevalence of obesity rises. Accurate assessment of sleep disordered breathing and its impact on the integrity of sleep architecture can only be achieved by polysomnography,32 which is not universally available in tertiary paediatric centres in the UK (CM Hill, communication to the Paediatric Special Interest Group, British Sleep Society Annual Meeting, 2005). Even with the availability of polysomnography, the clinical thresholds for intervention33 (generally adenotonsillectomy) remain controversial and have been guided in the past by complications such as systemic and pulmonary hypertension and failure to thrive rather than by neurocognitive considerations.
In 1892 Osler noted that children with tonsillar hypertrophy were “stupid looking” and slow to respond to questions.34 It took another 80 years before Guilleminault and colleagues re‐ignited medical interest in this area by reporting impairment of school performance in these children.35 Since then a burgeoning literature has provided more extensive and specific information about the nature of these impairments.
Memory
Recent neuropsychological research describes impairment in specific memory domains (episodic and working memory) in adults with obstructive sleep apnoea.36 Evidence of memory impairment in studies of children is conflicting, partially due to the fact that studies utilise different age groups and examine different types of memory. Gottlieb et al did not show any differences between 5 year olds with and without sleep disordered breathing on NEPSY memory measures.37 Conversely, in a prospective study of children aged 6–12, Kaemingk et al reported significant differences between sleep disordered breathing and control groups matched for age, socio‐economic status and ethnicity38 using the Children's Auditory Verbal Learning Test, a test of learning of and memory for words. Working memory deficits have also been described in children with poorer sleep efficiency and longer sleep latency39 and this may be consistent with constrained frontal cortex function.
However, a generalised effect on cognitive function and behaviour rather than a specific effect on memory is likely in children, in whom functional brain specialisation is still developing. Specifically, it is not clear if children who have disrupted sleep, due to repeated arousals or apnoea or both, have a primary deficit in one domain of cognitive function, with secondary implications for other domains, or if there is a generalised constraint on cognitive and behavioural function. Executive dysfunction has been reported in young children with sleep disordered breathing,40 but these higher order cortical functions continue to mature throughout adolescence commensurate with protracted maturation of the frontal lobes. Other domains of function such as language and perception have been less well examined. Thus, it is hard to confirm that memory is particularly vulnerable.
Intellectual ability and school performance
A number of clinical studies have identified reduced IQ indices in children with sleep disordered breathing compared to controls.31,41 However, population studies are contradictory, and it is important to point out that IQ does not typically fall below the average range. Kaemingk et al failed to demonstrate differences in full scale, performance or verbal IQ between socio‐economically equivalent children (mean age 8.6 years) with obstructive sleep apnoea compared to primary snorers.38 Conversely, O'Brien et al using the Differential Ability Scales reported significantly poorer performance in General Conceptual Ability and Verbal Cluster domains for primary snorers compared to controls aged 5–7 years.42
The evidence that sleep disordered breathing impacts on school performance is more convincing. Gozal et al demonstrated that frequent loud snoring in the pre‐school years was significantly more common in 7th and 8th grade North American children in the bottom 25th centile compared to peers matched for socio‐demographic factors who were in the top 25th centile for school performance.43 The authors suggested a learning debt consequent on sleep‐disordered breathing in early childhood. The same group studied 7th grade children in the lowest 10th percentile of class performance. A sub‐group, identified by clinical assessment as having obstructive sleep apnoea, were advised to seek the opinion of ENT professionals.44 Twenty four children underwent adenotonsillectomy and 30 parents did not seek referral. The following year the children who had undergone surgery demonstrated significant improvement in their school grades compared with the children who had not undergone surgery. This offered ecologically valid data that performance difficulties may be reversible.
Possible causes of memory and learning impairment in sleep disordered breathing
Sleep disordered breathing may result in intermittent hypoxia and/or sleep fragmentation due to arousal. Some children may experience frequent respiratory related arousal, significantly interrupting the integrity of their sleep architecture and limiting REM sleep but yet experience no hypoxia. Others may experience varying degrees of hypoxia (this latter group are readily identified by overnight pulse oximetry), while some experience a combination of both. The reason why some children become hypoxic and others arouse from sleep in response to airway compromise is unclear. It is well established that intermittent hypoxia is an important mediator of neurocognitive deficit in children.45,46,47 Animal models simulating isolated intermittent hypoxia have shown neuronal cell loss in brain areas critical for executive function and memory, namely the pre‐frontal cortex and hippocampus.48 Despite the absence of sleep disturbance in these models, the study rats showed deficits in spatial memory tasks.
However, studies of childhood sleep‐disordered breathing fail to consistently show strong correlations between overnight polysomnographic variables and measurable neurocognitive outcomes,38,49 suggesting a complex relationship between clinically measurable pathophysiological processes and neurocognitive impairment. This is supported by a recent finding that primary snoring, in the absence of measurable nocturnal hypoxia at the time of assessment, is associated with adverse neurobehavioural characteristics. O'Brien et al identified reduced attention and increased social problems and anxiety/depressive symptoms in 5–7 year old primary snorers.42 Clearly, even subtle disruption of sleep may be sufficient to generate adverse daytime effects. It is feasible that subtle intrusion of wake activity which is not detected by standard EEG analysis occurs in these milder spectrum disorders and is sufficient to impair normal sleep physiology at a neural level. Chervin et al demonstrated a variation of EEG power during non‐apnoeic respiratory cycles that altered following adenotonsillectomy and postulated that these may represent micro‐arousals.50
A limitation of much paediatric sleep research is that children are only assessed on one night. This reflects the cost and compliance issues of polysomnography. However, adenoidal hypertrophy develops over years and has a tendency to worsen with intercurrent upper respiratory tract infection. Intermittent chronic hypoxia cannot be accurately reflected by single time point sampling. Technologies that allow longitudinal study may shed light on pathways to learning impairment in sleep disordered breathing.
Cognitive reserve theory may also help to explain why there are few correlations between polysomnographic and neuropsychology measures. Cognitive reserve suggests that any compromise of brain function will manifest less in the behaviour of those with higher compared to lower levels of intelligence, as those with higher levels of intelligence have more to lose before the deficit becomes apparent. In one study, adults were divided into high (>90th percentile) and low intelligence groups. In the high intelligence group, there was no difference between obstructive sleep apnoea patients and controls on measures of attention, whereas there were significant group differences in the low functioning group. The authors concluded that high intelligence has a protective effect.51 To our knowledge, there has been no similar analysis in child studies, but, as IQ is typically within the average to higher average range,52 cognitive reserve may partially account for lack of correlations with polysomnographic measures of snoring severity. It is possible that if only those children who are of lower intelligence (determined by a median split of any one sample) are included in the analysis, then significant correlations may emerge.
Unravelling the mechanisms leading to neurocognitive dysfunction in sleep disordered breathing remains a challenge to sleep medicine research. What is clear at the present time is that children have reversible but potentially long‐lasting neurocognitive impairment, albeit subtle, from what has previously been considered benign symptomatology.
Summary and implications for practice
In summary, recent advances in neuroimaging have permitted exploration of sleep beyond the frontier of surface electrophysiology and have demonstrated that certain sleep stages are distinct in terms of their functional neuroanatomy and neurochemistry. Healthy sleep appears to be a pre‐condition for learning and in turn consolidates and enhances memories, helping to integrate them into existing neural networks. In this context, features of sleep electrophysiology are seen in a new light, no longer mere patterns by which to identify sleep architecture but electrical reflections of nocturnal synaptogenesis. It is possible that in mild sleep disordered breathing, disrupted sleep micro‐architecture is sufficient to impair memory processing with consequences for more general cognitive function and behaviour in children, although this requires further investigation. This raises important questions about our attitude to and management of behavioural sleep disorders and medical conditions that interrupt the subtle rhythms and balance of sleep. Even more important are the implications for vulnerable populations of children with atypical brains who have additional risk factors for sleep disruption (eg, physical, behavioural, co‐morbid epilepsy). Assuming that an important mechanism of sleep is to consolidate learning through synchronous firing of neurons across brain regions, it is perhaps unsurprising that sleep in children with epilepsy has been investigated as one cause of their cognitive deficit.53 Abnormal sleep may also contribute to learning deficits in seizure‐free children with more generalised developmental disorders such as Down syndrome.54
Increasingly, sleep is vulnerable to disruption from features of our environment that would not have concerned our ancestors, such as 24 h light and sound stimuli. There is evidence for long‐term memory deficits in humans55 exposed to environmental noise, such as air traffic. Children's bedrooms may be poor sleep environments, stocked with capability for 24 h stimulation and communication, for example music systems, mobile phones, and the internet. Paradoxically, then, the ontogeny of human brain development is facilitating greater intelligence and simultaneously creating the means by which to constrain its function. As a society and as paediatricians serving the healthy development of children, we need to wake up to the importance of sleep and its disorders.56
Acknowledgements
Thanks are due to sleep technologist, Annette Paul, for assistance with the figures for this article.
Footnotes
Competing interests: None.
References
- 1.Stores G, Crawford C. Medical student education in sleep and its disorders. J R Coll Physicians Lond 199832(2)149–153. [PMC free article] [PubMed] [Google Scholar]
- 2.Aserinsky E, Kleitman N. Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science 1953118273–274. [DOI] [PubMed] [Google Scholar]
- 3.Quan S F, Goodwin J L, Babar S I.et al Sleep architecture in normal Caucasian and Hispanic children aged 6–11 years recorded during unattended home polysomnography: experience from the Tucson Children's Assessment of Sleep Apnea Study (TuCASA). Sleep Med 20034(1)13–19. [DOI] [PubMed] [Google Scholar]
- 4.Nicolas A, Petit D, Rompre S.et al Sleep spindle characteristics in healthy subjects of different age groups. Clin Neurophysiol 2001112(3)521–527. [DOI] [PubMed] [Google Scholar]
- 5.Espana R A, Scammell T E. Sleep neurobiology for the clinician. Sleep 200427(4)811–820. [PubMed] [Google Scholar]
- 6.Muzur A, Pace‐Schott E F, Hobson J A. The prefrontal cortex in sleep. Trends Cogn Sci 20026(11)475–481. [DOI] [PubMed] [Google Scholar]
- 7.Benington J H, Frank M G. Cellular and molecular connections between sleep and synaptic plasticity. Prog Neurobiol 200369(2)71–101. [DOI] [PubMed] [Google Scholar]
- 8.Mirmiran M, Maas Y G, Ariagno R L. Development of fetal and neonatal sleep and circadian rhythms. Sleep Med Rev 20037(4)321–334. [DOI] [PubMed] [Google Scholar]
- 9.Walker M P, Stickgold R. Sleep, memory, and plasticity. Annu Rev Psychol 200657139–166. [DOI] [PubMed] [Google Scholar]
- 10.Born J, Rasch B, Gais S. Sleep to remember. Neuroscientist 200612(5)410–424. [DOI] [PubMed] [Google Scholar]
- 11.Mirmiran M, Uylings H B. The environmental enrichment effect upon cortical growth is neutralized by concomitant pharmacological suppression of active sleep in female rats. Brain Res 1983261(2)331–334. [DOI] [PubMed] [Google Scholar]
- 12.Drummond S P, Brown G C, Gillin J C.et al Altered brain response to verbal learning following sleep deprivation. Nature 2000403655–657. [DOI] [PubMed] [Google Scholar]
- 12a. Sterpenich V, Albouy G, Boly M L.et al The role of sleep in the consolidation of emotional memories in humans: a fMRI study. J Sleep Res 200615(Suppl 1) 190 [Google Scholar]
- 13.Rauske P L, Shea S D, Margoliash D. State and neuronal class‐dependant reconfiguration in the avian song system. J Neurophysiol 200389(3)1688–1701. [DOI] [PubMed] [Google Scholar]
- 14.Frank M G, Issa N P, Stryker M P. Sleep enhances plasticity in the developing visual cortex. Neuron 200130(1)275–287. [DOI] [PubMed] [Google Scholar]
- 15.Cheour M, Martynova O, Näätänen R.et al Speech sounds learned by sleeping newborns. Nature 2002415599–600. [DOI] [PubMed] [Google Scholar]
- 16.Plihal W, Born J. Effects of early and late nocturnal sleep on declarative and procedural memory. J Cogn Neurosci 19979534–547. [DOI] [PubMed] [Google Scholar]
- 17.Gais S, Born J. Low acetylcholine during slow wave sleep is critical for declarative memory consolidation. Proc Natl Acad Sci U S A 20041012140–2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huber R, Ghilardi M F, Massimini M.et al Local sleep and learning. Nature 2004430(6995)78–81. [DOI] [PubMed] [Google Scholar]
- 19.Schabus M, Hödlmoser K, Gruber G.et al Sleep spindle‐related activity in the human EEG and its relation to general cognitive and learning abilities. Eur J Neurosci 2006231738–1746. [DOI] [PubMed] [Google Scholar]
- 20.Sejnowski T J, Destexhe A. Why do we sleep? Brain Res 2000886208–223. [DOI] [PubMed] [Google Scholar]
- 21.Clemens Z, Fabó P, Halász P. Overnight verbal memory retention correlates with the number of sleep spindles. Neuroscience 2005132529–535. [DOI] [PubMed] [Google Scholar]
- 22.Clemens Z, Fabó P, Halász P. Twenty‐four hours retention of visuo‐spatial memory correlates with the number of parietal sleep spindles. Neurosci Lett 200640352–56. [DOI] [PubMed] [Google Scholar]
- 23.Peigneux P, Laureys S, Fuchs S.et al Are spatial memories strengthened in the human hippocampus during slow wave sleep? Neuron 200444(3)535–545. [DOI] [PubMed] [Google Scholar]
- 24.Wagner U, Hallschmid M, Rasch B.et al Brief sleep after learning keeps emotional memories alive for years. Biol Psychiatry 200660(7)788–790. [DOI] [PubMed] [Google Scholar]
- 25.Vertes R P, Siegel J M. Time for the sleep community to take a critical look at the purported role of sleep in memory processing. Sleep 200528(10)1228–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stickgold R, Walker M P. Sleep and memory: the ongoing debate. Sleep 200528(10)1225–1227. [DOI] [PubMed] [Google Scholar]
- 27.Castronovo V, Zucconi M, Nosetti L.et al Prevalence of habitual snoring and sleep‐disordered breathing in pre‐school aged children in an Italian community. J Pediatr 2003142(4)364–365. [DOI] [PubMed] [Google Scholar]
- 28.Sogut A, Altin R, Uzun L.et al Prevalence of obstructive sleep apnea syndrome and associated symptoms in 3‐11‐year‐old Turkish children. Pediatr Pulmonol 200539(3)251–256. [DOI] [PubMed] [Google Scholar]
- 29.Kaditis A G, Finder J, Alexopoulos E I.et al Sleep‐disordered breathing in 3,680 Greek children. Pediatr Pulmonol 200437(6)499–509. [DOI] [PubMed] [Google Scholar]
- 30.Marcus C L. Sleep disordered breathing in children. Am J Crit Care Med 2001116416–30. [DOI] [PubMed] [Google Scholar]
- 31.Rhodes S K, Shimoda K C, Waid L R.et al Neurocognitive deficits in morbidly obese children with obstructive sleep apnea. J Pediatr 1995127(5)741–744. [DOI] [PubMed] [Google Scholar]
- 32.American Academy of Pediatrics Clinical practice guideline; diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 2002109(4)704–712. [DOI] [PubMed] [Google Scholar]
- 33.American Thoracic Society Cardiorespiratory sleep studies in children: establishment of normative data and polysomnographic predictors of morbidity. Am J Respir Crit Care Med 19991601381–1387. [DOI] [PubMed] [Google Scholar]
- 34.Osler W.The principles and practice of medicine. New York: Appleton, 1892335–339.
- 35.Guilleminault C, Eldridge F L, Simmons F B.et al Sleep apnea in eight children. Pediatrics 197658(1)23–30. [PubMed] [Google Scholar]
- 36.Naegele B, Launois S H, Mazza S.et al Which memory processes are affected in patients with obstructive sleep apnea? An evaluation of 3 types of memory. Sleep 200629533–544. [DOI] [PubMed] [Google Scholar]
- 37.Gottlieb D J, Chase C, Vezina R M.et al Sleep‐disordered breathing symptoms are associated with poorer cognitive function in 5‐year‐old children. J Pediatr 2004145(4)458–464. [DOI] [PubMed] [Google Scholar]
- 38.Kaemingk K L, Pasvogel A E, Goodwin J L.et al Learning in children and sleep disordered breathing: findings of the Tucson Children's Assessment of Sleep Apnea (TuCASA) prospective cohort study. J Int Neuropsychol Soc 20039(7)1016–1026. [DOI] [PubMed] [Google Scholar]
- 39.Steenari M R, Vuontela V, Paavonen E J.et al Working memory and sleep in 6‐ to 13‐year‐old schoolchildren. J Am Acad Child Adolesc Psychiatry 20034285–92. [DOI] [PubMed] [Google Scholar]
- 40.Khierandish L, Gozal D. Neurocognitive dysfunction in children with sleep disorders. Dev Sci 20069(4)388–399. [DOI] [PubMed] [Google Scholar]
- 41.Kennedy J D, Blunden S, Hirte C.et al Reduced neurocognition in children who snore. Pediatr Pulmonol 200437(4)330–337. [DOI] [PubMed] [Google Scholar]
- 42.O'Brien L M, Mervis C B, Holbrook C R.et al Neurobehavioral implications of habitual snoring in children. Pediatrics 2004114(1)44–49. [DOI] [PubMed] [Google Scholar]
- 43.Gozal D, Pope D W., Jr Snoring during early childhood and academic performance at ages thirteen to fourteen years. Pediatrics 2001107(6)1394–1399. [DOI] [PubMed] [Google Scholar]
- 44.Gozal D. Sleep‐disordered breathing and school performance in children. Pediatrics 1998102(3 Pt 1)616–620. [DOI] [PubMed] [Google Scholar]
- 45.Hogan A M, de Haan M, Datta A.et al Hypoxia: an acute, intermittent and chronic challenge to cognitive development. Dev Sci 20069335–337. [Google Scholar]
- 46.Bass J L, Corwin M, Gozal D.et al The effect of chronic or intermittent hypoxia on cognition in childhood: a review of the evidence. Pediatrics 2004114(3)805–816. [DOI] [PubMed] [Google Scholar]
- 47.Urschitz M S, Wolff J, Sokollik C.et al Nocturnal arterial oxygen saturation and academic performance in a community sample of children. Pediatrics 2005115(2)e204–e209. [DOI] [PubMed] [Google Scholar]
- 48.Gozal D, Daniel J M, Dohanich G P. Behavioral and anatomical correlates of chronic episodic hypoxia during sleep in the rat. J Neurosci 200121(7)2442–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chervin R D, Ruzicka D L, Giordani B J.et al Sleep‐disordered breathing, behavior, and cognition in children before and after adenotonsillectomy. Pediatrics 2006117(4)e769–e778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chervin R D, Burns J W, Subotic N S.et al Correlates of respiratory cycle‐related EEG changes in children with sleep‐disordered breathing. Sleep 200427(1)116–121. [DOI] [PubMed] [Google Scholar]
- 51.Alchanatis M, Zias N, Deligiorgis N.et al Sleep apnea‐related cognitive deficits and intelligence: an implication of cognitive reserve theory. J Sleep Res 20051469–75. [DOI] [PubMed] [Google Scholar]
- 52.Hill C M, Hogan A M, Onugha N.et al Increased cerebral blood flow velocity in children with mild sleep disordered breathing. Pediatrics 2006118(4)e1100–e1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nieuwenhuis L, Nicolai J. The pathophysiological mechanisms of cognitive and behavioural disturbances in children with Landau‐Kleffner syndrome or epilepsy with continuous spike‐and‐waves during slow‐wave sleep. Seizure 200615249–258. [DOI] [PubMed] [Google Scholar]
- 54.Diomedi M, Curatolo P, Scalise A.et al Sleep abnormalities in mentally retarded autistic subjects: Down's syndrome with mental retardation and normal subjects. Brain Dev 199921548–553. [DOI] [PubMed] [Google Scholar]
- 55.Schapkin S A, Falkenstein M, Marks A.et al Executive brain functions after exposure to nocturnal traffic noise: effects of task difficulty and sleep quality. Eur J Appl Physiol 200696693–702. [DOI] [PubMed] [Google Scholar]
- 56.Kotagal S, Pianosi P. Sleep disorders in children and adolescents. BMJ 2006332828–832. [DOI] [PMC free article] [PubMed] [Google Scholar]