Abstract
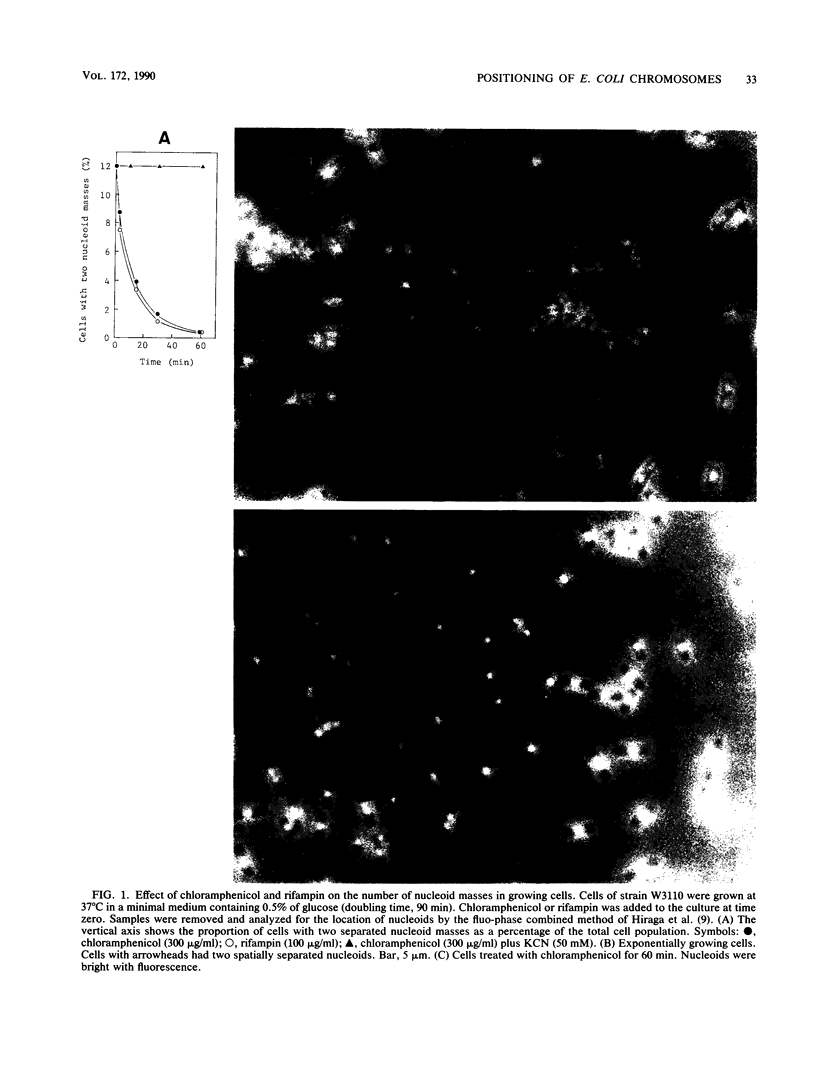
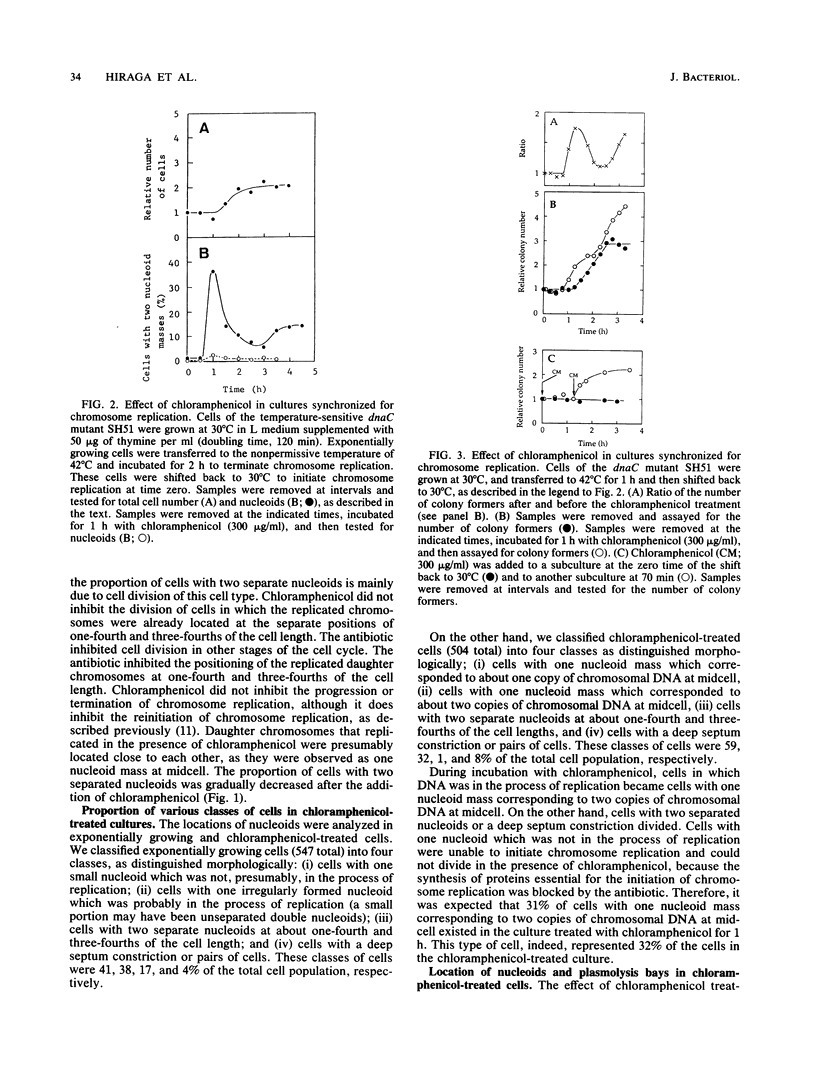
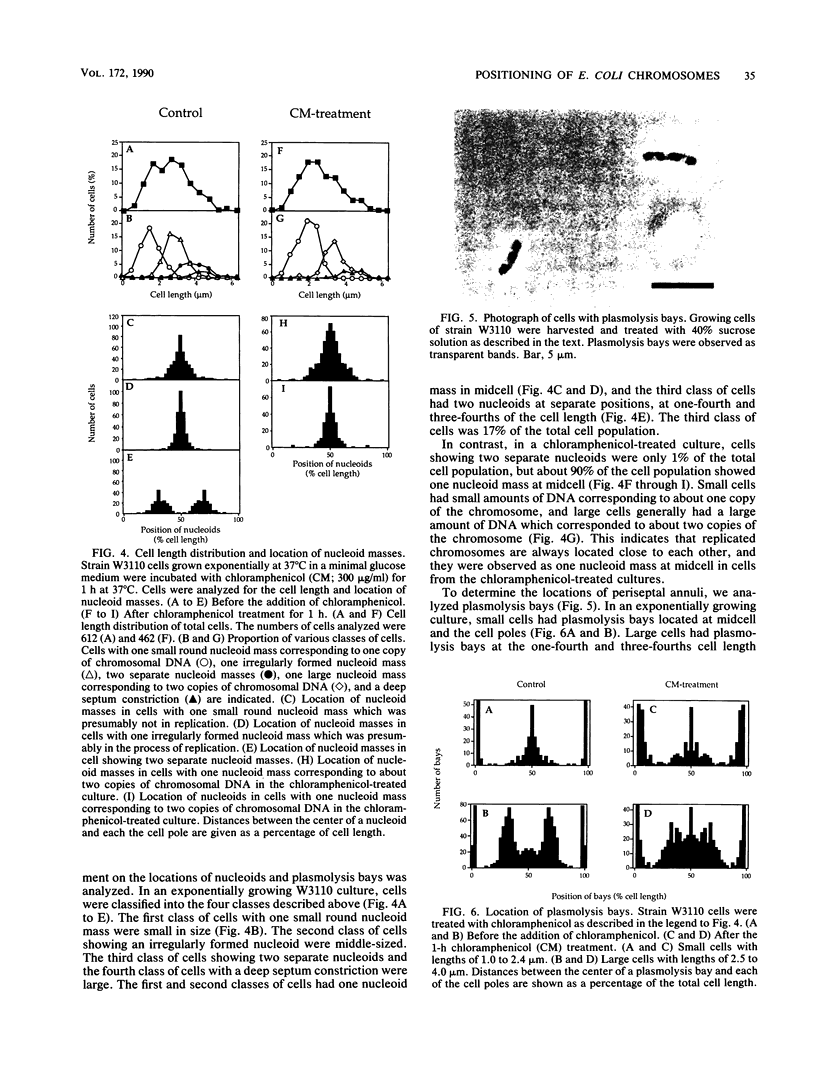
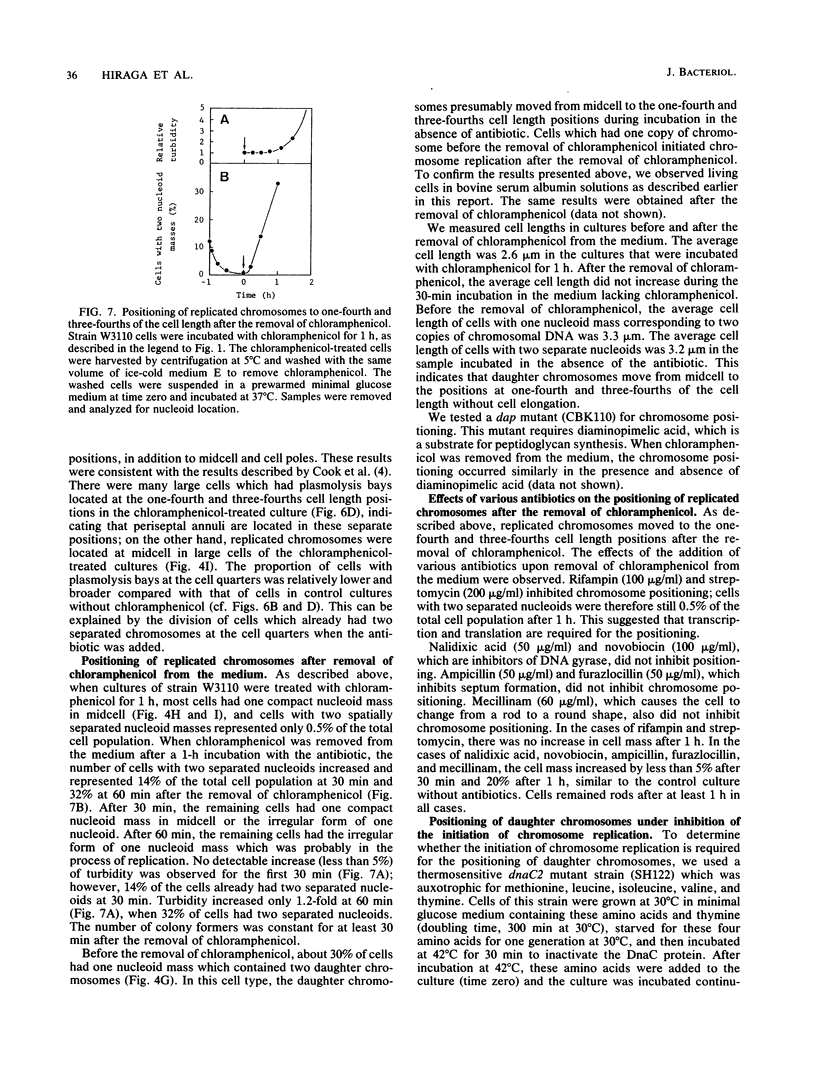
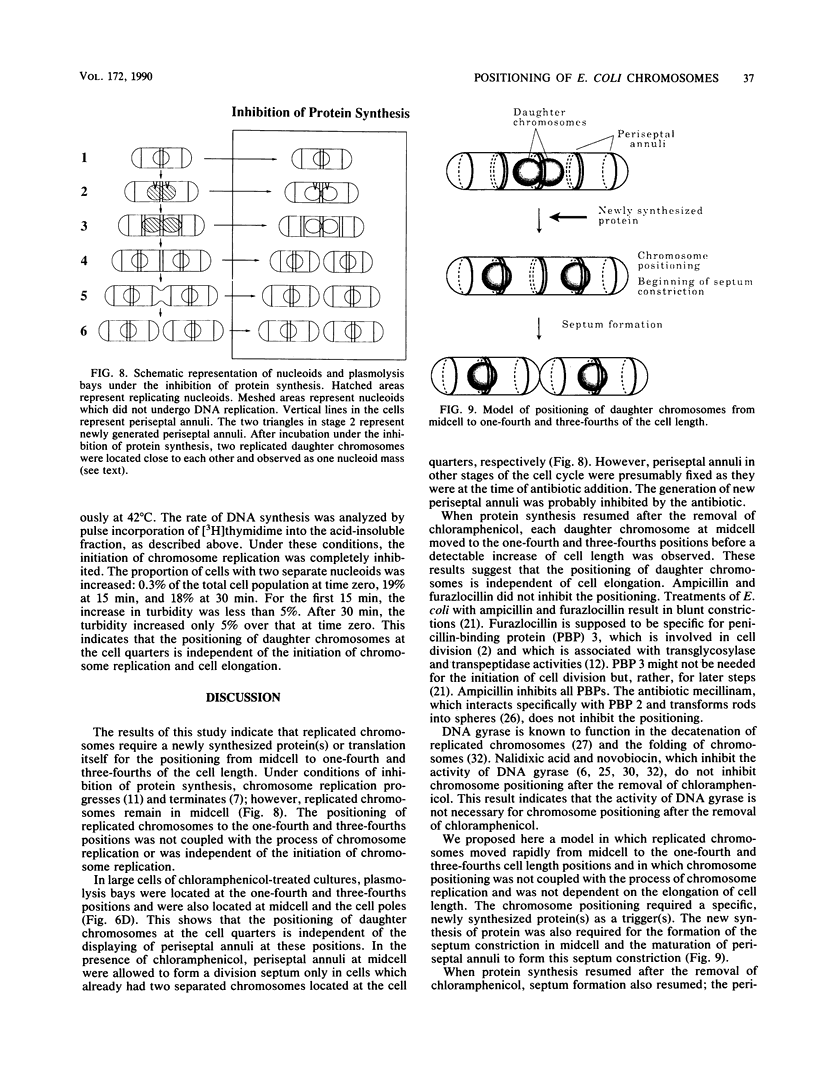
The positioning of replicated chromosomes at one-fourth and three-fourths of the cell length was inhibited when protein synthesis was inhibited by chloramphenicol or rifampin or by starvation for amino acids. Under these conditions, the progress of chromosome replication continued and replicated chromosomes were located close to each other as one nucleoid mass at midcell. Cells which already had two separate daughter chromosomes located at the cell quarters divided into two daughter cells under these conditions. When protein synthesis resumed, daughter chromosomes moved from midcell to the cell quarters, respectively, before any detectable increase in cell length was observed. The chromosome positioning occurred even under inhibition of the initiation of chromosome replication and under inactivation of DNA gyrase. The chromosome positioning presumably requires new synthesis of a particular protein(s) or translation itself.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe M., Brown C., Hendrickson W. G., Boyd D. H., Clifford P., Cote R. H., Schaechter M. Release of Escherichia coli DNA from membrane complexes by single-strand endonucleases. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2756–2760. doi: 10.1073/pnas.74.7.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botta G. A., Park J. T. Evidence for involvement of penicillin-binding protein 3 in murein synthesis during septation but not during cell elongation. J Bacteriol. 1981 Jan;145(1):333–340. doi: 10.1128/jb.145.1.333-340.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carl P. L. Escherichia coli mutants with temperature-sensitive synthesis of DNA. Mol Gen Genet. 1970;109(2):107–122. doi: 10.1007/BF00269647. [DOI] [PubMed] [Google Scholar]
- Cook W. R., Kepes F., Joseleau-Petit D., MacAlister T. J., Rothfield L. I. Proposed mechanism for generation and localization of new cell division sites during the division cycle of Escherichia coli. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7144–7148. doi: 10.1073/pnas.84.20.7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S., Helmstetter C. E. Chromosome replication and the division cycle of Escherichia coli B/r. J Mol Biol. 1968 Feb 14;31(3):519–540. doi: 10.1016/0022-2836(68)90425-7. [DOI] [PubMed] [Google Scholar]
- Drlica K. Biology of bacterial deoxyribonucleic acid topoisomerases. Microbiol Rev. 1984 Dec;48(4):273–289. doi: 10.1128/mr.48.4.273-289.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman N., Rosner E., Ron E. Z. Termination of DNA replication is required for cell division in Escherichia coli. J Bacteriol. 1989 Jan;171(1):74–79. doi: 10.1128/jb.171.1.74-79.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstetter C. E. DNA synthesis during the division cycle of rapidly growing Escherichia coli B/r. J Mol Biol. 1968 Feb 14;31(3):507–518. doi: 10.1016/0022-2836(68)90424-5. [DOI] [PubMed] [Google Scholar]
- Hiraga S., Niki H., Ogura T., Ichinose C., Mori H., Ezaki B., Jaffé A. Chromosome partitioning in Escherichia coli: novel mutants producing anucleate cells. J Bacteriol. 1989 Mar;171(3):1496–1505. doi: 10.1128/jb.171.3.1496-1505.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraga S., Ogura T., Mori H., Tanaka M. Mechanisms essential for stable inheritance of mini-F plasmid. Basic Life Sci. 1985;30:469–487. doi: 10.1007/978-1-4613-2447-8_34. [DOI] [PubMed] [Google Scholar]
- Hiraga S., Saitoh T. Initation of DNA replication in Escherichia coli. I. Characteristics of the initation process in dna mutants. Mol Gen Genet. 1974;132(1):49–62. doi: 10.1007/BF00268230. [DOI] [PubMed] [Google Scholar]
- Ishino F., Matsuhashi M. Peptidoglycan synthetic enzyme activities of highly purified penicillin-binding protein 3 in Escherichia coli: a septum-forming reaction sequence. Biochem Biophys Res Commun. 1981 Aug 14;101(3):905–911. doi: 10.1016/0006-291x(81)91835-0. [DOI] [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- MacAlister T. J., Cook W. R., Weigand R., Rothfield L. I. Membrane-murein attachment at the leading edge of the division septum: a second membrane-murein structure associated with morphogenesis of the gram-negative bacterial division septum. J Bacteriol. 1987 Sep;169(9):3945–3951. doi: 10.1128/jb.169.9.3945-3951.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson N. H. A model of bacterial DNA segregation based upon helical geometry. J Theor Biol. 1985 Jan 7;112(1):25–39. doi: 10.1016/s0022-5193(85)80115-6. [DOI] [PubMed] [Google Scholar]
- Mori H., Kondo A., Ohshima A., Ogura T., Hiraga S. Structure and function of the F plasmid genes essential for partitioning. J Mol Biol. 1986 Nov 5;192(1):1–15. doi: 10.1016/0022-2836(86)90459-6. [DOI] [PubMed] [Google Scholar]
- Ogura T., Hiraga S. Partition mechanism of F plasmid: two plasmid gene-encoded products and a cis-acting region are involved in partition. Cell. 1983 Feb;32(2):351–360. doi: 10.1016/0092-8674(83)90454-3. [DOI] [PubMed] [Google Scholar]
- Olijhoek A. J., Klencke S., Pas E., Nanninga N., Schwarz U. Volume growth, murein synthesis, and murein cross-linkage during the division cycle of Escherichia coli PA3092. J Bacteriol. 1982 Dec;152(3):1248–1254. doi: 10.1128/jb.152.3.1248-1254.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter A., Hirota Y., Jacob F. DNA-membrane complex and nuclear segregation in bacteria. Cold Spring Harb Symp Quant Biol. 1968;33:669–676. doi: 10.1101/sqb.1968.033.01.076. [DOI] [PubMed] [Google Scholar]
- Sargent M. G. Control of cell length in Bacillus subtilis. J Bacteriol. 1975 Jul;123(1):7–19. doi: 10.1128/jb.123.1.7-19.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaeppi J. M., Karamata D. Cosegregation of cell wall and DNA in Bacillus subtilis. J Bacteriol. 1982 Dec;152(3):1231–1240. doi: 10.1128/jb.152.3.1231-1240.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder M., Drlica K. DNA gyrase on the bacterial chromosome: DNA cleavage induced by oxolinic acid. J Mol Biol. 1979 Jun 25;131(2):287–302. doi: 10.1016/0022-2836(79)90077-9. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Temperature-sensitive cell division mutants of Escherichia coli with thermolabile penicillin-binding proteins. J Bacteriol. 1977 Jul;131(1):293–305. doi: 10.1128/jb.131.1.293-305.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steck T. R., Drlica K. Bacterial chromosome segregation: evidence for DNA gyrase involvement in decatenation. Cell. 1984 Apr;36(4):1081–1088. doi: 10.1016/0092-8674(84)90058-8. [DOI] [PubMed] [Google Scholar]
- Valkenburg J. A., Woldringh C. L., Brakenhoff G. J., van der Voort H. T., Nanninga N. Confocal scanning light microscopy of the Escherichia coli nucleoid: comparison with phase-contrast and electron microscope images. J Bacteriol. 1985 Feb;161(2):478–483. doi: 10.1128/jb.161.2.478-483.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. C. DNA topoisomerases. Annu Rev Biochem. 1985;54:665–697. doi: 10.1146/annurev.bi.54.070185.003313. [DOI] [PubMed] [Google Scholar]
- Yang Y., Ames G. F. DNA gyrase binds to the family of prokaryotic repetitive extragenic palindromic sequences. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8850–8854. doi: 10.1073/pnas.85.23.8850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zusman D. R., Carbonell A., Haga J. Y. Nucleoid condensation and cell division in Escherichia coli MX74T2 ts52 after inhibition of protein synthesis. J Bacteriol. 1973 Sep;115(3):1167–1178. doi: 10.1128/jb.115.3.1167-1178.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]