Abstract
Background and aims
Little is known about the familial characteristics of children with severe receptive specific language impairment (SLI). Affected children are more likely to have long‐term problems than those with expressive SLI but to date they have only been described as small cohorts within SLI populations. We therefore aimed to describe the clinical and familial characteristics of severe receptive SLI as defined by a rigorous phenotype and to establish whether non‐word repetition showed a relationship with language impairment in these families.
Methods
Cross‐sectional study of children who met ICD‐10 (F80.2) criteria for receptive SLI at school entry, their siblings and genetic parents with standardised measures of language and non‐verbal IQ, phonological auditory memory and speech sound inventory.
Results
At a mean of 6 years after school entry with a severe receptive SLI, the 58 participants had a normal mean and standard deviation non‐verbal IQ, but only 3% (two) had attained language measures in the normal range. One third still had severe receptive language impairment. One third of siblings not known to be affected had language levels outside the normal range. Phonological auditory memory was impaired in most family members.
Conclusion
Severe receptive SLI is nearly always associated with an equally severe reduction in expressive language skills. Language impairment in siblings may go undetected and yet they are at high risk. Family members had weak phonological auditory memory skills, suggesting that this could be a marker for language acquisition difficulties. Receptive SLI rarely resolves and trials of therapy are urgently needed.
Keywords: receptive specific language impairment
Specific language impairment (SLI) is a developmental disorder where children fail to acquire language at the normal rate but for whom there is no identifiable medical or neurological aetiology.1,2 SLI is common, affecting 6–8% of children at school entry.3,4 For many preschool children with SLI the prognosis is good, with 44% showing resolution, particularly if they have expressive language impairment only.5,6 However, the prognosis is believed to be much poorer for children with receptive language impairment in which comprehension is also affected. Follow‐up studies of children with SLI which have included those with receptive problems have suggested that there are persisting language, literacy and behaviour problems in later childhood and adult life despite speech and language therapy and placement in specialised educational settings such as language units. The level of non‐verbal intelligence and language comprehension is considered to have a role in this poorer prognosis.7,8,9,10
An active ascertainment study of SLI suggested that only a minority of affected children have an accompanying speech sound or pronunciation difficulty or have been identified as having language delay by their parents.11 Presumably parents are then unable to bring concerns of poor language acquisition to the attention of primary care professionals during developmental surveillance. The process of identification is further frustrated by the difficulties inherent in identifying abnormalities of language acquisition in surveillance consultations, even when the health visitors have been specially trained to do so.12
The acquisition of language is highly heritable and particularly so for those children with the slowest rates of language development.13,14 Therefore, it might be reasonable to measure language skills in siblings of families in which there is a proband already identified as having SLI. However, the majority of familial studies of SLI have defined caseness on the basis of a language composite measure which combines both receptive and expressive levels and have therefore included a majority of children with expressive difficulties only.15,16 Preliminary conclusions suggest that although there is a high familial incidence of SLI, the rate is much less for children with receptive disorder.17
In this report our objective is to describe the receptive and expressive language of a group of children with carefully defined receptive SLI, some 6 years after entry to school. We also examined familial characteristics to determine the incidence of language impairment in siblings and whether this was recognised by their parents. We measured phonological auditory memory, through non‐word repetition for other family members, which has been shown to be heritable in twin studies.18 Non‐word repetition has also acted as a quantitative phenotype to establish linkage to chromosomes 19 and 16 in a large genetic study of children with SLI19 and we aim to establish whether non‐word repetition showed a relationship with language impairment in these families.
Methods
Participants
Ethical approval was granted by the three health authorities in South, Central and South Eastern Scotland. Speech and language therapists and paediatricians referred English‐speaking monolingual children with SLI including a receptive component to the study. All available biological parents and siblings of the probands also took part.
Proband eligibility was determined by the phenotype of the ICD‐10 receptive language disorder (F80.2) (box 1).20
Box 1 ICD‐10 (F80.2) criteria for receptive specific language impairment (SLI)
A. Language comprehension, as assessed on standardised tests, is below the 2 standard deviation (SD) limit for the child's age.
B. Receptive language skills are at least 1 SD below non‐verbal IQ as assessed on standardised tests.
C. There are no neurological, sensory or physical impairments that directly affect receptive language nor is there a pervasive developmental disorder.
D. Most commonly used exclusion clause: non‐verbal IQ is below 70 on a standardised test.
This eligibility had to be relevant at the time the child was assessed by a multi‐disciplinary team as requiring special educational support on entering school, which is usually at the age of 5 years in Scotland. Criterion A was established from a retrospective analysis of the standardised assessment results recorded in the child's clinical notes. Adherence to criterion B for most children was again based on their having a documented measure of non‐verbal IQ (NVIQ) from their clinical notes at the time that they entered school. For one education authority, this measure was not available because it did not form part of the assessment screen for language unit entry. Therefore, these children were regarded as provisionally eligible based on the referring clinician's judgement with acceptance contingent on a child having a measured normal NVIQ during the cross‐sectional component of the study. Criterion C was addressed by both retrospective analysis of clinical case notes and current clinical history conducted by the researchers. This established whether the child had had a normal hearing test at the time of diagnosis. As the children were participating in a genetic study, we elected to exclude those who had been born preterm,21 those who had epilepsy22 and those born to epileptic mothers23 because of the reported associations in these conditions with language delay. No clinical investigation such as karyotype or brain imaging was conducted by the research team, but no child required to be excluded on the basis of abnormal results of such investigations that had been conducted by the clinical team or referrer.
Assessments
The parents were interviewed before the assessments to establish whether they considered that they themselves or the probands' siblings had or had had a language learning impairment, which was defined as a difficulty in speech and language and/or literacy skills. Receptive and expressive language skills for the probands and siblings who were aged 5–16.9 years were assessed using the Clinical Evaluation of Language Fundamentals‐Revised (CELF‐R) test.24 This assessment consists of six subtests, three of which combine to give a composite receptive language score (RLS) and three a composite expressive language score (ELS). There are relatively few standardised tests for assessing language abilities in school‐aged children and the CELF‐R is widely used and accepted in the UK for this purpose. However, the test is standardised on American children and information on UK norms is limited, although a study of 20 Scottish children aged 12 years showed that receptive subtest scores were not significantly different.25 NVIQ was assessed using the age‐appropriate version of the Raven's Matrices.26,27
Auditory memory was measured for all family members aged over 4 years using a research version of a non‐word repetition test (NWR).28 This task is a measure of phonological auditory memory and a poor performance is considered a good clinical indicator of SLI as it can continue to be impaired even when the overt language deficit has resolved.17 All family members had their speech sound inventory determined by the Edinburgh Articulation Test (EAT).29 This allowed for a qualitative judgement as to whether any potential errors made in the non‐word memory test were typical of a person's habitual speech sounds to ensure that the speaker was not penalised for making accent‐specific productions. The standardisation of the EAT is based on a sample of children aged 3–6 years. All speakers above the age of 6 years who had a raw score of 52 or lower (ie, the standard score equivalent of 85 at 6 years) were considered to have a speech sound disorder in accordance with the authors' suggestion.25 The measure of the CELF language scores and the NWR have both been informative in a systematic genome‐wide analysis in SLI.19,30
Statistical analysis
Quantitative measurements were approximately normally distributed, so parametric methods were used throughout. Associations between different measurements within each category for the study participants were conducted by two‐sample t tests or Pearson correlation as appropriate. Multiple linear regression was used to test for the effects of different factors on NVIQ after adjusting for age. Paired t tests with the family as the unit, were used to compare probands with other family members using the mean scores for all of those tested in the case of siblings.
Results
A total of 355 children were referred to the study. Of these, 266 were excluded, 141 because, although they had a receptive language impairment, it was less marked than 2 SD below the mean and therefore did not meet the ICD‐10 (F80.2) criteria. A further 78 children were excluded because their NVIQ was below the normal range and 47 were excluded under criterion C. Of the remaining 89 families invited onto the study, 22 declined and nine subsequently withdrew, leaving 58 probands and their families to participate. There were no children from ethnic minorities. There was no significant difference in age and gender of the participants who declined or withdrew. The participant group characteristics are shown in table 1.
Table 1 Participant group characteristics.
| Probands (n = 58) | Siblings (n = 98) | Mothers (n = 57) | Fathers (n = 51) | |
|---|---|---|---|---|
| Age (study entry), years | ||||
| Mean (SD) | 8.8 (3.3) | 9.6 (5.5) | 38.4 (5.8) | 40.8 (5.6) |
| Median | 7.7 | 7.5 | 37.9 | 40.8 |
| Range | 4.3–16.8 | 0.3–27.4 | 23.9–57.0 | 28.7–50.3 |
| Duration (years) from | 5.9 (2.8) | |||
| multi‐disciplinary | ||||
| assessment of special | ||||
| educational needs to | ||||
| study entry, mean (SD) | ||||
| Gender male:female | 2.2:1 | 1.13:1 | ||
| Number with SLI by | 35 | 4 | 10 | |
| parental report |
The proband adherence to ICD‐10 (F80.2) criteria had been established during the late preschool year at the approach of school entry with the exception of one child who had a late diagnosis of SLI at the age of 8 years. All probands were receiving or had recently had specialist educational support. Parents reported language and literacy difficulties for 35 of the siblings and four of the mothers gave this as a self‐report and 10 of the fathers. Table 2 shows the results of the assessment battery for the probands.
Table 2 Proband assessment profile expressed in standard scores.
| Non‐verbal IQ (Raven's) | (n = 53)* |
| Mean (SD) | 100 (14) |
| Range | 75–125 |
| Language measures (CELF‐R) | (n = 54)* |
| Receptive language composite | |
| Mean (SD) | 72.7 (12) |
| Range | 50–107 |
| Expressive language composite | |
| Mean (SD) | 64.2 (10.6) |
| Range | 50–97 |
| Auditory memory (NWR) | (n = 53)* |
| Mean (SD) | 69.9 (16.4) |
| Range | 55–130 |
*Data missing due to proband being too young (1) or too old (3) to comply with test standardisation, emigration (1), non‐cooperation with specific test (4) or missing data (1).
CELF‐R, Clinical Evaluation of Language Fundamentals‐Revised; NWR, non‐word repetition test; SD, standard deviation.
Proband phenotype
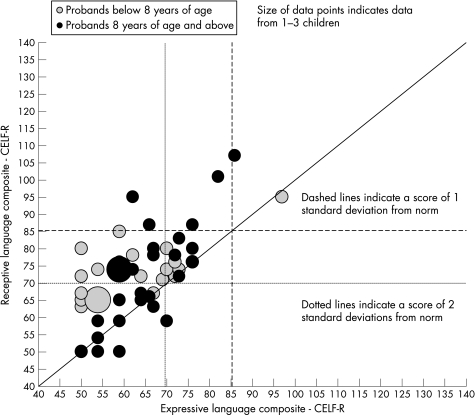
At a mean of 6 years from the initial multi‐disciplinary assessment and diagnosis of receptive SLI, cross‐sectional study demonstrated that the proband's language levels remained significantly impaired. A total of 35% of the probands had receptive language measures that were below 2 SD from the mean and thus continued to meet ICD‐10 (F80.2) criteria for receptive SLI. An additional 28 (52%) of probands had a receptive deficit that lay between 1 and 2 SD below the mean. Expressive language was even more severely affected and 35 (60%) had expressive language below 2 SD from the mean, with a further 17 (29%) between 1 and 2 SD below. The expressive language was positively correlated with receptive language (p>0.001, r = 0.60) (fig 1) and only two (3%) of the children had attained both receptive and expressive ability within normal limits at the cross‐sectional point of the study. Fourteen of 51 probands had a speech sound disorder. There was no significant correlation between the school entry scores for the Reynell Language Scales31 and the CELF‐R scores at the point of the study. This remained true after the groups were split around the median follow‐up time, so that there was no evidence that those with shorter time intervals of follow‐up had more association between the two scores.
Figure 1 Comparison of the receptive and expressive language standard scores of probands.
The language measures were not related to age (receptive language vs age non‐significant, p = 0.829, r = −0.0301; expressive language vs age non‐significant, p = 0.303, r = 0.1427). NVIQ was normally distributed and after adjusting for age in a multiple regression there was no significant relationship with language measures.
Family members
The sibling assessment profiles are shown in table 3.
Table 3 Sibling assessment profile expressed in standard scores.
| Non‐verbal IQ (Raven's) | n = 72 |
| Mean (SD) | 96 (14.72) |
| Range | 75–125 |
| Language measures (CELF‐R) | n = 62 |
| Receptive language composite | |
| Mean (SD) | 89.9 (16.6) |
| Range | 50–128 |
| Expressive language composite | |
| Mean (SD) | 81.0 (15.3) |
| Range | 50–124 |
| Auditory memory (NWR) | n = 82 |
| Mean (SD) | 81.7 (17.6) |
| Range | 55–125 |
CELF‐R, Clinical Evaluation of Language Fundamentals‐Revised; NWR, non‐word repetition test; SD, standard deviation.
A total of 62 of 98 siblings were the correct age to complete the assessment of the CELF‐R (20 siblings were too young and 12 were too old for the assessment battery and four were non‐compliant). Figure 2 shows that although the siblings had significantly higher language scores than their probands (p>0.001), the distribution of their standard scores was below that expected in the normal population. Six of the 62 had both a receptive and expressive language standard score below 70 and thus would have met ICD‐10 (F80.2) criteria for receptive SLI. Four of these children had been reported to have difficulties in their language acquisition or literacy development by their parents. In addition, one other child had a receptive language standard score below 70, thus fulfilling ICD‐10 criteria for receptive SLI but their expressive language was better developed and their parents had not reported any difficulties that they recognised in the acquisition of language or literacy. There were a further seven children who had expressive language standard scores that were below 70 and six of these siblings had been recognised by their parents to have some sort of language learning problem affecting the acquisition of spoken language or literacy. Therefore, even though four children met criteria for SLI they had not been recognised by their parents to have a language learning difficulty. Three of these children had a measured speech sound disorder on the EAT.
Figure 2 Comparison of the receptive and expressive language standard scores of siblings.
Phonological auditory memory for probands, siblings and parents
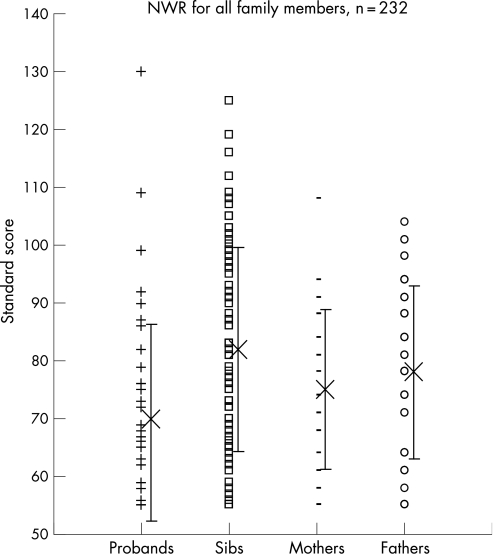
Both parents participated in the study for 50 families, only the mother took part for seven, and only the father for one. Forty nine mothers and 41 fathers were available to complete the EAT and none had a speech disorder on this measure. Three mothers had reported language learning difficulties: one an SLI and two dyslexia. Ten of the fathers described language learning difficulties with SLI in one, dyslexia in five, phonological disorder in one and a mixed language learning impairment in three. The probands' non‐word repetition scores as a measure of phonological auditory memory were not related to receptive language ability (NS, p = 0.082, r = 0.25) or age (NS, p = 0.056, r = −0.27) and were significantly related to expressive language (p = 0.005, r = 0.39) and NVIQ (p = 0.013, r = 0.35). The non‐word repetition standard scores for the siblings also correlated with expressive language measures (p>0.001) but did not correlate with the siblings' receptive language scores. Looking at the family members as a whole, the non‐word repetition standard scores were substantially below the normal range for probands, siblings and parents (fig 3). There were significant correlations between the proband's standard scores and that of their family members (the average of their siblings (p>0.001, r = 0.50), their mothers (p = 0.002, r = 0.42) and their fathers (p = 0.011, r = 0.37)). There was no significant correlation between the maternal and the paternal scores (p = 0.07, r = 0.28). The parents' reporting of their own affected status was not reflected in their non‐word repetition score (mothers, p = 0.37, t = 0.91; fathers, p = 0.55, t = 0.60). The siblings' non‐word repetition standard scores, however, did correlate with the reports from their parents that they were affected with a language learning difficulty (p = 0.004). Overall, 40 (69%) of the probands had a first‐degree relative with either a language learning impairment described from the history or a directly measured impaired language standard score.
Figure 3 Non‐word repetition (NWR) test as a measure of auditory memory expressed as a standard score for all family members.
Discussion
Receptive SLI, in which a child experiences difficulties in acquiring language comprehension despite normal non‐verbal intelligence, is an important condition to identify because it has long‐term adverse sequelae on language, learning and behaviour.8,9,10,32 The definition of the condition is debated both in terms of where one places the exact cut‐off points for the exclusionary criteria for language impairment severity and NVIQ and what constitutes a predisposing neurological impairment.33 However, in this paper we are reporting on a group of children with a severe form of the disorder which conforms to strict research ICD‐10 criteria. This is the largest group of children with severe receptive SLI reported to date in the literature and the 65% participation in this family genetic study was very high compared with that described in the literature for other conditions such as asthma.34 This rigour of phenotyping identified a group in whom a language learning difficulty occurred in 69% of first‐degree relatives, which is much higher than previously thought to be the case in receptive SLI.17
The ratio of boys to girls in our study group was just over 2 to 1. There is no other directly similar group with which to compare this finding. As the children in this report were identified as being in need of a language unit type level of educational support, the closest comparison might be the cohort of children reported in a number of publications32,35 who were a random sample of 50% of all year 2 children attending language units in the UK. The ratio of boys to girls was over 3 to 1 in these studies, but the cohort included children with expressive disorder only, as well as those with moderate learning difficulties and autism spectrum disorders. The other literature that explores gender ratios for SLI includes epidemiological studies, but again the definition of SLI is usually based on a composite of receptive and expressive language skills and is less severe. With this approach the prevalence in boys and girls is very similar at 0.08 and 0.06, respectively.3
Although the children were identified by their receptive language impairment, they all had equally severe expressive language impairments. The same pattern was observed in affected siblings. There are a number of studies reviewed by Bishop36 which suggest that receptive language impairment may be conceptualised as being at the severe end of SLI in contrast to isolated expressive disorder which characterises the milder form. This relationship between receptive and expressive language is in keeping with the apparent genetic similarities of linkage in the different forms of receptive and expressive SLI, although the work in this area to date is not powered per se to compare the genetics between the two.19,30
In our study we examined the capacity of phonological working memory through an NWR for all family members who were old enough to complete it. This measures short‐term storage and verbal processing, has been reported as deficient in SLI and demonstrates a consistent linkage with the SLI1 region of chromosome 16q.19,30,36 It has not been specifically described in receptive language impairment, but our study demonstrates that not only is it reduced in affected probands, but it is generally depressed in siblings and parents. These findings are in keeping with the evidence that although performance on the test by young children can be constrained by the immature phonological and articulatory systems, the highly consistent pattern of associations between non‐word repetition and language learning across different ages and groups suggests that speech‐motor outputs cannot explain the individual performance.37 Although many of the adults in our study performed poorly on this measure of non‐word repetition, none of them had a speech output problem as formally measured by a standardised articulation test.
Although performance on an NWR is highly heritable and associates with poorer language acquisition, it is possible to have difficulties with phonological memory and yet not develop SLI,36 and so other factors appear involved. However, although our understanding of the genetics of speech and language disorders is advancing rapidly since the first reporting of the Fox P2 gene on 7q31 in a family with a severe speech disorder,38,39 with subsequently linkage sites reported for SLI,19,28,40 our present state of knowledge does not allow for genetic confirmation of SLI. Therefore, our findings suggest that siblings of children affected by receptive SLI should have a formal assessment of their language skills by a speech and language therapist. As our findings confirm that children in families affected by receptive SLI also have expressive difficulties, the identification of affected siblings has implications for their treatment. A recent Cochrane report emphasised that although more research was needed to identify the best way forward for treating receptive language difficulties,41 there were significant benefits demonstrable for expressive language difficulties from speech and language therapy. The importance of our findings is that the incidence of language impairment is so high in siblings that they might be best served by undergoing formal language assessment given the difficulties of case recognition and diagnosis.11,12,33,42
What is already known on this topic
Language delay affects 6–8% of preschool children and, although nearly half will attain normal language levels by 5 years of age, studies of heterogeneous groups of children with either receptive and/or expressive specific language impairment (SLI) suggest that a persisting difficulty in verbal comprehension at school entry is associated with a poorer prognosis for complete language recovery.
SLI has a familial incidence, but this is considered less important in receptive SLI.
What this study adds
This is the first study to report clinical and familial characteristics for a group of children with tightly defined receptive SLI and demonstrates a high familial incidence of the condition, with 64% of children having at least one language impaired sibling who may not have been recognised by their parents as being affected.
Only 3% of children with severe receptive SLI achieved language in the normal range at follow‐up and screening of siblings should be considered.
In conclusion, we acknowledge that this present study is not prospective and so there is the possibility of a bias towards more severely affected children participating. Nevertheless, the very high rate of continuing severe receptive and expressive difficulties at a mean of 6 years after identification of the SLI at school entry is of concern, particularly if extrapolated into adult life.10 We are also unable to comment on outcome and familial and clinical features for children who have either a less severe receptive language impairment or one that might be arising from a wide range of underlying aetiologies, as these children were excluded under the rigorous subject eligibility that we had to adhere to in order to arrive at an informative phenotype for genetic study. What we have demonstrated is that for families with a child recognised to have a severe receptive language impairment at school entry, there is a high rate of SLI in siblings which may not be evident clinically or apparent to their parents, and that there may be merit in identifying affected siblings through formal language testing. Also poor phonological memory is a characteristic for these family members. Finally, the prognosis for recovery of receptive and expressive language appears poor and affected children are likely to require long‐term support.
Acknowledgements
This research was supported by a grant from the Chief Scientist Office.
Abbreviations
CELF‐R - Clinical Evaluation of Language Fundamentals‐Revised
EAT - Edinburgh Articulation Test
NVIQ - non‐verbal IQ
NWR - non‐word repetition test
SD - standard deviation
SLI - specific language impairment
Footnotes
Competing interests: None declared.
References
- 1.Rapin I. Practitioner review of developmental language disorders: a clinical update. J Child Psychol Psychiatry 199637643–655. [DOI] [PubMed] [Google Scholar]
- 2.Stark R E, Tallal R P. Selection of children with specific language deficits. J Speech Hear Disord 198146114–180. [DOI] [PubMed] [Google Scholar]
- 3.Tomblin J B, Records N L, Buckwalter P.et al Prevalence of specific language impairment in kindergarten children. J Speech Lang Hear Res 1997401245–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Law J, Boyle J, Harris F.et al Screening for speech and language delay: a systematic review of the literature. Health Technol Assess 199821–180. [PubMed] [Google Scholar]
- 5.Bishop D, Edmundson A. Language impaired 4‐year‐olds: distinguishing transient from persistent impairment. J Speech Hear Disord 198752156–173. [DOI] [PubMed] [Google Scholar]
- 6.Bishop D, Edmundson A. Specific Language Impairment as a maturation lag: evidence from language and motor development.Dev Med Child Neurol 198729442–449. [DOI] [PubMed] [Google Scholar]
- 7.Conti‐Ramsden G, Botting N, Faragher B. Psycholinguistic markers for specific language impairment (SLI). J Child Psychol Psychiatry 200142741–748. [DOI] [PubMed] [Google Scholar]
- 8.Stothard S, Snowling M, Bishop D.et al Language‐impaired preschoolers: a follow‐up into adolescence. J Speech Lang Hear Res 199841407–418. [DOI] [PubMed] [Google Scholar]
- 9.Johnson C J, Beitchman J H, Escobar M.et al Fourteen‐year follow‐up of children with and without speech/language impairments: speech/language stability and outcomes. J Speech Lang Hear Res 199942744–760. [DOI] [PubMed] [Google Scholar]
- 10.Clegg J, Hollis C, Mawhood L.et al Developmental language disorders ‐ a follow‐up in later adult life. Cognitive, language and psychosocial outcomes. J Child Psychol Psychiatry 200546(2)128–149. [DOI] [PubMed] [Google Scholar]
- 11.Shriberg L D, Tomblin J B, McSweeney J L. Prevalence of speech delay in 6 year old children and comorbidity with language impairment. J Speech Lang Hear Res 1999421461–1481. [DOI] [PubMed] [Google Scholar]
- 12.Laing G J, Law J, Levin A.et al Evaluation of a structured test and a parent led method for screening for speech and language problems: prospective population based study. BMJ 20023251152–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dale P, Simonoff E, Bishop D.et al Genetic influence on language delay in two‐year old children. Nat Neurosci 19981324–328. [DOI] [PubMed] [Google Scholar]
- 14.Bishop D V M, North T, Donlan C. Genetic basis of specific language impairment: evidence from a twin study. Dev Med Child Neurol 19953756–71. [DOI] [PubMed] [Google Scholar]
- 15.Tallal P, Ross R, Curtiss S. Familial aggregation in specific language impairment. J Speech Hear Disord 198954167–173. [DOI] [PubMed] [Google Scholar]
- 16.Tomblin J B. Familial concentration of developmental language impairment. J Speech Hear Disord 198954287–295. [DOI] [PubMed] [Google Scholar]
- 17.Lahey M, Edwards J. Specific language impairment: preliminary investigation of factors associated with family history and with patterns of language performance. J Speech Hear Res 199538643–657. [DOI] [PubMed] [Google Scholar]
- 18.Bishop D V M, North T, Donlan C. Nonword repetition as a behavioural marker for inherited language impairment: evidence from a twin study. J Child Psychol Psychiatry 199637391–403. [DOI] [PubMed] [Google Scholar]
- 19.The SLI Consortium ( S L I C ) Highly significant linkage to SLI1 locus in expanded sample of individuals affected by a specific language impairment. Am J Hum Genet 200474(6)1225–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization International Statistical Classification of Diseases and Related Health Problems: diagnostic criteria for research, tenth revision (ICD‐10). Geneva: World Health Organization, 1993
- 21.Aram D M, Hack M, Hawkins S.et al Very‐low‐birthweight children and speech and language development. J Speech Hear Res 1991341169–1179. [DOI] [PubMed] [Google Scholar]
- 22.Parkinson G M. High incidence of language disorder in children with focal epilepsies. Dev Med Child Neurol 200244533–537. [DOI] [PubMed] [Google Scholar]
- 23.James F, Fairgrieve S, Lynch S A.et al Prospective study of development of infants born to mothers with epilepsy. J Neurol Neurosurg Psychiatry 200272135 [Google Scholar]
- 24.Semel E, Wiig E, Secord W.Clinical Evaluation of Language Fundamentals‐Revised. New York, NY: Psychological Corporation, 1987
- 25.Young F, Gibbon F. Normative Scottish data on the CELF‐R UK: a pilot study. Int J Lang Commun Disord 199833345–350. [DOI] [PubMed] [Google Scholar]
- 26.Raven J, Court J, Raven J.Coloured progressive matrices. Oxford: Oxford Psychologists Press, 1995
- 27.Raven J, Court J, Raven J.Standard progressive matrices. Oxford: Oxford Psychologists Press, 1996
- 28.Gathercole S E, Willis C S, Baddeley A D.et al The children's test of nonword repetition: a test of phonological working memory. Memory 19942103–127. [DOI] [PubMed] [Google Scholar]
- 29.Anthony A, Bogle D, Ingram T T S.et alThe Edinburgh Articulation Test textbook. Edinburgh: Churchill Livingstone, 1971
- 30.The SLI Consortium A genomewide scan identifies two novel loci involved in specific language impairment. Am J Hum Genet 200270384–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reynell J, Huntley M.Reynell Developmental Language Scales II. Windsor: NFER‐Nelson, 1987
- 32.Conti‐Ramsden G, Botting N, Simkin Z.et al Follow‐up of children attending infant language units: outcomes at 11 years of age. Int J Lang Commun Disord 200136207–219. [PubMed] [Google Scholar]
- 33.Tager‐Flusberg H, Cooper J. Present and future possibilities for defining a phenotype for a specific language impairment. J Speech Lang Hear Res 1999421275–1278. [DOI] [PubMed] [Google Scholar]
- 34.Lenney W, Child C. Family genetic studies. Arch Dis Child 200287272–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Botting N. Non‐verbal cognitive development and language impairment. J Child Psychol Psychiatry 200546(3)317–326. [DOI] [PubMed] [Google Scholar]
- 36.Bishop D V M. Developmental cognitive genetics: how psychology can inform genetics and vice‐versa. Q J Exp Psychol 200659(7)1153–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gathercole S. Nonword repetition and word learning: the nature of the relationship. Appl Psycholinguist 200627(4)513–543. [Google Scholar]
- 38.Fisher S E, Vargha‐Khadem F, Watkins K E.et al Localisation of a gene implicated in a severe speech and language disorder. Nat Genet 199818168–170. [DOI] [PubMed] [Google Scholar]
- 39.Lai C S L, Fisher S E, Hurst J.et al A forkhead‐domain gene is mutated in a severe speech and language disorder. Nature 2001413519–523. [DOI] [PubMed] [Google Scholar]
- 40.Bartlett C W, Flax J F, Logue M W.et al A major susceptibility locus for specific language impairment is located on 13q21. Am J Hum Genet 20027145–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Law J, Garrett Z, Nye C. Speech and language therapy interventions for children with primary speech and language delay or disorder (Cochrane Review). In: Cochrane Library, Issue 3. Oxford, UK: Update Software, 2003 [DOI] [PMC free article] [PubMed]
- 42.Plante E, Shenkman K, Clark M M. Classification of adults for family studies of developmental language disorders. J Speech Hear Res 199639(3)661–667. [DOI] [PubMed] [Google Scholar]