Abstract
Objective
Children with nephrotic syndrome (NS) are usually treated with long‐term low dose alternate day prednisolone with or without glucocorticoid sparing therapy, such as levamisole or ciclosporin, to maintain remission. The degree of hypothalamic‐pituitary‐adrenal axis (HPA) suppression with such therapeutic strategies has not been studied systematically. HPA suppression could cause a relapse or adrenal crisis.
Study design
To study the risks of HPA suppression, a modified low dose synacthen test (0.5 μg) was administered to 32 patients (22 male,10 female) with a mean age of 9.7 years (range 3.8–17.6 years) with NS receiving long‐term alternate day prednisolone for over 12 months. Twelve patients received alternate day prednisolone, 11 alternate prednisolone+levamisole and nine alternate prednisolone+ciclosporin. All patients were followed up for 3 years and the relapse rate noted.
Results
20/32 (62.5%) patients had a peak serum cortisol concentration of <500 nmol/l, which suggested suboptimal cortisol secretion and possible HPA suppression. 10/12 children in the prednisolone group and 8/11 in the levamisole group had a suboptimal cortisol response compared with 2/9 in the ciclosporin group. During follow‐up, the 20 children who had a suboptimal cortisol response had significantly more relapses (95 relapses) compared to the 12 children with a normal cortisol response who had 24 relapses (p = 0.01).
Conclusions
Children with NS receiving long‐term alternate day prednisolone therapy are at risk of developing HPA suppression and should be evaluated using the modified synacthen test. Children with evidence of HPA suppression are at a greater risk of relapse.
Keywords: cortisol, synacthen test, glucocorticoid therapy, proteinuria, glucocorticoid sparing therapy
Nephrotic syndrome (NS) is the most common glomerular disorder in childhood and is characterised by heavy proteinuria, hypoproteinaemia and oedema.1 Over 90% of cases in children are due to minimal change disease and most will respond to glucocorticoid therapy.2 However, this therapy is not curative and over 70% of children subsequently relapse.1 Each relapse is associated with an increased risk of morbidity from infection, thromboembolism and hypovolaemic shock.3,4 The aim of the management of NS in children is to induce and maintain complete remission with resolution of proteinuria and oedema without serious adverse side effects.5
Despite a paucity of knowledge regarding the underlying molecular mechanism responsible for NS, numerous therapeutic regimens have been proposed to reduce the relapse rate and minimise glucocorticoid toxicity based on the results of clinical trials and case cohort studies.6 Children who relapse frequently following glucocorticoid therapy will benefit from a maintenance prednisolone regimen of 0.1–1 mg/kg on alternate days.1,5 Children who encounter glucocorticoid toxicity or who continue to have relapses may benefit from a glucocorticoid sparing agent such as levamisole, cyclophosphamide or ciclosporin.6,7,8 Once disease activity has been stable for 6–12 months, the current practice is to taper the dose of glucocorticoids.1,6
In 1977 Leisti et al reported that children who develop severe hypothalamic‐pituitary‐adrenal axis (HPA) suppression were more likely to relapse early and also demonstrated that supplementation with a small dose of glucocorticoid could prevent some of the relapses.9,10,11 These studies suggested that HPA suppression could be an important risk factor triggering NS relapse, possibly due to inadequate glucocorticoid response to infections and stress. However, these observations failed to change the management of NS as the HPA axis was evaluated by the insulin‐hypoglycaemia test, a test which itself is associated with significant morbidity and rare mortality.
There are few data on the degree of HPA suppression in children with NS receiving long‐term alternate day prednisolone therapy. Prednisolone is principally bound to plasma albumin and it is the unbound fraction that is metabolically active. Therefore, the efficacy of glucocorticoids prescribed for the hypo‐albuminaemic patient is likely to be enhanced.12 Administration of ciclosporin with alternate day prednisolone has been reported to increase the risk of HPA axis suppression in renal transplant patients, but the effect in patients with NS has not been evaluated.13 Moreover, the effect of levamisole on prednisolone metabolism is also unknown. A better understanding of the degree of HPA suppression in children with NS who receive different regimens of alternate day prednisolone with or without adjuvant therapy would be useful for the clinician in order to determine future management.
The usefulness of the adrenocorticotrophin hormone test in the evaluation of the HPA axis in patients with secondary hypoadrenalism has been widely discussed.14,15 However, it has been questioned whether the use of a supra‐physiological dose, such as 250 μg, would give false positive results.16,17,18 Recently, an adrenocorticotrophin hormone (synacthen) test using a physiological dose (0.5–1 μg) has been found to be a more sensitive, safe and inexpensive method for assessing HPA function.19,20,21
This study was designed to evaluate the status of the HPA axis by performing a low dose synacthen test (LDST) in patients receiving long‐term alternate day prednisolone with or without adjuvant therapy such as levamisole or ciclosporin and to describe the association between HPA axis suppression and NS relapse.
Methods
Study population
Children between 1 and 16 years of age attending the outpatient department at Great Ormond Street Hospital for Children, London, UK with steroid sensitive or frequently relapsing NS were considered for recruitment. Children with frequently relapsing or steroid dependant disease received long‐term maintenance treatment with alternate day prednisolone. The dose of prednisolone was titrated to the lowest that was sufficient to maintain remission in order to minimise glucocorticoid toxicity (maximum 1 mg/kg/body weight on alternate days). When undesirable glucocorticoid related side effects were encountered, levamisole, an anti‐helminthic drug with immunomodulatory effects and steroid sparing properties, was prescribed at a dosage of 2.5 mg/kg/alternate day in conjunction with alternate day prednisolone. The dose of alternate day prednisolone was then tapered to the lowest amount required to maintain remission. Ciclosporin was generally reserved for children who demonstrate steroid dependence following a course of cyclophosphamide. The usual dose of ciclosporin was 3–5 mg/kg/day to achieve a 12 h trough blood level of 50–150 μg/l, which was prescribed with alternate day prednisolone.
Patients receiving maintenance alternate day prednisolone for a minimum of 12 months and who had been on the same dose for at least for the preceding 3 months were recruited. Depending upon immunosuppression, the patients were subdivided into three subgroups:
prednisolone 0.1–1 mg/kg/alternate day,
prednisolone 0.1–1 mg/kg/alternate day with ciclosporin,
prednisolone 0.1–1 mg/kg/alternate day with levamisole.
Prior to enrolment written informed consent was obtained from the parents as well as the child where appropriate. A cannula was inserted into a large vein in the mid arm at 12:00 h after applying a local anaesthetic cream (Ametop; Smith and Nephew Healthcare, Hull, UK) over the skin to minimise discomfort. An intravenous injection of synacthen (0.5 μg) was administered at 14:00 h and blood samples drawn at 0, 10, 20, 30, 40, 50 and 60 min for estimation of serum cortisol concentration. Serum cortisol levels were measured by fluoro‐immunometric assay performed on the Immunolite 2000 analyser (Diagnostic Products, Los Angeles, CA, USA). A peak plasma cortisol concentration of >500 nmol/l was considered to be indicative of normal adrenal function. All patients were followed up for a period of 3 years and the relapse rate was noted. Proteinuria of 2+ or more for 3 consecutive days was diagnostic of relapse.1 Relapses were treated with the standard relapse regimen.1 The immunosuppression of these children was managed by a single clinician in order to maintain a uniform approach. Ethical approval for this study was obtained from the Clinical Research Ethics Committee of Great Ormond Street Hospital for Children and the Institute of Child Health, University College London, London, UK.
Statistical analysis
ANOVA was used to identify any differences in the alternate day prednisolone dosage in subgroups and the Bonferroni test was used for further analysis to identify exactly which subgroups differed significantly. The χ2 test was used to compare the proportion of children who had a suboptimal cortisol response. Student's t test was used to compare the relapse rate in patients with a normal cortisol response and those with a suboptimal cortisol response. Statistical analysis was performed using the computer program package SSPS (version 11.0, 2001).
Results
Thirty two patients (22 male, 10 female) with a mean age of 9.7 years (range 3.8–17.6 years) underwent evaluation. Fifteen patients were of Asian ethnicity, 14 were Caucasian and three were Afro‐Caribbean. Twelve patients were receiving alternate day prednisolone (P), 11 alternate P+levamisole and nine alternate P+ciclosporin.
The mean alternate day prednisolone dose, duration of alternate day prednisolone therapy and the total prednisolone dosage over the preceding 1 year for the three groups are shown in table 1.
Table 1 Prednisolone dosage and cortisol response in the three groups.
| Mean dosage (SD), mg/kg | Total mean dose (SD), mg/year | Mean duration (SD), years | Peak cortisol response, <500 nmol/l | Maximum mean response (SD), nmol/l | |
|---|---|---|---|---|---|
| Prednisolone (P) | 0.52 (0.28) | 211.5 (49.9) | 1.23 (0.36) | 10/12 | 320 (117.9) |
| P+levamisole | 0.27 (0.15) | 164.7 (95.8) | 1.48 (0.21) | 8/11 | 333 (227.8) |
| P+ciclosporin | 0.41 (0.2) | 183.6 (75.2) | 1.62 (0.24) | 2/9 | 464 (175.7) |
| Total group | 0.4 (0.22) | 186.0 (75.6) | 1.43 (0.26) | 20/32 | 372 (179.7) |
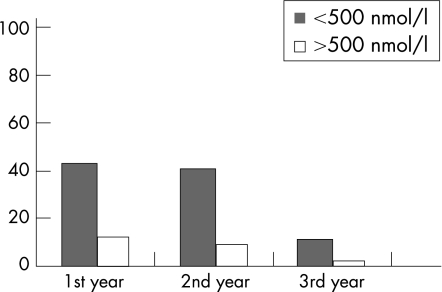
The difference in alternate day prednisolone dosage in the subgroups was significant (ANOVA, p = 0.01, F = 5.13). Multiple comparisons performed using the Bonferroni test identified a significant difference between the alternate day prednisolone group and the P+levamisole group (p = 0.01), while there was no difference in the dosage in the alternate day prednisolone group and the P+ciclosporin group (p = 0.09), or in the P+levamisole group and the P+ciclosporin group (p = 0.08). There was no significant difference in the duration of alternate day prednisolone therapy or in the total prednisolone dosage over the preceding 1 year between the three groups. Overall, 20/32 (62.5%) patients had a peak plasma cortisol concentration below 500 nmol/l: 10/12 (83.3%) children in the prednisolone group, 9/11 (81.8%) in the P+levamisole group and 2/9 (22.2%) in the P+ciclosporin group (χ2 10.46; p = 0.005). Of the 20 children who had evidence of a subnormal HPA axis, all had at least one relapse during the follow‐up period: there were 43, 41 and 11 relapse episodes (total 95) in the 1st, 2nd and 3rd years of follow‐up, respectively. In contrast, only seven out of 12 children who had a normal cortisol response had a relapse during follow‐up period: there were 12, nine and three relapse episodes (total 24) in the 1st, 2nd and 3rd years of follow‐up, respectively (fig 1). Thus children who have evidence of HPA suppression are at greater risk of relapse (95 vs 24 relapses) compared to children with a normal cortisol response (Student's t test, p = 0.01).
Figure 1 The difference in the relapse rate based upon the response to the low dose synacthen test over 3 years.
Discussion
Children with frequently relapsing or steroid dependant disease are treated with long‐term maintenance prednisolone as opposed to repeated standard relapse treatment.1 The side effects of glucocorticoids are numerous, well recognised and of great concern to the patient and their family.1,5 When unacceptable glucocorticoid related adverse events occur, these patients are considered for glucocorticoid sparing therapy.1,6 However, the degree of glucocorticoid related adverse events is mostly based upon clinical observations rather than an objective assessment.1 Moreover, HPA axis suppression in this group of patients is not evaluated routinely, whereas much attention has been given to HPA axis suppression in patients receiving inhaled glucocorticoids.22
The established reference test for assessing the HPA axis is to measure the cortisol response to insulin‐induced hypoglycaemia. This test is unpleasant for the patient, potentially dangerous, resource intensive, contraindicated in patients with epilepsy and not advisable in children.22 Various studies have evaluated the plasma cortisol concentration in response to a standard synacthen dose of 250 μg, a supraphysiological dose originally designed as a test for primary adrenal failure.23 Several groups have reduced this dose and demonstrated that the cortisol response to 0.5–1 μg is equivalent to that obtained with 250 μg in normal subjects.24 Over the last few years, a number of studies have examined the clinical usefulness of the LDST (0.5–1 μg) compared to the conventional dose (250 μg) test in patients receiving long‐term glucocorticoid therapy and in patients with pituitary disease23,24 and have demonstrated that the LDST produced fewer false positive results than the conventional dose test.23,24
Our data indicate that 62.5% (20/32) of children receiving alternate day prednisolone for steroid dependant NS had a suboptimal cortisol response, suggesting HPA axis suppression. Similar proportions of children in the prednisolone group (10/12) and in the P+levamisole group (9/11) had a subnormal cortisol response, whereas in the P+ciclosporin group, only 2/9 had evidence of HPA suppression in spite of receiving a higher dose of alternate day prednisolone. This could be due to interference by ciclosporin in prednisolone metabolism. However, these results contrast with findings in renal transplant patients receiving alternate day prednisolone in whom ciclosporin therapy increased the risk of HPA suppression; the reasons for these differences are not clear and need further evaluation. What is apparent is the importance of evaluating HPA function in children receiving long‐term alternate day prednisolone for maintenance of remission in NS. However, the ideal timing and the advantages of performing LDST at regular intervals for the early identification of patients with HPA axis suppression needs further evaluation.
The results of this study also confirm the findings of Finnish studies9,10 that children with HPA axis suppression are at greater risk of relapse than those with a normal HPA axis following glucocorticoid therapy (fig 1). A marked reduction in the relapse rate was observed during the third year of follow‐up, which could be due disease stability achieved with immunosuppressive therapy and also the natural improvement of the disease with time.2
It could be argued that children with more severe disease received a higher dose of prednisolone resulting in HPA axis suppression and thus the observed higher rate of relapse is due to the initial disease severity rather than HPA axis suppression. However, glucocorticoid sparing therapy, such as levamisole and ciclosporin, was introduced for more severe cases and therefore it is unlikely that the initial disease severity had a significant influence on the relapse rate.
While the exact mechanism underlying NS is not clear, the current understanding of the pathogenesis of idiopathic NS is that it is probably the result of a primary immune disturbance.25 There is strong evidence that proteinuria, which is the hallmark of this condition, is mediated by cytokines, namely interleukin 2, 4 and 13.26 Relapses are often triggered by viral infections, which possibly result in the release of cytokines, causing immunoregulatory imbalance.27 Immunosuppressive drugs which induce a clinical remission of NS, such as prednisolone and ciclosporin, affect cytokine production. It is therefore possible that patients with HPA axis suppression have a greater cytokine response to infections resulting in NS relapse. Children with evidence of HPA axis suppression should therefore be considered for glucocorticoid sparing therapy which will help the HPA axis to recover.
The results of this study indicate that children with NS receiving long‐term alternate day prednisolone with or without steroid sparing therapy are at risk of developing HPA suppression, placing them at a greater risk of NS relapse.
What is already known on this topic
Alternate day prednisolone is known to reduce the risk of adrenal suppression in nephrotic syndrome (NS).
Long‐term alternate day prednisolone helps to maintain remission in frequently relapsing NS.
Adjuvant therapy such as levamisole and ciclosporin has steroid sparing properties.
What this study adds
A significant proportion of children receiving long‐tern alternate day prednisolone for NS have evidence of adrenal suppression.
Children with evidence of adrenal suppression are at greater risk of NS relapse.
Acknowledgements
We thank Carol Hutchinson, Clinical Research Nurse, Nephro‐Urology Unit, Great Ormond Street Hospital for Children for helping with collecting blood samples during LDST and Tracey Calvert, Clinical Research Nurse, Nephro‐Urology Unit, Great Ormond Street Hospital for Children for helping with data collection. We also thank Pallegoda V Kumarasiri, Senior Lecturer in Community Medicine, University of Peradeniya, Sri Lanka for assistance with statistical analysis.
Abbreviations
HPA - hypothalamic‐pituitary‐adrenal axis
LDST - low dose synacthen test
NS - nephrotic syndrome
Footnotes
Competing interests. None.
References
- 1. Consensus statement on management and audit potential for steroid responsive nephrotic syndrome. Report of a workshop by the British Association for Paediatric Nephrology and Research Unit, Royal College of Physicians. Arch Dis Child 199470151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trompeter R S, Lloyd B W, Hicks J.et al Long‐term outcome for children with minimal change nephrotic syndrome. Lancet 1985i368–370. [DOI] [PubMed] [Google Scholar]
- 3.Hoyer P F, Gonda S, Barthels M.et al Thromboembolic complications in children with nephrotic syndrome. Risk and incidence. Acta Paediatr Scand 198675804–807. [DOI] [PubMed] [Google Scholar]
- 4.Kresnsky A M, Ingelfinger J R, Grupe W E. Peritonitis in childhood nephrotic syndrome. Am J Dis Child 1982136732–736. [DOI] [PubMed] [Google Scholar]
- 5.Abeyagunawardena A, Brogan P A, Trompeter R S.et al Immunosuppressive therapy of childhood idiopathic nephrotic syndrome. Expert Opin Pharmacother 20023(5)513–519. [DOI] [PubMed] [Google Scholar]
- 6.Abeyagunawardena A, Dillon M J, Rees L.et al The use of steroid sparing agents in steroid sensitive nephrotic syndrome. Pediatr Nephrol 200318919–924. [DOI] [PubMed] [Google Scholar]
- 7.Dayal U, Dayal A K, Shastry J C.et al Use of levamisole in maintaining remission in steroid‐sensitive nephrotic syndrome in children. Nephron 199466408–412. [DOI] [PubMed] [Google Scholar]
- 8.Hulton S A, Neuhaus T J, Dillon M J.et al Long‐term cyclosporin A treatment of minimal‐change nephrotic syndrome of childhood. Pediatr Nephrol 19948401–403. [DOI] [PubMed] [Google Scholar]
- 9.Leisti S, Hallman N, Koskimies O.et al Association of postmedication hypocortisolism with early first relapse of idiopathic nephrotic syndrome. Lancet 19772(8042)795–796. [DOI] [PubMed] [Google Scholar]
- 10.Leisti S, Koskmies O. Risk of relapse in steroid‐sensitive nephrotic syndrome: effect of stage of post‐prednisolone adrenocortical suppression. J Pediatr 1983103553–557. [DOI] [PubMed] [Google Scholar]
- 11.Leisti S, Koskimies O, Perheentupa J.et al Idiopathic nephrotic syndrome. Prevention of early relapse. BMJ 19781892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis G P, Jusko W J, Burke C W.et al Prednisolone side effects and serum‐protein levels. Lancet 1971ii778–781. [DOI] [PubMed] [Google Scholar]
- 13.Oka K, Shimodaira H, Hirano T.et al Comparison of adrenal functions in kidney transplant recipients in different long‐term immunosuppressive treatment: prednisolone and azathioprine versus prednisolone and cyclosporin. Transplantation 199353(3)603–609. [DOI] [PubMed] [Google Scholar]
- 14.Lindholm J, Kehlet H, Blichert‐Toft M.et al Reliability of the 30 minute ACTH test in assessing hypothalamic pituitary adrenal axis function. J Clin Endocrinol Metab 197847272–274. [DOI] [PubMed] [Google Scholar]
- 15.Stewart P M, Corrie J, Seckl J R.et al A rational approach for assessing the hypothalamo‐pituitary‐adrenal axis. Lancet 198811208–1210. [DOI] [PubMed] [Google Scholar]
- 16.Soule S G, Fahie‐Wilson M, Tomlinson S. Failure of the short ACTH test to unequivocally diagnose longstanding symptomatic hypoadrenalism. Clin Endocrinol (Oxf) 199644137–140. [DOI] [PubMed] [Google Scholar]
- 17.Streeten D H P, Anderson G H, Bonaventyra M M. The potential for serious consequences from misinterpreting normal responses to the rapid adrenocorticotrophin test. J Clin Endocrinol Metab 199681285–290. [DOI] [PubMed] [Google Scholar]
- 18.Ammari F, Issa B G, Millward E.et al A comparison between the short ACTH and insulin stress test for assessing hypothalamo‐pituitary‐adrenal function. Clin Endocrinol (Oxf) 199644473–476. [DOI] [PubMed] [Google Scholar]
- 19.Weintrob N, Sprecher E, Josefsberg Z.et al Standard and low dose short adrenocorticotrophin test compared with insulin‐induced hypoglycaemia for assessment of the hypothalamo‐pituitary‐adrenal axis in children with idiopathic multiple pituitary hormone deficiencies. J Clin Endocrinol Metab 19988388–92. [DOI] [PubMed] [Google Scholar]
- 20.Crowley S, Hindmarsh P C, Honour J W.et al Reproducibility of the cortisol response to stimulation with a low dose of ACTH(1–24): the effect of basal cortisol levels and comparison of low‐dose with high‐dose secretary dynamics. J Endocrinol 1992136167–172. [DOI] [PubMed] [Google Scholar]
- 21.Agwu J C, Spoudeas H, Hindmarsh P C.et al Test of adrenal insufficiency. Arch Dis Child 199980330–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahachoklertwattana P, Sudkronrayudh K, Direkwattanachai C.et al Decreased cortisol response to insulin induced hypoglycaemia in asthmatics treated with inhaled fluticasone propionate. Arch Dis Child 200489(11)1055–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tordjman K, Jaffe A, Grazas N.et al The role of the low dose (1 μg) adrenocorticotrophin test in the evaluation of patients with pituitary diseases. J Clin Endocrinol Metab 1995801301–1305. [DOI] [PubMed] [Google Scholar]
- 24.Talwar V, Lodha S, Dash R J. Assessing the hypothalamo‐pituitary‐adrenocortical axis using physiological doses of adrenocorticotrophic hormone. Q J Med 199891285–290. [DOI] [PubMed] [Google Scholar]
- 25.Shalhoub R J. Pathogenesis of lipoid nephrosis: a disorder of T‐cell function. Lancet 1974ii556–560. [DOI] [PubMed] [Google Scholar]
- 26.Yap H, Cheung W, Murugasu M.et al Th1 and Th2 cytokine mRNA profiles in childhood nephrotic syndrome: evidence for increased IL‐13 mRNA expression in relapse. J Am Soc Nephrol 199910529–537. [DOI] [PubMed] [Google Scholar]
- 27.MacDonald N, Wolfish N, Maclaine P.et al Role of respiratory viruses in exacerbations of primary nephrotic syndrome. J Pediatr 1986108378–382. [DOI] [PubMed] [Google Scholar]