Abstract
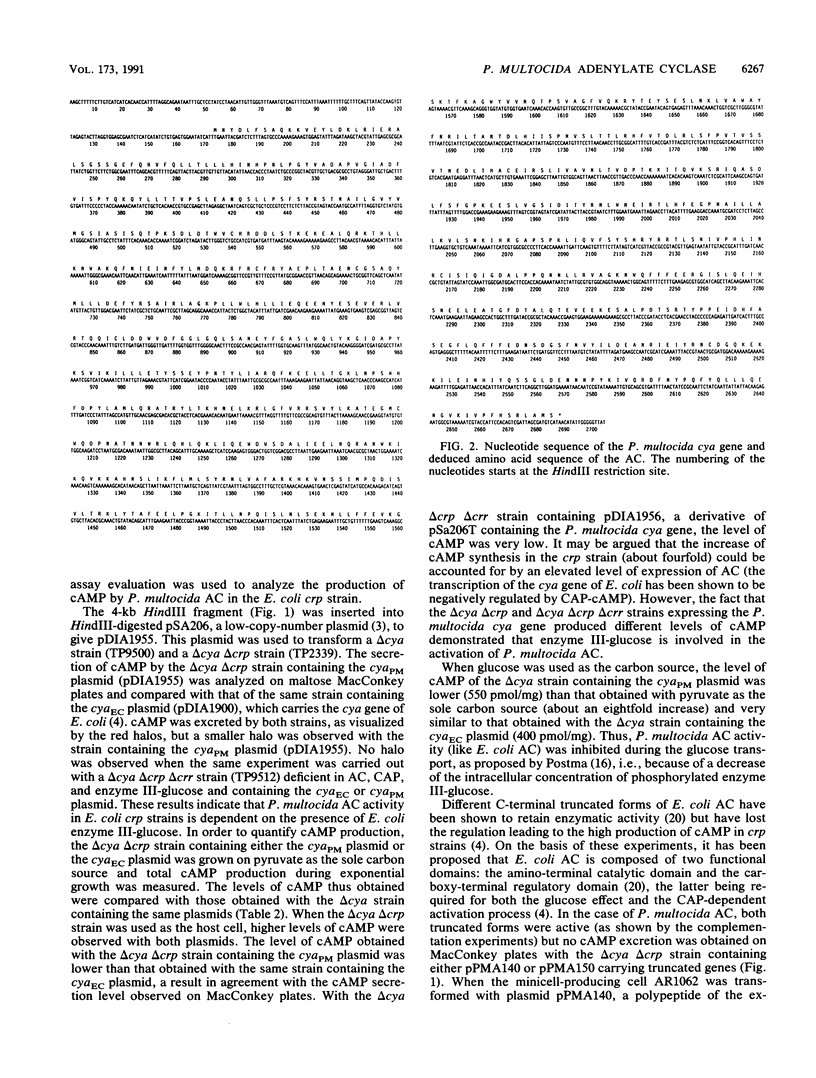
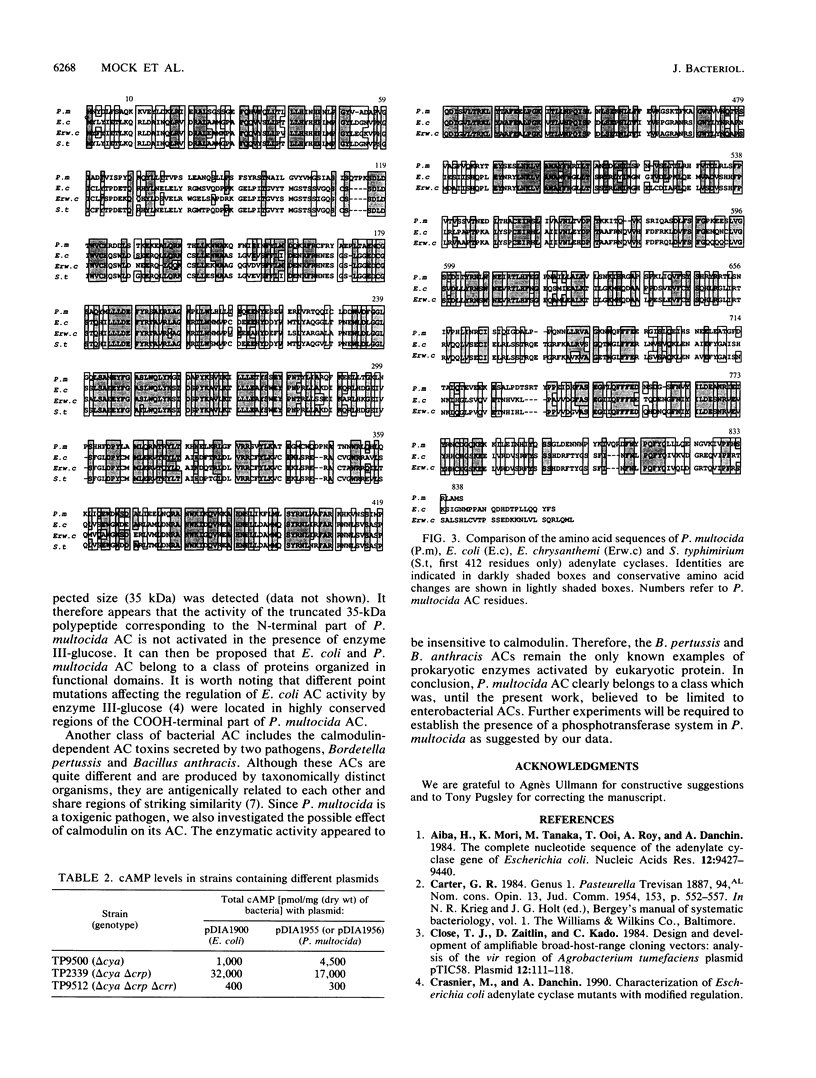
The Pasteurella multocida adenylate cyclase gene has been cloned and expressed in Escherichia coli. The primary structure of the protein (838 amino acids) deduced from the corresponding nucleotide sequence was compared with that of E. coli. The two enzymes have similar molecular sizes and, based on sequence conservation at the protein level, are likely to be organized in two functional domains: the amino-terminal catalytic domain and the carboxy-terminal regulatory domain. It was shown that P. multocida adenylate cyclase synthesizes increased levels of cyclic AMP in E. coli strains deficient in the catabolite gene activator protein compared with wild-type strains. This increase does not occur in strains deficient in both the catabolite gene activator protein and enzyme III-glucose, indicating that a protein similar to E. coli enzyme III-glucose is involved in the regulation of P. multocida adenylate cyclase. It also indicates that the underlying process leading to enterobacterial adenylate cyclase activation has been conserved through evolution.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiba H., Mori K., Tanaka M., Ooi T., Roy A., Danchin A. The complete nucleotide sequence of the adenylate cyclase gene of Escherichia coli. Nucleic Acids Res. 1984 Dec 21;12(24):9427–9440. doi: 10.1093/nar/12.24.9427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close T. J., Zaitlin D., Kado C. I. Design and development of amplifiable broad-host-range cloning vectors: analysis of the vir region of Agrobacterium tumefaciens plasmid pTiC58. Plasmid. 1984 Sep;12(2):111–118. doi: 10.1016/0147-619x(84)90057-x. [DOI] [PubMed] [Google Scholar]
- Crasnier M., Danchin A. Characterization of Escherichia coli adenylate cyclase mutants with modified regulation. J Gen Microbiol. 1990 Sep;136(9):1825–1831. doi: 10.1099/00221287-136-9-1825. [DOI] [PubMed] [Google Scholar]
- Danchin A., Lenzen G. Structure and evolution of bacterial adenylate cyclase: comparison between Escherichia coli and Erwinia chrysanthemi. Second Messengers Phosphoproteins. 1988;12(1):7–28. [PubMed] [Google Scholar]
- Escuyer V., Duflot E., Sezer O., Danchin A., Mock M. Structural homology between virulence-associated bacterial adenylate cyclases. Gene. 1988 Nov 30;71(2):293–298. doi: 10.1016/0378-1119(88)90045-5. [DOI] [PubMed] [Google Scholar]
- Fandl J. P., Thorner L. K., Artz S. W. Mutations that affect transcription and cyclic AMP-CRP regulation of the adenylate cyclase gene (cya) of Salmonella typhimurium. Genetics. 1990 Aug;125(4):719–727. doi: 10.1093/genetics/125.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feucht B. U., Saier M. H., Jr Fine control of adenylate cyclase by the phosphoenolpyruvate:sugar phosphotransferase systems in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1980 Feb;141(2):603–610. doi: 10.1128/jb.141.2.603-610.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser D. E., Yamazaki H. Construction of an Escherichia coli strain which excretes abnormally large amounts of adenosine 3',5'-cyclic monophosphate. Can J Microbiol. 1978 Nov;24(11):1423–1425. doi: 10.1139/m78-228. [DOI] [PubMed] [Google Scholar]
- Guidi-Rontani C., Danchin A., Ullmann A. Isolation and characterization of an Escherichia coli mutant affected in the regulation of adenylate cyclase. J Bacteriol. 1981 Dec;148(3):753–761. doi: 10.1128/jb.148.3.753-761.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Postma P. W. Regulation of sugar transport in Salmonella typhimurium. Ann Microbiol (Paris) 1982 Mar-Apr;133(2):261–267. [PubMed] [Google Scholar]
- Postma P. W., Schuitema A., Kwa C. Regulation of methyl beta-galactoside permease activity in pts and crr mutants of Salmonella typhimurium. Mol Gen Genet. 1981;181(4):448–453. doi: 10.1007/BF00428734. [DOI] [PubMed] [Google Scholar]
- Rambach A., Hogness D. S. Translation of Drosophila melanogaster sequences in Escherichia coli. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5041–5045. doi: 10.1073/pnas.74.11.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy P., Peterkofsky A., McKenney K. Translational efficiency of the Escherichia coli adenylate cyclase gene: mutating the UUG initiation codon to GUG or AUG results in increased gene expression. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5656–5660. doi: 10.1073/pnas.82.17.5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A., Danchin A., Joseph E., Ullmann A. Two functional domains in adenylate cyclase of Escherichia coli. J Mol Biol. 1983 Mar 25;165(1):197–202. doi: 10.1016/s0022-2836(83)80251-4. [DOI] [PubMed] [Google Scholar]
- Roy A., Haziza C., Danchin A. Regulation of adenylate cyclase synthesis in Escherichia coli: nucleotide sequence of the control region. EMBO J. 1983;2(5):791–797. doi: 10.1002/j.1460-2075.1983.tb01502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorner L. K., Fandl J. P., Artz S. W. Analysis of sequence elements important for expression and regulation of the adenylate cyclase gene (cya) of Salmonella typhimurium. Genetics. 1990 Aug;125(4):709–717. doi: 10.1093/genetics/125.4.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubay G., Schwartz D., Beckwith J. Mechanism of activation of catabolite-sensitive genes: a positive control system. Proc Natl Acad Sci U S A. 1970 May;66(1):104–110. doi: 10.1073/pnas.66.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]


