Abstract
Background
Pyridoxine‐dependent seizures (PDS) is a rare, autosomal recessively inherited disorder. Recently α‐aminoadipic semialdehyde (α‐AASA) dehydrogenase deficiency was identified as a major cause of PDS, which causes accumulation of both α‐AASA and pipecolic acid (PA) in body fluids.
Methods
We studied urinary and plasma α‐AASA and PA levels in 12 Dutch clinically diagnosed patients with PDS.
Results
α‐AASA was elevated in both urine and plasma in 10 patients. In these patients plasma PA levels were also elevated but urinary PA levels were normal.
Discussion
In all patients with clinically definite PDS, and in most patients with probable or possible PDS, the clinical diagnosis of PDS could be confirmed at the metabolite level. Non‐invasive urinary screening for α‐AASA accumulation provides a reliable tool to diagnose PDS and can save these patients from the classical and potentially dangerous pyridoxine withdrawal test to prove PDS.
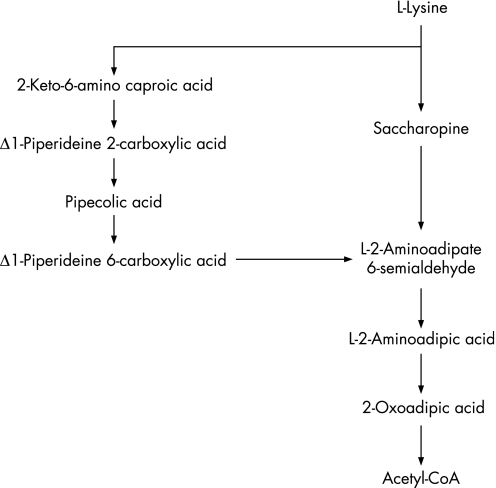
Pyridoxine‐dependent seizures (PDS) is a rare, autosomal recessively inherited disorder usually presenting very shortly after birth and in some cases in the womb. For 50 years PDS has been a clinical and biochemical conundrum which has puzzled physicians and scientists.1 Plecko et al and Willemsen et al observed isolated pipecolic acid (PA) elevations in the plasma and cerebrospinal fluid of PDS patients, yet the biochemical relationship with pyridoxine metabolism remained unclear.2,3,4 Recently, α‐aminoadipic semialdehyde (α‐AASA) dehydrogenase deficiency due to pathogenic mutations in the ALDH7A1 gene, was shown to be a major cause of PDS.5 In mammals, the essential amino acid L‐lysine is degraded via PA into the intermediate α‐AASA, which is subsequently oxidised to L‐2‐aminoadipic acid, a reaction catalysed by the enzyme α‐AASA dehydrogenase (EC 1.2.1.31, also named antiquitin) (fig 1). In PDS patients, the lack of α‐AASA dehydrogenase leads to an accumulation of α‐AASA and PA in body fluids. α‐AASA is in spontaneous reversible equilibrium with piperideine‐6‐carboxylate (P6C) in the cytosol. Accumulated P6C irreversibly reacts with active pyridoxine, ie, pyridoxal‐5‐phosphate (P5P), by forming a Knoevenagel condensation product. This irreversible reaction causes a secondary deficit of P5P in affected children, which subsequently leads to epileptic seizures. Restoration of the P5P pool can easily be achieved by oral pyridoxine supplementation, which resolves the seizures.
Figure 1 Metabolic pathway of L‐lysine.
Recently, we reported on the epidemiology and clinical features of PDS in the Netherlands in this journal.6 In that paper the classical clinical criteria according to Baxter were used to establish the diagnosis of PDS. We therefore re‐evaluated that series of PDS patients by measuring their levels of α‐AASA and PA in urine and plasma.
Methods
We re‐evaluated all children (n = 11) with a diagnosis of definite, probable or possible PDS from a recently described Dutch cohort of 13 patients.6 These patients and their parents were invited to visit our hospital and were informed of the novel insights into the pathophysiology of PDS. All except one patient (patient 11) underwent further diagnostic work‐up by laboratory investigations of urine and blood. Furthermore, we were able to include a recently born sibling of patient 10 in the present study (patient 12).
α‐AASA in urine and plasma was measured by liquid chromatography‐tandem mass spectrometry as previously published.5 Quantitative determination of PA in urine and plasma was performed by stable isotope dilution gas chromatography‐mass spectrometry.7
Results
Urine and/or blood samples were obtained from 11 patients. The results of α‐AASA and PA measurements are given in table 1. α‐AASA was elevated in the urine and plasma of 10 patients. PA in plasma was elevated in all patients with elevated (plasma and urine) α‐AASA, while urinary PA concentrations were normal in all patients.
Table 1 Results of α‐AASA and PA measurements.
| Patient | Sibling | Birth year | PDS (Baxter criteria) | α‐AASA urine (mmol/mol cr) | α‐AASA plasma (μmol/l) | PA urine (mmol/mol cr) | PA plasma (μmol/l) | PDS (confirmed metabolically) |
|---|---|---|---|---|---|---|---|---|
| 1 | 5 | 1991 | Possible | 16 | 8.0 | 0.11 | 6.5 | Y |
| 2 | 1992 | Definite | 4.7 | 0.9 | 0.27 | 5.8 | Y | |
| 3 | 7 | 1992 | Definite | 29 | 5.7 | 0.00 | 4.6 | Y |
| 4 | 6 | 1992 | Definite | 4.0 | 1.1 | 0.02 | 7.0 | Y |
| 5 | 1 | 1993 | Possible | 24 | 5.0 | 0.11 | 5.0 | Y |
| 6 | 4 | 1994 | Probable | 0.2 | <0.2 | 0.02 | 2.2 | N |
| 7 | 3 | 1995 | Probable | 20 | 5.8 | 0.01 | 5.1 | Y |
| 8 | 1998 | Possible | 12 | 2.4 | 0.07 | 5.5 | Y | |
| 9 | 2001 | Definite | 9.6 | 0.8 | 2.0 | 22 | Y | |
| 10 | 12 | 2003 | Probable | 39 | 6.1 | 0.08 | 7.8 | Y |
| 11 | 2003 | Possible | NA | NA | NA | NA | N | |
| 12 | 10 | 2004 | 75 | 5.2 | 1.37 | 11 | Y |
N, no; NA, not available; Y, yes.
Control α‐AASA concentrations are <0.2 μmol/l for plasma and <1 mmol/mol creatinine for urine.
Control values for PA in urine are 0.55–24.1 mmol/mol creatinine (<6 months of age) and 0.01–1.54 mmol/mol creatinine (>6 months of age).
For PA in plasma, control values are 3.75–10.8 μmol/l (<1 week of age), and 0.7–2.46 μmol/l (>1 week of age).
Discussion
In this study, in all patients with a definite diagnosis of PDS according to the criteria published by Baxter,8 α‐AASA dehydrogenase deficiency could be proven at the metabolite level by demonstrating elevated concentrations of α‐AASA (plasma and urine) and PA (plasma). The diagnosis was also confirmed in two out of three patients with probable PDS and in three out of four patients with possible PDS.
The diagnosis of probable PDS could not be confirmed in one patient (patient 6). She is a younger sister of a girl with a definite clinical diagnosis of PDS and a metabolically confirmed diagnosis of α‐AASA dehydrogenase deficiency (patient 4). She had subtle neonatal seizures with only minimal epileptic discharges on a 24‐h EEG, which responded to 100 mg of pyridoxine given intravenously. She is performing well at school. Her normal development makes a diagnosis of PDS unlikely since most PDS patients suffer from, at least a mild, encephalopathy with learning difficulties.5 However, the nature of the neonatal seizure‐like period remains unexplained. We advised a trial period of pyridoxine withdrawal but could not convince the parents to stop treatment. DNA analysis of patients included in our study are pending.
In patient 11, originally diagnosed with possible PDS, pyridoxine was recently withdrawn without recurrence of seizures. We consider PDS a very unlikely diagnosis in this patient because of the above observation and the fact that the child is developing well. The parents did not want to cooperate with further metabolic investigations.
In our first report,6 we described two patients (patients 12 and 13 in that paper) who did not meet the criteria for definite, probable or possible PDS. In both patients we have now demonstrated normal α‐AASA concentrations in plasma and urine, as would be expected (data not shown).
This report is the first nationwide population‐based study on metabolically confirmed PDS. Our results show that at least 10 children with PDS were born in the Netherlands between January 1991 and December 2004. As 2 764 697 children were born in the Netherlands during this time (adapted from http://statline.cbs.nl), the birth incidence of biochemically proven PDS in the Netherlands is at least 1: 276 000 children. This study further shows that most patients, namely nine out of 11 (82%), were diagnosed correctly using the criteria proposed by Baxter. Thus, in circumstances where metabolic examination of α‐AASA and/or PA is not possible, applying the clinical criteria proposed by Baxter seems a reliable method to establish a diagnosis of PDS.
The concentrations of α‐AASA and PA, in urine as well as in plasma, vary considerably in patients with PDS. A remarkably wide range of α‐AASA and PA levels in patients has also been found by Mills et al in their first report on α‐AASA dehydrogenase deficiency in PDS.5 We have no clear explanation for this wide range. Hypothetically it might reflect different levels of α‐AASA dehydrogenase residual activity, dietary protein (L‐lysine) intake, or the amount of supplemental pyridoxine. It is tempting to speculate that optimum treatment (ie, pyridoxine dosage) in PDS might be achieved by focusing on the concentrations of α‐AASA and PA.
Conclusion
Metabolic investigations of urinary concentrations of α‐AASA provide a reliable tool to prove PDS associated with α‐AASA dehydrogenase deficiency at the metabolite level. The potentially dangerous trial of withdrawal of pyridoxine, classically used to prove PDS, can now be avoided. The novel insights into the pathophysiological processes that underlie PDS further provide us with tools to better estimate the true incidence of PDS (at least 1:276 000 newborns in The Netherlands according to this study) and will hopefully lead to an optimum treatment regime for this serious neurometabolic disorder.
What is already known on this topic
PDS is a rare disease caused by alpha‐aminoadipic semialdehyde (α‐AASA) dehydrogenase deficiency.
Epidemiological data on PDS are rare and are based on clinical criteria for PDS.
What this study adds
Non‐invasive, urinary screening for α‐AASA accumulation provides a reliable tool to diagnose PDS.
The birth incidence of metabolically confirmed PDS in this nationwide Dutch study is estimated to be at least 1:276 000.
Acknowledgements
We would like to thank all replying clinicians for their cooperation, in particular the following for kindly providing the patient data: Dr W Baerts, Isala Klinieken, Zwolle; Dr F van Berkestijn, Universitair Medisch Centrum, Utrecht; Dr AN Bosschaart and Dr RFHM Tummers, Medisch Spectrum Twente, Enschede; Dr I de Coo, Erasmus Medisch Centrum, Rotterdam; Dr GAPT Hurkx, Elkerliek Ziekenhuis, Helmond; Dr R Kohl, Het Spittaal, Zuthpen; Dr LAEM Laan, Leids Universitair Medisch Centrum, Leiden; Dr A van der Wagen, Streekziekenhuis Midden‐Twente, Hengelo.
Abbreviations
α‐AASA - α‐aminoadipic semialdehyde
PA - pipecolic acid
PDS - pyridoxine‐dependent seizures
P5P - pyridoxal‐5‐phosphate
P6C - piperideine‐6‐carboxylate
Footnotes
Competing interests: None.
References
- 1.Baxter P. Pyridoxine‐dependent seizures: a clinical and biochemical conundrum. Biochim Biophys Acta 20031647(1–2)36–41. [DOI] [PubMed] [Google Scholar]
- 2.Plecko B, Hikel C, Korenke G C.et al Pipecolic acid as a diagnostic marker of pyridoxine‐dependent epilepsy. Neuropediatrics 200536(3)200–205. [DOI] [PubMed] [Google Scholar]
- 3.Plecko B, Stockler‐Ipsiroglu S, Paschke E.et al Pipecolic acid elevation in plasma and cerebrospinal fluid of two patients with pyridoxine‐dependent epilepsy. Ann Neurol 200048(1)121–125. [PubMed] [Google Scholar]
- 4.Willemsen M A, Mavinkurve‐Groothuis A M, Wevers R A.et al Pipecolic acid: a diagnostic marker in pyridoxine‐dependent epilepsy. Ann Neurol 200558(4)653. [DOI] [PubMed] [Google Scholar]
- 5.Mills P B, Struys E, Jakobs C.et al Mutations in antiquitin in individuals with pyridoxine‐dependent seizures. Nat Med 200612(3)307–309. [DOI] [PubMed] [Google Scholar]
- 6.Been J V, Bok L A, Andriessen P.et al Epidemiology of pyridoxine dependent seizures in the Netherlands. Arch Dis Child 200590(12)1293–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kok R M, Kaster L, de Jong A P.et al Stable isotope dilution analysis of pipecolic acid in cerebrospinal fluid, plasma, urine and amniotic fluid using electron capture negative ion mass fragmentography. Clin Chim Acta 1987168(2)143–152. [DOI] [PubMed] [Google Scholar]
- 8.Baxter P. Epidemiology of pyridoxine dependent and pyridoxine responsive seizures in the UK. Arch Dis Child 199981(5)431–433. [DOI] [PMC free article] [PubMed] [Google Scholar]