Abstract
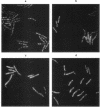
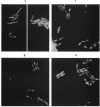
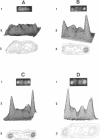
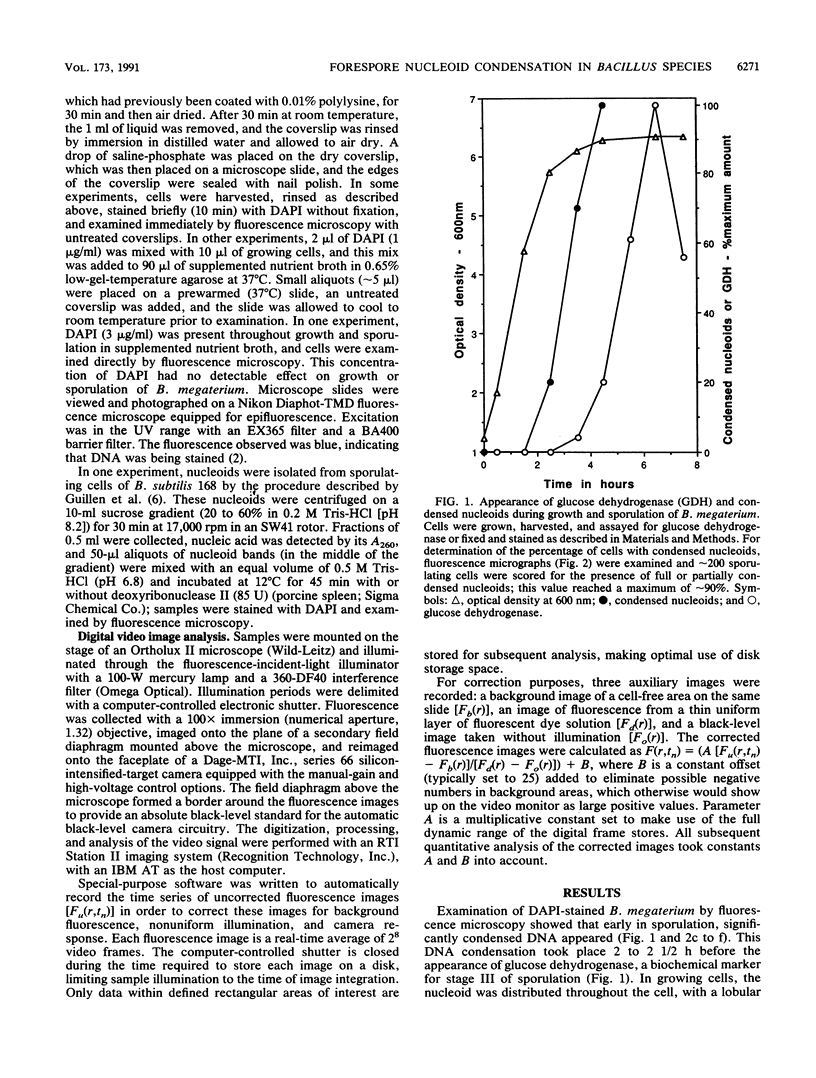
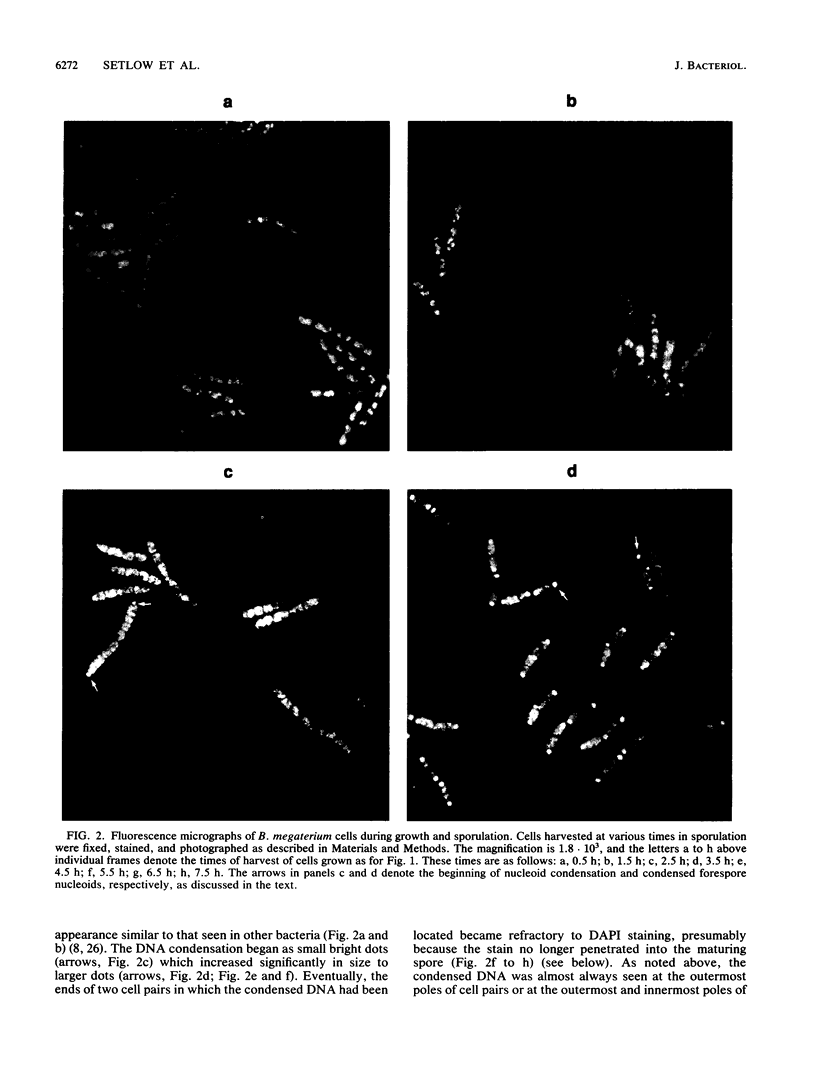
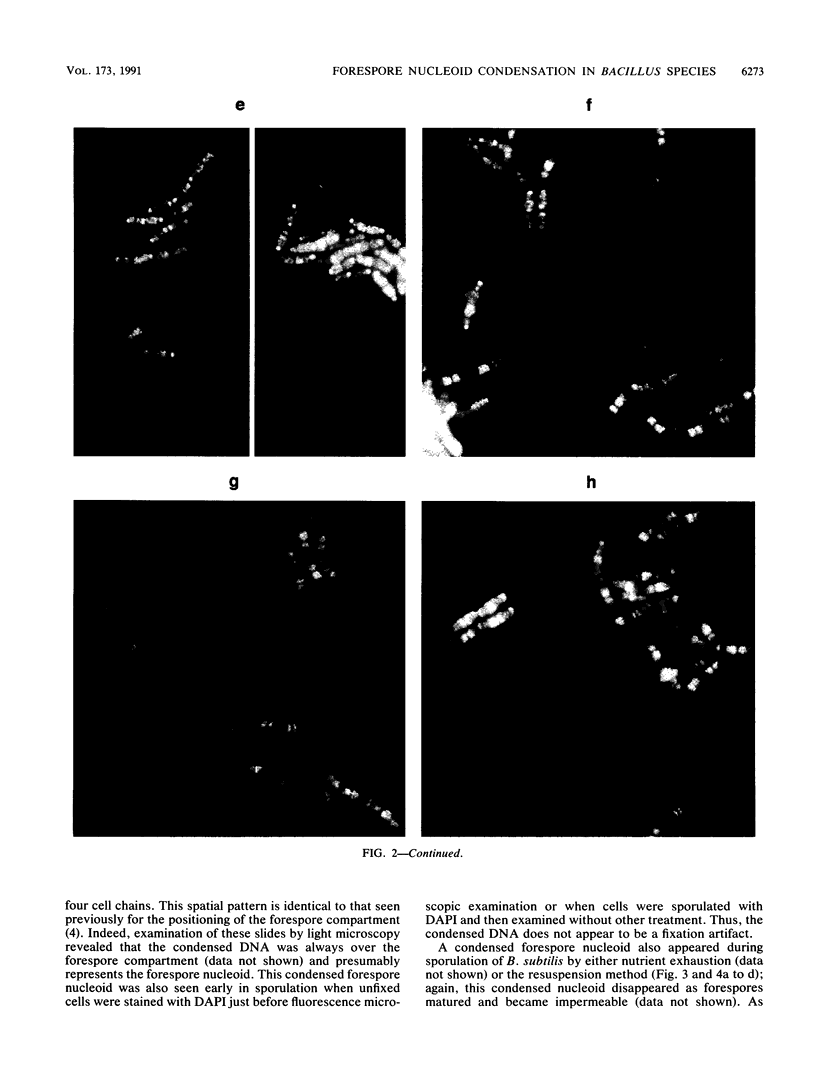
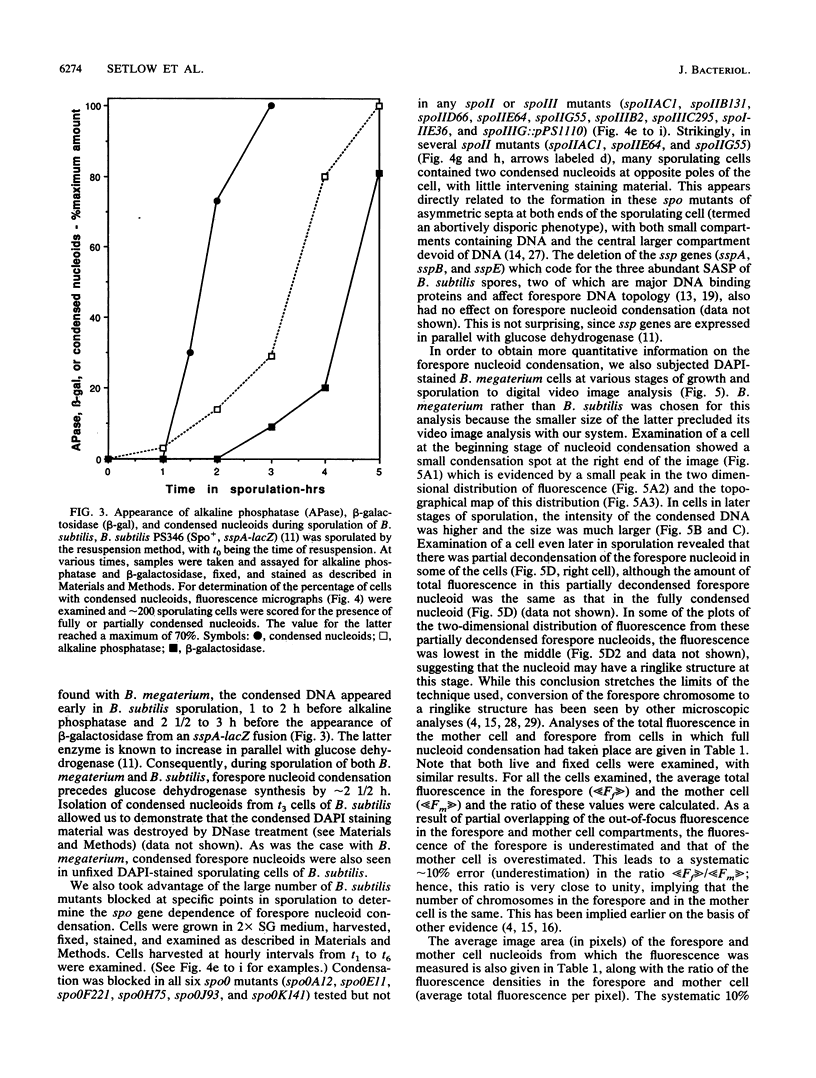
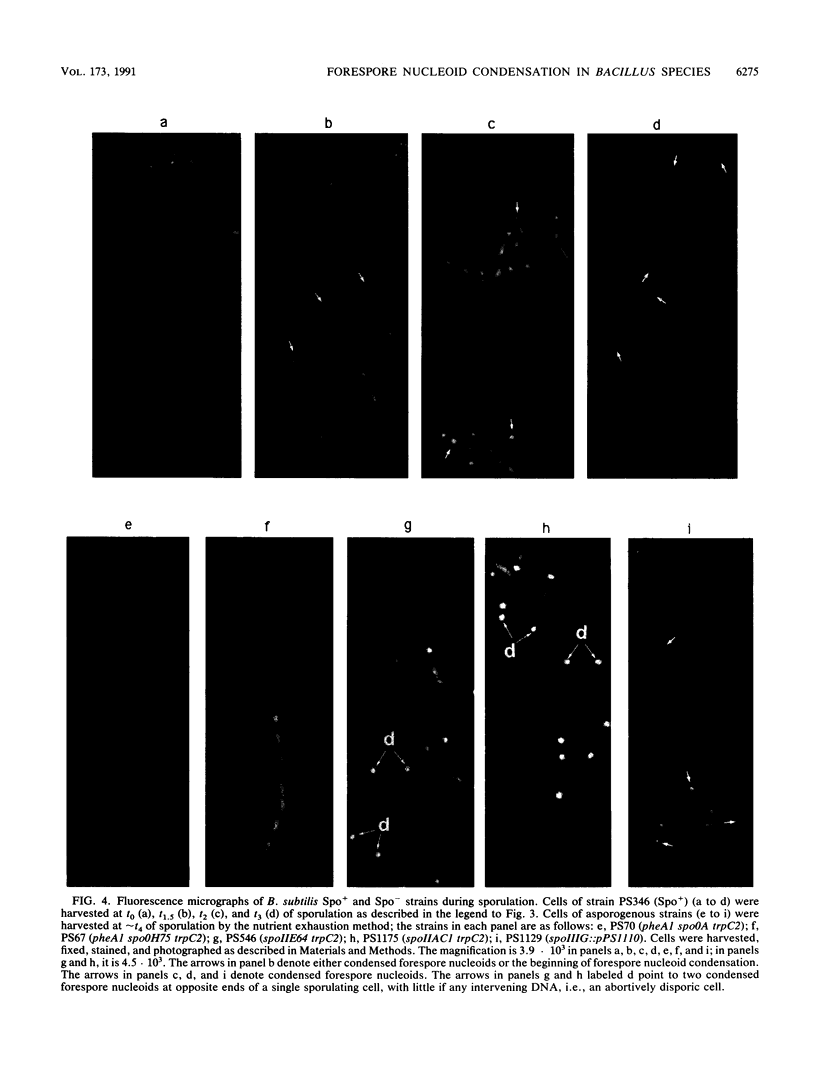
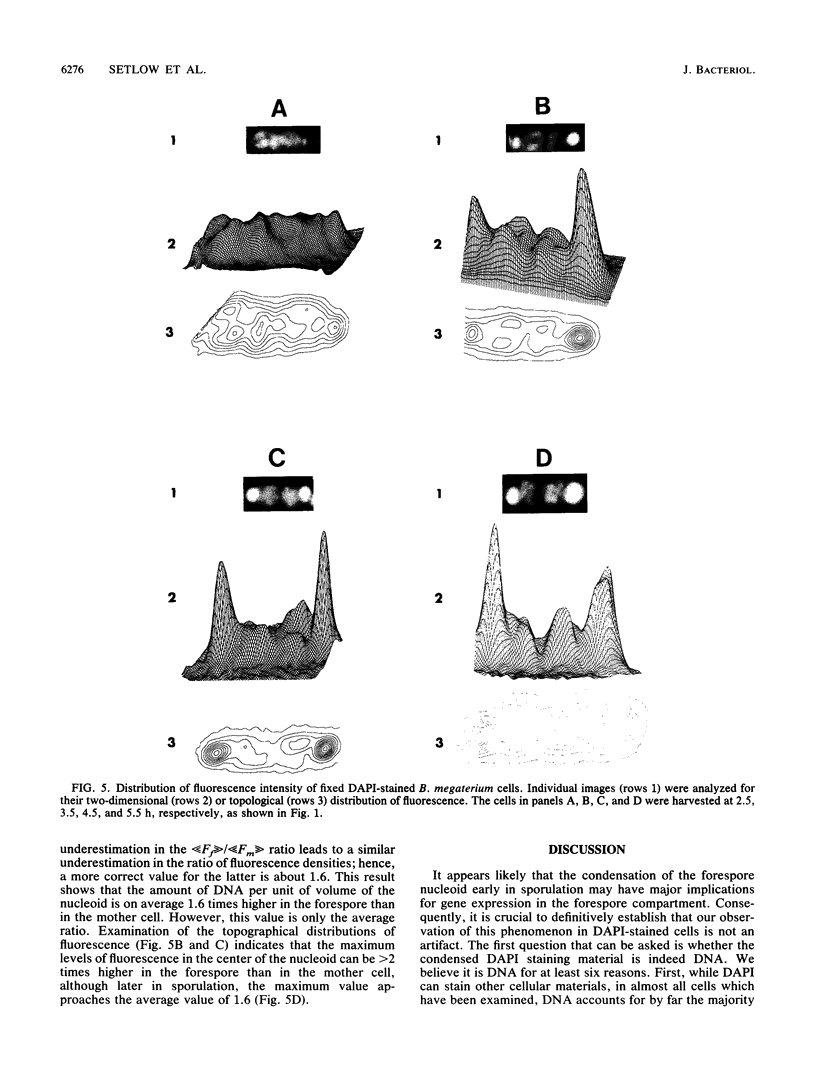
Fluorescence microscopic examination coupled with digital videoimage analysis of 4',6-diamidino-2-phenylindole-stained sporulating cells of Bacillus megaterium or Bacillus subtilis revealed a striking condensation of the forespore nucleoid. While both mother cell and forespore compartments had equal amounts of DNA, the forespore nucleoid became greater than 2-fold more condensed than the mother cell nucleoid. The condensation of the forespore nucleoid began after only the first hour of sporulation, 2 to 3 h before expression of most forespore-specific genes including those for small, acid-soluble spore proteins, and was abolished in spo0 mutants but not in spoII or spoIII mutants. It is possible that this striking condensation of forespore DNA plays some role in modulating gene expression during sporulation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brehm S. P., Le Hegarat F., Hoch J. A. Developmental modulation of deoxyribonucleic acid-binding proteins of Bacillus subtilis during sporulation stages. J Bacteriol. 1974 Dec;120(3):1443–1450. doi: 10.1128/jb.120.3.1443-1450.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman A. W., Maguire M. J., Coleman J. R. Mithramycin- and 4'-6-diamidino-2-phenylindole (DAPI)-DNA staining for fluorescence microspectrophotometric measurement of DNA in nuclei, plastids, and virus particles. J Histochem Cytochem. 1981 Aug;29(8):959–968. doi: 10.1177/29.8.6168681. [DOI] [PubMed] [Google Scholar]
- Errington J., Mandelstam J. Variety of sporulation phenotypes resulting from mutations in a single regulatory locus, spoIIA, in Bacillus subtilis. J Gen Microbiol. 1983 Jul;129(7):2091–2101. doi: 10.1099/00221287-129-7-2091. [DOI] [PubMed] [Google Scholar]
- Freese E. Sporulation of bacilli, a model of cellular differentiation. Curr Top Dev Biol. 1972;7:85–124. doi: 10.1016/s0070-2153(08)60070-8. [DOI] [PubMed] [Google Scholar]
- Goldrick S., Setlow P. Expression of a Bacillus megaterium sporulation-specific gene during sporulation of Bacillus subtilis. J Bacteriol. 1983 Sep;155(3):1459–1462. doi: 10.1128/jb.155.3.1459-1462.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillen N., Le Hegaret F., Fleury A. M., Hirschbein L. Folded chromosomes of vegetative Bacillus subtilis: composition and properties. Nucleic Acids Res. 1978 Feb;5(2):475–489. doi: 10.1093/nar/5.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett R. H., Setlow P. Properties of spores of Bacillus subtilis strains which lack the major small, acid-soluble protein. J Bacteriol. 1988 Mar;170(3):1403–1404. doi: 10.1128/jb.170.3.1403-1404.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobot J. A., Bjornsti M. A., Kellenberger E. Use of on-section immunolabeling and cryosubstitution for studies of bacterial DNA distribution. J Bacteriol. 1987 May;169(5):2055–2062. doi: 10.1128/jb.169.5.2055-2062.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M., Wilson K. S. On the DNA binding protein II from Bacillus stearothermophilus. II. The amino acid sequence and its relation to those of homologous proteins from other prokaryotes. J Biol Chem. 1983 Mar 25;258(6):4007–4011. [PubMed] [Google Scholar]
- Mason J. M., Hackett R. H., Setlow P. Regulation of expression of genes coding for small, acid-soluble proteins of Bacillus subtilis spores: studies using lacZ gene fusions. J Bacteriol. 1988 Jan;170(1):239–244. doi: 10.1128/jb.170.1.239-244.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson W. L., Setlow P. Dramatic increase in negative superhelicity of plasmid DNA in the forespore compartment of sporulating cells of Bacillus subtilis. J Bacteriol. 1990 Jan;172(1):7–14. doi: 10.1128/jb.172.1.7-14.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggot P. J., Coote J. G. Genetic aspects of bacterial endospore formation. Bacteriol Rev. 1976 Dec;40(4):908–962. doi: 10.1128/br.40.4.908-962.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RYTER A. ETUDE MORPHOLOGIQUE DE LA SPORULATION DE BACILLUS SUBTILIS. Ann Inst Pasteur (Paris) 1965 Jan;108:40–60. [PubMed] [Google Scholar]
- Salti V., Le Hégarat F., Fontaine Y., Hirschbein L. Purification and properties of the DNA-binding protein HPB12 from Bacillus subtilis nucleoid. Biochim Biophys Acta. 1989 Nov 2;1009(2):161–167. doi: 10.1016/0167-4781(89)90096-1. [DOI] [PubMed] [Google Scholar]
- Salti V., Le Hégarat F., Hirschbein L. Isolation and characterization of small heat-stable acid-soluble DNA-binding proteins from Bacillus subtilis nucleoids. J Gen Microbiol. 1985 Mar;131(3):581–590. doi: 10.1099/00221287-131-3-581. [DOI] [PubMed] [Google Scholar]
- Setlow P., Kornberg A. Biochemical studies of bacterial sporulation and germination. XVII. Sulfhydryl and disulfide levels in dormancy and germination. J Bacteriol. 1969 Dec;100(3):1155–1160. doi: 10.1128/jb.100.3.1155-1160.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P. Small, acid-soluble spore proteins of Bacillus species: structure, synthesis, genetics, function, and degradation. Annu Rev Microbiol. 1988;42:319–338. doi: 10.1146/annurev.mi.42.100188.001535. [DOI] [PubMed] [Google Scholar]
- Sterlini J. M., Mandelstam J. Commitment to sporulation in Bacillus subtilis and its relationship to development of actinomycin resistance. Biochem J. 1969 Jun;113(1):29–37. doi: 10.1042/bj1130029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D. X., Cabrera-Martinez R. M., Setlow P. Control of transcription of the Bacillus subtilis spoIIIG gene, which codes for the forespore-specific transcription factor sigma G. J Bacteriol. 1991 May;173(9):2977–2984. doi: 10.1128/jb.173.9.2977-2984.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D. X., Stragier P., Setlow P. Identification of a new sigma-factor involved in compartmentalized gene expression during sporulation of Bacillus subtilis. Genes Dev. 1989 Feb;3(2):141–149. doi: 10.1101/gad.3.2.141. [DOI] [PubMed] [Google Scholar]
- Valkenburg J. A., Woldringh C. L., Brakenhoff G. J., van der Voort H. T., Nanninga N. Confocal scanning light microscopy of the Escherichia coli nucleoid: comparison with phase-contrast and electron microscope images. J Bacteriol. 1985 Feb;161(2):478–483. doi: 10.1128/jb.161.2.478-483.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUNG I. E. CHARACTERISTICS OF AN ABORTIVELY DISPORIC VARIANT OF BACILLUS CEREUS. J Bacteriol. 1964 Jul;88:242–254. doi: 10.1128/jb.88.1.242-254.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUNG I. E., FITZ-JAMES P. C. Chemical and morphological studies of bacterial spore formation. III. The effect of 8-azaguanine on spore and parasporal protein formation in Bacillus cereus var. alesti. J Biophys Biochem Cytol. 1959 Dec;6:499–506. doi: 10.1083/jcb.6.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]