Abstract
Objectives
Hypospadias, a common birth defect, has shown widespread variation in reported rates and temporal trends across countries over the last 30 years. The aim of this study was to determine the prevalence and trends of hypospadias in an Australian population.
Design
Population‐based study of all male infants born in Western Australia (WA) between 1980 and 2000 diagnosed with hypospadias and notified to the WA Birth Defects Registry.
Main outcome measures
Prevalence of hypospadias, birth outcome and association with other congenital anomalies, stratified by degree‐of‐severity.
Results
1788 cases of hypospadias were registered in WA in 1980–2000 with an overall prevalence of 34.8 (95% confidence interval (CI): 33.2 to 36.4) cases per 10 000 births. The prevalence increased by 2.0% per annum (95% CI: 1.2% to 2.8%) from 27.9 in 1980 to 43.2 per 10 000 births in 2000 (p<0.001). Hypospadias was mild in 84% of cases, moderate‐severe in 11% and unspecified in 5%, with the number of moderate‐severe hypospadias almost doubling over time (p<0.01). There were 1465 (82%) cases of isolated hypospadias and 323 (18%) had co‐existing anomalies. Infants with co‐existing genital (relative risk (RR) 4.5; 95% CI: 3.3 to 6.1) or non‐genital (RR 1.5; 95% CI: 1.0 to 2.2) anomalies were more likely to have moderate‐severe hypospadias compared with isolated cases.
Conclusion
Hypospadias affects one in 231 births and has been reported to have increased significantly over the last 20 years. Future investigation of the aetiology of hypospadias is important to identify potentially modifiable risk factors and ensure optimal male reproductive health in the future.
Keywords: hypospadias, prevalence, infant, congenital abnormality, Australia
Hypospadias is a congenital malformation of the male genitalia characterised by incomplete development of the urethra in which the opening is located on the ventrum rather than at the tip of the penis.1 Severity depends on the location of the urethral opening and, in most cases, the opening is located on the glans.2,3,4 Moderate or severe forms of hypospadias occur when the opening is situated more proximally on the penile shaft, at the base of the penis or, in rare cases, in the scrotum or perineum.1,5
The aetiology of hypospadias is unknown. However, possible causal mechanisms include lapse or disturbance in endogenous hormonal production, principally testosterone production during fetal development,6,7 or exposure to exogenous oestrogenic hormones in pregnancy.8,9 Insufficient amounts of androgen production may be due to early placental dysfunction and some studies have shown hypospadias to be associated with low birth weight.10,11 Hypospadias has also been found to have an increased risk of familial incidence12 and environmental exposure to endocrine‐disrupting chemicals including pollutants, industrial chemicals and pesticides and dietary phyto‐oestrogens (soya) have also been identified as risk factors.8,13,14
Hypospadias has been found to occur in up to four in every 1000 births. However, widespread variation in the rates and temporal trend over various time periods and across countries have been reported over the last 30 years.2,3,4,15 The aim of this investigation was to conduct a population‐based study to determine the prevalence and trends of hypospadias in an Australian population.
Methods
Study population and data sources
The study population consisted of all male infants born in Western Australia (WA) between 1980 and 2000 and diagnosed with hypospadias. Study data were obtained from the Western Australia Birth Defects Registry (WABDR), a population‐based notification system of malformations in WA established in 1980.16 The WABDR collates information on all reported malformations occurring in live births and stillbirths in WA and in pregnancies terminated because of fetal abnormalities, and includes malformations diagnosed in children up to 6 years of age. The registry uses multiple sources of notification to ascertain cases of birth defects, including statutory sources (midwives' notification of birth form, hospital morbidity data, death certificates) and a large number of voluntary sources (for example, private practitioners, diagnostic and treatment centres, child health nurses)16; the cases are registered by year of birth. Each individual birth defect is coded by the WABDR according to the British Paediatric Association International Classification of Diseases, 9th revision system (BPA‐ICD9) with up to 10 diagnostic categories available to allow coding of multiple birth defects per case.
Hypospadias cases were identified as infants with a WABDR code for hypospadias (75260, 75263–75269). These codes are based on the BPA‐ICD9 code for hypospadias (752.6) which are then modified by the WABDR by using the fifth digit to classify the degree of severity of hypospadias cases based on the location of the urethral opening.1 Cases with glanular (75263) or coronal (75264) forms of hypospadias were classified as mild, moderate severity was considered when the urethral opening was located subcoronally on the penile midshaft (75267) and severe was classified where the meatus opened on the scrotum or below in the cases of penoscrotal (75265) or perineal (75268) hypospadias. Confirmed hypospadias cases with degree of severity not otherwise specified (75260, 75266, 75269) were also included in the analysis. Epispadias (75261), a different and very rare condition where the urethra opens on the dorsum of the penis, and chordee (75262), curvature of the penis that is not always associated with hypospadias, were both excluded from the case definition. During the 21‐year study period, two infants were diagnosed with epispadias (one per 257 000 births). Chordee was associated with 43% of cases with hypospadias and a further 154 cases of isolated chordee were registered (three per 10 000 births).
Study outcomes
Overall prevalence and trends of hypospadias, birth outcome (live born, stillborn, termination), degree of severity and association with other congenital anomalies (74000–75999) were examined. Moderate and severe forms of hypospadias were combined due to small numbers. Rates were also examined for cases of isolated hypospadias and cases with additional co‐existing malformations and stratified by degree‐of‐severity. Isolated cases may have had chordee or hooded foreskin. Infants with hypospadias and other associated congenital anomalies were divided into two groups: cases with other types of genital anomalies such as undescended testis or anomaly of the testis or scrotum (75250–75299), excluding epispadias (75261), chordee (75262) and hooded foreskin (75286), and cases with any other type of congenital anomaly (excluding genital anomalies).
Analysis
Calculation of prevalence rates was based on the cumulative number of registered hypospadias cases divided by all births in the WA population and expressed per 10 000 births in the relevant year(s). Denominator data were obtained from the WA Department of Health and consisted of all live births and stillbirths of 20 weeks' gestation or more born in WA. For comparative purposes, prevalence rates were also determined using all male births in WA, 1980–2000 as the denominator and by calculating hypospadias rates using the European Surveillance of Congenital Anomalies (EUROCAT) definition, which excludes glanular cases from totals.3 Trends in prevalence rates over the study period were assessed using Poisson regression analysis to estimate the average yearly change in the rate of hypospadias and associated 95% confidence interval. All data were analysed using SAS, release 9.1 (SAS Institute, Cary, NC, USA).
The study protocol was approved by the ethics committee of the Women's and Children's Health Service and the Confidentiality of Health Information Committee for the Department of Health, Western Australia.
Results
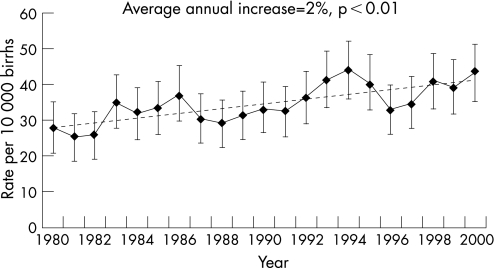
A total of 1788 cases of hypospadias were registered in WA out of 514 120 births between 1980 and 2000, representing an overall prevalence rate of 34.8 cases per 10 000 births (95% confidence interval (CI): 33.2 to 36.4). The trend in the prevalence of hypospadias increased significantly by 2.0% (95% CI: 1.2% to 2.8%) per year during the study period from 27.9 in 1980 to 43.2 per 10 000 births in 2000 (p<0.001) (fig 1). The average prevalence using only male infants in the denominator was 67.7 (95% CI: 64.6 to 70.8) per 10 000 male births. When applying the EUROCAT definition (excluding glanular cases), the average prevalence of hypospadias during 1980–2000 was 21.8 (95% CI: 20.5 to 23.1) per 10 000 births and the average annual prevalence increased significantly over the study period by 2.2% (95% CI: 1.2% to 3.2%) per year (p<0.01). Among all infants diagnosed with hypospadias, 1778 (99.5%) were live born, six (0.3%) stillborn and four (0.2%) were diagnosed following termination of pregnancy for another fetal anomaly.
Figure 1 Trend in the average annual prevalence of hypospadias in Western Australia, 1980–2000.
Hypospadias was diagnosed as mild in the majority of cases (84%), 11% were moderate‐severe and 5% had missing information for degree‐of‐severity. The average rates of mild and moderate‐severe forms of hypospadias in 1980–2000 were 29.1 and 3.8 per 10 000 births, respectively. The rate of mild hypospadias increased significantly by 50% over the study period at a rate of 1.6% per annum (95% CI: 0.7% to 2.4%; p<0.01) to 35 per 10 000 births. The prevalence of moderate‐severe hypospadias nearly doubled from 3.4 to 6.3 per 10 000 births in 2000 at a rate of 3.5% per annum (95% CI: 1.1% to 6.0%; p<0.01). The number of hypospadias cases with unspecified degree‐of‐severity also increased significantly over time (p = 0.02). There was a non‐significant but steady decline in the ratio of mild to moderate‐severe cases of hypospadias from 6.9 in 1980 to 5.6 in 2000 (p = 0.22). All infants with mild forms of hypospadias were live born. Stillbirths were more likely to occur among infants with moderate‐severe forms of hypospadias (n = 4/6) and three of the four terminations due to fetal malformation had no information on the degree‐of‐severity available.
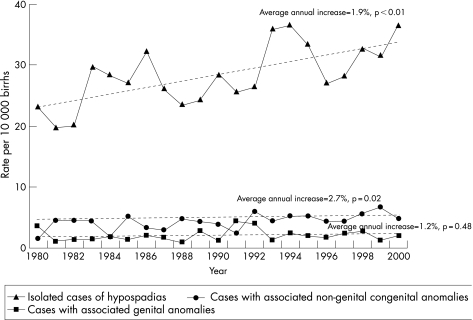
During 1980–2000, there were 1465 (82%) isolated cases of hypospadias and 323 (18%) infants had one or more additional anomalies diagnosed. Of the infants with multiple anomalies, 103 (5.8%) had a genital anomaly and 220 (12.3%) had other non‐genital malformations. The mean prevalence of isolated, genital and other non‐genital anomalies associated with hypospadias for the 21‐year period was 28.5 (95% CI 27 to 30), 2.0 (95% CI 1.6 to 2.4) and 4.3 (95% CI 3.7 to 4.8) per 10 000 births, respectively. Figure 2 highlights the significant increasing trend over time for isolated and other non‐genital co‐existing anomalies. Congenital anomalies of the genital organs, such as undescended testes, indeterminate sex and other non‐specified anomalies of genital organs (excluding hooded foreskin and chordee), were the most common types of malformations associated with hypospadias (n = 103) and showed a non‐significant increase over time.
Figure 2 Prevalence of isolated and co‐existing congenital anomalies associated with hypospadias, Western Australia, 1980–2000.
In addition to anomalies of genital organs, the most common types of co‐existing malformations included congenital anomalies of the urinary system (4.6%), musculoskeletal defects (4.3%) and cardiovascular defects (3.6%) (table 1). The rate of co‐existing anomalies associated with hypospadias was higher than in the general population of male births (table 1) and there was no trend in the proportion of co‐existing anomalies over time.
Table 1 Type and number of congenital anomalies associated with hypospadias and overall rate in the Western Australian population, 1980–2000.
| Diagnostic category of congenital anomalies | Number of hypospadias cases with co‐existing anomalies (n = 1788) | Proportion of hypospadias cases with co‐existing anomalies (n = 1788) | Proportion of male birth defects in the Western Australian population (n = 264 160) |
|---|---|---|---|
| n (%) | Rate per 1000 hypospadias births | Rate per 1000 male births | |
| Nervous system defects | 30 (1.7) | 16.8 | 4.3 |
| Congenital anomalies of eye | 10 (0.6) | 5.6 | 1.3 |
| Congenital anomalies of ear, face and neck | 49 (2.7) | 27.4 | 3.6 |
| Cardiovascular defects | 64 (3.6) | 35.8 | 10.2 |
| Respiratory system defects | 6 (0.3) | 3.4 | 1.2 |
| Gastrointestinal defects | 50 (2.8) | 28.0 | 7.8 |
| Urogenital anomalies* | 802 (44.9) | 448.5 | 7.7 |
| Undescended testis† | 66 (3.7) | 36.9 | 12.4 |
| Musculoskeletal defects | 76 (4.3) | 42.5 | 13.4 |
| Congenital anomalies of integument | 31 (1.7) | 17.3 | 3.7 |
| Chromosome defects | 22 (1.2) | 12.3 | 3.2 |
| Other | 57 (3.2) | 31.9 | 2.6 |
*Excluding hypospadias and undescended testis and including chordee, epispadias and hooded foreskin; †treated cases only.
Some cases may have more than one co‐existing congenital anomaly, so categories are not mutually exclusive.
The association of hypospadias with other congenital anomalies varied by degree‐of‐severity. Isolated cases were more likely to have a mild form of hypospadias (87%), while cases with non‐genital or genital anomalies had lower rates of mild hypospadias at 80% and 52%, respectively. Compared with cases of isolated hypospadias, infants with anomalies of genital organs were 4.5 (95% CI: 3.3 to 6.1) times more likely to have a moderate or severe form of hypospadias. The proportion of moderate or severe cases of hypospadias was also significantly higher among boys with other congenital anomalies than among those with isolated hypospadias (RR 1.5; 95% CI: 1.0 to 2.2). Of the cases with associated genital anomalies, 39% (40/103) had the most severe form of hypospadias and nearly half consisted of infants with undescended testis (19/40).
Discussion
The prevalence of hypospadias in WA has risen significantly at an average rate of 2% per annum over the last 20 years, with the rate of moderate or severe hypospadias almost doubling. Hypospadias is the second most common birth defect occurring among infant boys in WA,16 and in 2000 was diagnosed for one in 231 all births (one in every 118 male births) in WA. Our prevalence of hypospadias was akin to rates reported over similar periods by two United States (US) surveillance systems, and in Washington, Atlanta and Rotterdam,2,4,17,18 but was more than double the rate found in California and Hokkaido, Japan.19,20 The overall hypospadias rate of 16 per 10 000 births reported by the European (EUROCAT) and English and Welsh congenital anomaly surveillance systems (excluding glanular hypospadias) was also lower than the comparative rate of 21.6 per 10 000 births in WA.3 Except for one US study,2 no other studies have found an increasing trend in rates of hypospadias throughout the 1980s and 1990s. Although many countries experienced rising rates of hypospadias in the 1960–70s, many have reported a levelling off in rates in the 1980s or decreasing prevalence over recent years.3,15,19 Comparison of the prevalence of hypospadias in other states of Australia revealed a similar rate and increasing trend in South Australia21 and lower rates in Victoria and New South Wales.22,23
What is already known on this topic
There has been widespread variation in the definition, rates and temporal trend of hypospadias over various time periods and across countries.
Studies suggest increasing numbers of hypospadias may be associated with declining male reproductive health.
What this study adds
This population‐based study overcomes many of the limitations of previous studies and is a comprehensive assessment of the prevalence and trends of hypospadias over a 20‐year period.
Hypospadias is the second most common birth defect, affects 1 in 231 all births (one in every 118 male births) in Western Australia and has increased significantly by 2% per annum over the last 20 years.
Future research to explore the aetiology of hypospadias is important to identify potentially modifiable risk factors and ensure optimal male reproductive health in the future.
Differences in the reported prevalence and trends of hypospadias may be due to variation in case ascertainment and reporting across countries and jurisdictions. Each setting has its own individual data sources, such as birth defects registries, that rely on notifications from one or various sources and with various periods of follow‐up.2,3,22,23 Hence, reporting may be limited, particularly if notifications are based on surgical cases and if surgery, usually performed between 6 and 18 months of age,5 is conducted after the period of follow‐up. Variation in case definition across a number of countries is also common, particularly for minor forms of hypospadias, and in some cases there is poor collection of information on severity of hypospadias.2,3,19,20
In a number of settings hypospadias cases are sourced from hospital discharge data and based on hospital admissions for hypospadias repair.17,18,24 Hospital data are limited to those infants that have surgery and may not include minor cases. Rates of hypospadias may also be difficult to compare over time because of differences in indication and threshold for surgery between and within countries and surgeons. However, recent changes in surgical practice have led to a growing trend to recommend surgical repair for all cases of hypospadias, particularly minor cases that would have not been operated on in the past.25 Hence, in the future we may observe less variability between hospital‐ or population‐based rates and a potential rise in rates of hypospadias in the 2000s.
Additional evidence of a real increasing trend of hypospadias in WA was the steady (although non‐significant) decline in the ratio of mild to moderate‐severe cases of hypospadias from 6.9 in 1980 to 5.6 in 2000. As proximal cases are easier to diagnose and more likely to be notified and degree‐of‐severity has been classified by the WABDR since 1980, these results indicate a real increase in the rate of hypospadias and not just an increase or change in reporting of mild cases. Similar results were also found in Atlanta, although there was a high rate of unspecified hypospadias (∼60%) cases that limits the findings.2
As the increasing prevalence of hypospadias is not limited to isolated cases, results suggest that the causative factors are affecting embryonic fetal development more generally and are not just restricted to the urogenital system. Some authors suggest that increasing rates of hypospadias, and birth defects in general, may be due to environmental factors and greater exposure to endocrine‐disrupting chemicals in more industrialised and affluent countries.15
In addition to the greater prevalence of hypospadias, the increasing trend in testicular cancer26 and high rates of cryptorchidism, the most common birth defect in WA,16 have all been linked to the apparent decline in male reproductive health. These conditions are considered to represent a syndrome of disorders known as testicular dysgenesis syndrome, as they have been found to share a common origin of lowered androgen production, potentially due to fetal oestrogen exposure.13,27 Given the risk factors outlined above, increasing maternal age,28 greater use of assisted reproductive technology29 and the rising trend in concentration levels of some air pollutants in both metropolitan and regional areas of WA during the 1990s30 may have each contributed to the increasing prevalence of hypospadias in WA. However, due to limited data and consequently the main limitation to this study, we were unable to explore the association and contribution of maternal, paternal, genetic, environmental and geographical factors to rates and trends of hypospadias in WA, all of which require thorough assessment in future studies.
Ongoing follow‐up is also required to determine whether the trend in hypospadias continues to increase or has plateaued in WA.
The strength of this study is that data from the WABDR have a high level of ascertainment based on multiple sources of notifications, consistent definitions, case review and classification of severity of all cases of birth defects in WA in children up to 6 years of age.16 The results are also more reliable as they overcome many of the discrepancies of previous research characterised by limitations in data sources and differences in inclusion criteria, reporting systems, definitions and denominators between countries. Overall, the finding of a significant increasing trend in hypospadias and corresponding rising prevalence of oestrogenic exposures in WA suggests future investigation of the aetiology of hypospadias is important to identify potentially modifiable risk factors and ensure optimal male reproductive health in the future.
Acknowledgements
We would like to acknowledge the Telethon Institute for Child Health Research and the contributors to the Birth Defects Registry for provision of the data and would particularly like to thank Dr Clare Walker, Mr Peter Cosgrove, Ms Edwina Rudy and Ms Aandra Ryan for their contribution to the study. We also acknowledge the Confidentiality of Health Information Committee of the Department of Health in Western Australia for granting permission for us to use the data from the registries.
Abbreviations
BPA‐ICD9 - British Paediatric Association International Classification of Diseases, 9th revision system
EUROCAT - European Surveillance of Congenital Anomalies
RR - relative risk
US - United States
WA - Western Australia
WABDR - Western Australia Birth Defects Registry
95% CI - 95% confidence interval
Footnotes
Funding: Dr Nassar is supported by a Public Health Fellowship (404198) and Dr Bower by a Research Fellowship (353628) from the National Health and Medical Research Council of Australia. The funding source of this study had no role in the study design, data collection, data analysis, data interpretation, or the writing of the report.
Competing interests: None.
References
- 1.Duckett J W. Hypospadias. In: Walsh PC, Gittes RF, Perlmutter AD, et al eds. Campbell's urology. 5th edn. Philadelphia: WB Saunders, 19861969–1999.
- 2.Paulozzi L J, Erickson J D, Jackson R J. Hypospadias trends in two US surveillance systems. Pediatrics 1997100(5)831–834. [DOI] [PubMed] [Google Scholar]
- 3.Dolk H, Vrijheid M, Scott J E.et al Toward the effective surveillance of hypospadias. Environ Health Perspect 2004112(3)398–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pierik F H, Burdorf A, Nijman J M.et al A high hypospadias rate in The Netherlands. Hum Reprod 200217(4)1112–1115. [DOI] [PubMed] [Google Scholar]
- 5.Baskin L S, Ebbers M B. Hypospadias: anatomy, etiology, and technique. J Pediatr Surg 200641(3)463–472. [DOI] [PubMed] [Google Scholar]
- 6.Sharpe R M, Skakkebaek N E. Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract? Lancet 1993341(8857)1392–1395. [DOI] [PubMed] [Google Scholar]
- 7.Skakkebaek N E, Rajpert‐De Meyts E, Main K M. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod 200116(5)972–978. [DOI] [PubMed] [Google Scholar]
- 8.Colborn T, vom Saal F S, Soto A M. Developmental effects of endocrine‐disrupting chemicals in wildlife and humans. Environ Health Perspect 1993101(5)378–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klip H, Verloop J, van Gool J D.et al Hypospadias in sons of women exposed to diethylstilbestrol in utero: a cohort study. Lancet 2002359(9312)1102–1107. [DOI] [PubMed] [Google Scholar]
- 10.Krantz D, Goetzl L, Simpson J L.et al Association of extreme first‐trimester free human chorionic gonadotropin‐beta, pregnancy‐associated plasma protein A, and nuchal translucency with intrauterine growth restriction and other adverse pregnancy outcomes. Am J Obstet Gynecol 2004191(4)1452–1458. [DOI] [PubMed] [Google Scholar]
- 11.O'Leary P, Breheny N, Dickinson J E.et al First‐trimester combined screening for Down syndrome and other fetal anomalies. Obstet Gynecol 2006107(4)869–876. [DOI] [PubMed] [Google Scholar]
- 12.Weidner I S, Moller H, Jensen T K.et al Risk factors for cryptorchidism and hypospadias. J Urol 1999161(5)1606–1609. [PubMed] [Google Scholar]
- 13.Toppari J, Larsen J C, Christiansen P.et al Male reproductive health and environmental xenoestrogens. Environ Health Perspect 1996104(Suppl 4)741–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Safe S H. Environmental and dietary estrogens and human health: is there a problem? Environ Health Perspect 1995103(4)346–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paulozzi L J. International trends in rates of hypospadias and cryptorchidism. Environ Health Perspect 1999107(4)297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bower C, Rudy E, Ryan A.et alReport of the Birth Defects Registry of Western Australia, 1980–2004. Perth: King Edward Memorial Hospital, Women's and Children's Health Service, 2005
- 17.Gallentine M L, Morey A F, Thompson I M., Jr Hypospadias: a contemporary epidemiologic assessment. Urology 200157(4)788–790. [DOI] [PubMed] [Google Scholar]
- 18.Porter M P, Faizan M K, Grady R W.et al Hypospadias in Washington State: maternal risk factors and prevalence trends. Pediatrics 2005115(4)e495–e499. [DOI] [PubMed] [Google Scholar]
- 19.Carmichael S L, Shaw G M, Nelson V.et al Hypospadias in California: trends and descriptive epidemiology. Epidemiology 200314(6)701–706. [DOI] [PubMed] [Google Scholar]
- 20.Kurahashi N, Murakumo M, Kakizaki H.et al The estimated prevalence of hypospadias in Hokkaido, Japan. J Epidemiol 200414(3)73–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.South Australian Birth Defects Register 2003 annual report of the South Australian Birth Defects Register. Adelaide, 2005: South Australian Clinical Genetics Service, 2005
- 22.Riley M, Halliday J.Birth defects in Victoria 2001–2002. Melbourne: Victorian Perinatal Data Collection Unit, Victorian Government Department of Human Services, 2004
- 23.Centre for Epidemiology and Research, NSW Department of Health New South Wales mothers and babies 2004. NSW Public Health Bulletin 200516S4 [Google Scholar]
- 24.Aho M, Koivisto A M, Tammela T L.et al Is the incidence of hypospadias increasing? Analysis of Finnish hospital discharge data 1970–1994. Environ Health Perspect 2000108(5)463–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Belman A B. Hypospadias update. Urology 199749(2)166–172. [DOI] [PubMed] [Google Scholar]
- 26.Australian Institute of Health and Welfare and Australasian Association of Cancer Registries Cancer in Australia 2001. AIHW cat no CAN 23 (Cancer Series no 28). Canberra: AIHW, 2004
- 27.Sharpe R M. The ‘oestrogen hypothesis'‐ where do we stand now? Int J Androl 200326(1)2–15. [DOI] [PubMed] [Google Scholar]
- 28.Gee V, Godman K.Perinatal statistics in Western Australia, 2004. Twenty‐second annual report of the Western Australian Midwives' Notification System. Western Australia: Department of Health, 2006
- 29.Hansen M, Kurinczuk J J, Bower C.et al The risk of major birth defects after intracytoplasmic sperm injection and in vitro fertilization. N Engl J Med 2002346(10)725–730. [DOI] [PubMed] [Google Scholar]
- 30.Runnion T.Air quality in Perth, 1992–1999. Technical Report 109. Perth: Air Quality Management Branch, Department of Environmental Protection, 2001