Abstract
Background
Although three randomised control trials have shown that selective dorsal rhizotomy (SDR) reduces spasticity in children with cerebral palsy, a meta‐analysis of the results demonstrated that the procedure conferred only small functional benefit on the patient.
Aim
To determine whether applying strict criteria for patient selection as practised in Oswestry leads to improved outcomes, using gait analysis as an outcome measure.
Methods
Ambulant children with cerebral palsy were selected for SDR using very strict clinical criteria. Instrumented gait analysis was used as the main outcome measure.
Results
Of 53 children referred for the procedure, only 19 (35%) fulfilled our strict criteria for selection. These children underwent surgery and when pre‐ and post‐SDR data were compared, they showed improvement in cosmesis of gait, clinical examination and temporal, kinetic and kinematic parameters of gait. After SDR the children walked, on average, 0.15 m/s faster, with a step length improvement of 0.11 m. Changes were seen at hip, knee and ankle, with those at the knee being most marked. A 0.3 grade improvement in knee extensor power on clinical examination led to a 13° improvement in stance phase knee extension. Knees also became less stiff, with an 82°/s improvement in the rate of flexion into swing phase. A functional tool (the GMFCS) applied retrospectively also confirmed post‐operative improvement, with 15 of the 19 children improving by at least one level.
Conclusion
Application of strict selection criteria when considering children for SDR leads to encouraging results as demonstrated by gait analysis and other measures.
Keywords: selective dorsal rhizotomy, selection criteria, gait analysis, cerebral palsy
Objective assessment has always been a problem in measuring the outcome of a particular intervention in cerebral palsy and the procedure of selective dorsal rhizotomy (SDR) is no exception.1 Between 1982 and 1997 a large number of papers on SDR were published, mainly in North America, claiming good results in children with cerebral palsy. However, these reports were plagued by selection bias, the use of variable surgical techniques and subjective outcome measures.2,3,4 Three randomised control trials on SDR were then published in quick succession and all agreed that SDR led to a reduction in spasticity in the lower limbs, but they did not agree that the procedure led to long‐term functional benefit.5,6,7 All used the Gross Motor Function Measure (GMFM)8 as a functional outcome measure, but there were marked disparities in the choice of other outcome measures and none included full gait analysis. Meta‐analysis data derived from the three randomised control trials did eventually demonstrate that SDR conferred a small beneficial effect on gross motor function.9
Four areas of potential disparity in results from different centres are: (1) patient selection, (2) intraoperative techniques, (3) post‐operative physiotherapy regimes and (4) outcome measures. Cerebral palsy is a very heterogeneous condition, but early reports on SDR barely acknowledged this and made little attempt to select those children who would most benefit from the procedure. There has been increasing awareness in many centres of the importance of using selection criteria for this operation and we have focussed sharply on this aspect of our practice.
In recent years computerised gait analysis has provided some technical objectivity with which to measure outcomes10,11,12 and we have attempted to evaluate the effects of SDR on the cosmetic, clinical, technical and functional parameters of gait using a combination of videos, clinical assessment, instrumented three dimensional gait analysis and the Gross Motor Function Classification System (GMFCS).13 This paper is a report of the Oswestry experience and suggests that provided careful selection criteria are adhered to, the procedure should be regarded with positivity and optimism.
Methods
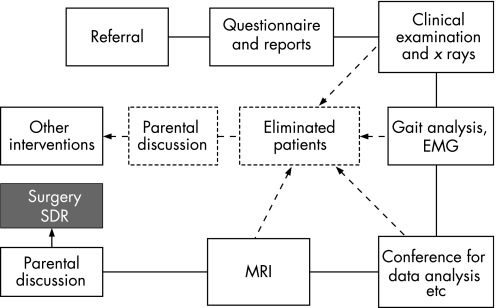
Fifty three ambulant children with lower limb spasticity were referred for SDR. The majority had cerebral palsy (hemiplegia, diplegia and quadriplegia), although four had hereditary spastic paraparesis. Approximately one third of the group used walking aids. The selection of children for SDR was a phased, fairly complex procedure (fig 1). Initially a questionnaire seeking information on the previous medical history including psychological and psychometric assessment was sought. Assessment by a multidisciplinary team (including a paediatric neurologist, orthopaedic surgeon and physiotherapist) was carried out and an x ray of the spine and hips was arranged.
Figure 1 Assessment procedure for children referred for consideration of SDR. Arrowed broken lines indicate potential points of elimination.
Gait assessment including video examination in three orthogonal planes walking barefoot and in shoes with or without walking aids (sticks, tripods, rollators), 3‐D instrumented gait analysis (Vicon, Oxford, UK) and dynamic electromyography of critical muscles was performed.
When all the data from the above procedures had been analysed, MRI of the brain and spinal cord of those children felt to be suitable for SDR was performed. The parents of those children who had not met the selection criteria were offered recommendations about alternative treatments. The important task of establishing goals in those children who fulfilled the criteria was undertaken before surgery was agreed.
During the operation laminotomies were performed and lumbar roots L1 to S1 were involved. Up to 50% of the dorsal rootlets at each level were transected and intraoperative EMG was used to complement the data already obtained from gait analysis in making decisions about the extent of surgery. Surgery was followed by 6 weeks of intensive inpatient physiotherapy. Post‐operative gait analysis was performed on average about 18 months after surgery at which time GMFCS scores provided data on functional parameters. The physiotherapist's assessment and the parents' report of their child's ability were used to provide post‐operative GMFCS scores. We obtained pre‐operative GMFCS scores for each child retrospectively from the initial physiotherapy assessment and the patient questionnaire on functional activities.
Approval from the ethics committee was not deemed necessary as the patients were undergoing routine clinical and gait laboratory assessment prior to decisions on orthopaedic intervention.
Patients
The criteria used to select our patients are shown in table 1.
Table 1 Criteria used to select patients for selective dorsal rhizotomy.
| History | Examination | Investigation |
|---|---|---|
| (1) Age range 5–10 | (1) Diagnosis spastic diplegia, severe hemiplegia, HSP | (1) No hip dysplasia |
| (2) Absence of chronic conditions, eg BPD, refractory | (2) Spasticity moderate to severe. | (2) No basal ganglia change on MRI |
| epilepsy, severe visual impairment, scoliosis | (3) Mean lower limb power >3 on MRC scale | (3) Weight not disproportionately greater |
| (3) Cognitive ability – IQ 70 or above | (4) Movement control at least moderate | than height |
| (4) Well motivated, emotionally robust child | (5) Balance at least moderate | |
| (5) No previous multilevel surgery | (6) Absence of severe fixed joint deformity | |
| (6) Good family/social support | (7) No involuntary movements or dystonia | |
BPD, broncho‐pulmonary dysplasia; HSP, hereditary spastic paraparesis.
Spasticity was assessed using the Ashworth scale, the Duncan Ely test (for spasticity in the rectus femoris), the quality of the deep tendon reflexes and also the presence or absence of sustained clonus. These latter two measures while not direct measures are usually associated findings in severe spasticity. Spasticity was categorised as mild, moderate or severe; patients needed a moderate or severe score to be considered for SDR.
Lower limb power was assessed using the MRC scale (0–5). A score of 3 to 3.5 indicated moderate weakness and a score of 4 or above indicated mild weakness. Patients with mild to moderate weakness were considered appropriate for SDR.
Movement control was assessed by noting the degree of synergy in muscles in the ipsilateral and contralateral legs during knee extension and flexion against resistance. Control impairment was mild if it involved movement in the knee and only one other joint in one leg, moderate if the whole leg was involved and severe if the contralateral leg was also involved. Children with mild or moderate impairment were considered suitable for SDR.
A patient unable to achieve and maintain high kneeling for 5 s was considered to have severely impaired balance. Maintaining high kneeling for 5 s or more indicated moderately good balance and maintaining high kneeling for more than 5 s with pertubation demonstrated good balance. Moderate or good balance was required for SDR.
From early studies it was clear to us that all children gained weight following SDR. We thus included a somewhat arbitrary criterion of excluding children whose weight was significantly disproportionate to height (ie, where weight was more than 20 centiles greater than height).
Statistics
Pre‐ and post‐operative clinical and gait analysis data were compared using paired t tests where valid; otherwise Wilcoxon signed‐rank tests were applied. Pre‐ and post‐operative GMFCS scores were also directly compared.
Results
Only 19 patients were selected for SDR as the procedure was thought to be appropriate in only 35% of referred children. This group comprised 17 children with diplegia, some of whom also had some upper limb involvement, one child with a hemiplegia and one with hereditary spastic paraparesis. Most of the 34 patients who were not selected were excluded on the basis of more than one criterion. The commonest reasons for rejection were insufficient spasticity and marked underlying muscle weakness.
The group chosen for SDR comprised 13 boys and six girls with an average age of 8 years 7 months. Three children needed walking frames at all times, 10 children used sticks or tripods some of the time but could undergo most aspects of gait analysis without walking aids and six children did not require any walking aids at all. Only 13 of the 19 children were able to fully comply with all components of the comprehensive pre‐operative gait assessment. Incomplete data sets were often caused by walking aids obscuring markers on the patients from the cameras. The six‐camera system used for the earlier patients was particularly sensitive to this. Some children became too tired to complete the assessment.
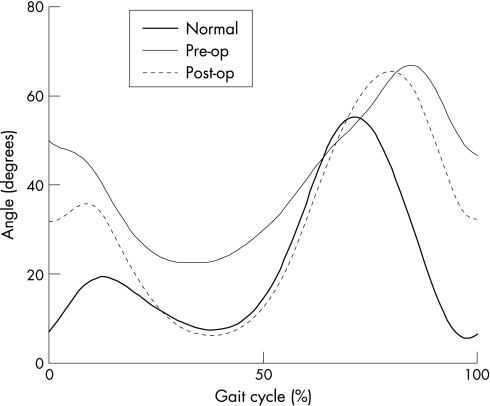
We considered cosmetic, clinical, technical and functional gait parameters. Clinical examination data were collected by a number of clinicians in routine clinical service. All, however, worked with standard, written protocols and recording forms as part of the departmental quality system. The best way of demonstrating cosmetic changes in gait following intervention is, of course, by comparing pre‐ and post‐operative videos (fig 2); all children demonstrated a subjectively more pleasing gait pattern post operatively. The 3D gait data provided objective evidence for these improvements.
Figure 2 Sample of knee flexion graph with pre‐ and post‐SDR graphs compared with normal.
The changes in clinical findings and technical or gait analysis results are reported in table 2.
Table 2 Significant changes in clinical examination measures and gait analysis data after SDR.
| Clinical examination measures | |||||
|---|---|---|---|---|---|
| Change (p value) | |||||
| Weight (kg) | +6.6 kg (p<0.001) | ||||
| Weight (centiles) | +11.3 (p<0.004) | ||||
| Height (m) | +0.10 (p<0.001) | ||||
| Average power (grades) | +0.4 (p<0.01) | ||||
| Right | Left | Average | |||
|---|---|---|---|---|---|
| Leg length (m) | +0.06 (p<0.001) | +0.06 (p<0.001) | +0.06 | ||
| Abduction range (degrees) | +7.1 (p<0.001) | +6.1 (p<0.001) | +6.6 | ||
| Hidden flexion/Thomas test (degrees) | −8.2 (p<0.001) | −7.9 (p<0.017) | −8.0 | ||
| Hip extensor power (grades) | +0.6 (p<0.03) | +0.6 (p<0.04) | +0.6 | ||
| Knee extensor power (grades) | +0.3 (p<0.005) | +0.3 (p<0.016) | +0.3 | ||
| Dorsiflexion with the knee bent (degrees) | +8.7 (p<0.001) | +8.9 (p<0.001) | +8.8 | ||
| Dorsiflexion with the knee straight (degrees) | +7.5 (p<0.001) | +7.1 (p<0.005) | +7.3 | ||
| Dorsiflexor power (grades) | +0.7 (p<0.004) | +0.4 (p<0.033) | +0.5 | ||
| Plantarflexion range (degrees) | −5.6 (p<0.012) | −5.3 (p<0.015) | −5.4 | ||
| Anteversion (degrees) | −8.5 (p<0.017) | −6.8 (p<0.04) | −7.6 | ||
| Gait analysis data | |||||
| Walking speed | +0.149 (p<0.002) | ||||
| Right | Left | Average | |||
|---|---|---|---|---|---|
| Step length (m) | +0.10 (p<0.001) | +0.12 (p<0.001) | +0.11 | ||
| Normalised step length (% height) | +5.7 (p<0.001) | +6.5 (p<0.001) | +6.1 | ||
| Knee flexion at initial contact (degrees) | −10.4 (p<0.001) | −8.8 (p<0.016) | −9.6 | ||
| Maximum knee extension (degrees) | +13.2 (p<0.001) | +12.5 (p<0.001) | +12.9 | ||
| Maximum knee flexion rate (degrees/s) | +62 (p<0.001) | +102 (p<0.001) | +82.2 | ||
| Maximum dorsiflexion (degrees) | +19.4 (p<0.01) | +9.0 (p<0.04) | +14.2 | ||
Data are only reported where both left and right limb groups showed significant changes. Left, right and average data are provided.
All the changes reported here are significant at p<0.05. The results are presented for the left and right limbs separately because the two sides were treated as two separate groups for the calculation of statistical significance. A change was only deemed significant if a consistent significant difference was seen for both left and right limb groups. It can be seen that there was a significant improvement in the range of movement at the hip, knee and ankle. Overall, the clinical examination showed that the children gained rather than lost strength, with significant improvements in the key extensor muscles of hip and knee.
The gait analysis results also showed improvements after SDR in walking speeds, step length and normalised step length (step length adjusted for growth in height). These parameters indicate an improvement in the functional aspect of gait.
The results reveal some interesting features when the individual joints are considered in more detail. At the hip, the significant relaxation of the hidden hip flexion contracture and improved hip extensor strength seen on clinical examination (table 2) did not translate into a statistically significant improvement in dynamic hip extension as measured in the laboratory. A small improvement was recorded (not shown here) which might achieve significance with a larger number of patients. Surprisingly, bony torsion (anteversion) did improve significantly after surgery.
At the knee, the improvements in gait kinematics are much more dramatic. Collated group results are presented in table 2, with a typical example of a single patient given in fig 2. The clinical examination revealed increased extensor strength (along with a 12.9° increase in range of movement (ROM)). Dynamically, extension improved at initial contact (9.6° ROM) and through stance phase. Most marked of all was the freeing of the knee during the stance/swing transition, assessed by the rate of knee flexion which increased by 82.2°/s. The effect of this can be observed in fig 2 as an increase in the slope of the knee flexion curve at the stance/swing transition.
Clinical examination of the ankle shows a shift in range rather than an increase. This shift is, however, in the right (“functional”) direction with a gain in dorsiflexion and corresponding loss of plantar flexion, which, combined with an increased strength of the dorsiflexors, is reflected in a better, more dorsiflexed, ankle posture during gait (improved by 14.2°).
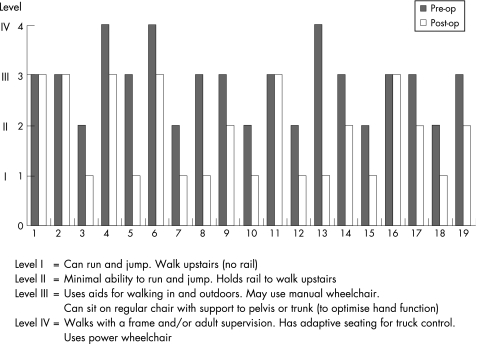
Pre‐ and post‐SDR functional ability can be compared using the GMFCS data in fig 3. There was an overall shift from higher to lower levels and no‐one was functionally worse after surgery, although the GMFCS data did not change in four children. However, 15 children experienced functional improvement of varying degrees after SDR.
Figure 3 Changes in the GMFCS of 19 patients following SDR.
Discussion
Although some parameters of gait analysis have been used in the past to assess the results of SDR,10,11,12 most outcome studies have employed the GMFM8 or resorted to subjective scales such as PEDI14 or Wee FIM.15 Of the three randomised controlled trials5,6,7 published on SDR between 1997 and 1998, only one incorporated some temporal parameters of gait into the outcome assessment.6
Our gait analysis results have provided a detailed description of the effects of SDR on gait, and as such they are a useful outcome measure. SDR has a beneficial effect on many of the key problem areas for the child with spastic diplegia affecting all the major lower limb joints. The changes seen have allayed our early reservations about the procedure and we are now reassured that a child with some muscle weakness will not see a marked worsening of their strength post‐operatively. It also appears that mild femoral anteversion may be resolved by the procedure, reducing the need for subsequent bony surgery. Calf muscle tightness also responded better than we would have predicted, given that our technique does not involve sectioning S2/S3 roots. Again, this has enabled us to be more optimistic about the outcome in children with plantar flexor spasticity. Of all the gait analysis results examined, the most dramatic effects have been seen at the knee. SDR is clearly a powerful procedure for combating crouch gait and knee stiffness.
All of our children have shown improvement in cosmetic, clinical and technical parameters of gait. Most have also shown undisputed functional improvement (15 out of 19). The GMFCS is a standardised method for describing the gross motor functional ability of children with cerebral palsy in one of five ordered levels and is age related. The GMFCS using an assessment questionnaire has recently been validated for use by parents.16 We acknowledge that the use of this tool as an outcome measure is somewhat controversial as it was designed to be a stable descriptor of function throughout the life of a child, irrespective of treatment interventions. Nevertheless, our results suggest that SDR has a dramatic effect on functional ability and has influenced the GMFCS.
Our complication rate for the procedure of SDR, in keeping with other recent series, has been low.3,17,18 One boy complained of transient numbness on the anterior aspect of one thigh and one girl of urinary incontinence over a 3‐month period about a year after her surgery. Two boys have asymptomatic, but clinically detectable, limited proprioceptive or cutaneous sensory loss in the legs. Three children have a mild “vertebral prominence” only (the practice of replacement laminotomy has clearly reduced the previous small incidence of post‐operative spinal deformity). The most serious complication was a hip subluxation requiring reconstruction (this has been reported by others previously19). Our most frequent and perhaps interesting post‐operative finding has been weight gain. Eighteen of our 19 children have crossed weight centile charts in an upward direction as opposed to height, which has largely progressed along the pre‐operative centile. Children with the greatest degree of spasticity pre‐operatively (and by definition the slowest walking speeds) have tended to gain most weight. We have reported this elsewhere and believe it is due to the fact that spasticity itself consumes energy.20 Even though mobility is improved post‐operatively, unless children modify their eating habits and reduce their calorie intake they are likely to gain excessive weight. This observation has taught us to be vigilant about using spasticity‐reducing treatments in children who are already overweight. We now advise the child and his family about diet as part of our routine pre‐operative investigation.
There have been large disparities in age ranges in published studies and little uniformity in attempting to grade severity or the ambulatory status of patients.21 We have attempted to be as precise as the situation allows in categorising the physical features of our patients (in terms of muscle strength, spasticity, balance, coordination, etc) and we consider that motivation, emotional robustness and family support are of prime importance when selecting patients for SDR.
The careful choice of patients has been fundamental to our good outcome and clearly, while not all children with cerebral palsy can benefit from SDR, we have demonstrated that for the type of child we have profiled, the outcome is likely to be advantageous.
What is already known on this topic
Selective dorsal rhizotomy (SDR) relieves lower limb spasticity.
SDR has been shown to confer a small functional benefit on children with cerebral palsy.
What this study adds
This is the first UK cohort studied.
Gait analysis as an outcome measure demonstrates SDR is an excellent procedure for combating crouch gait and knee stiffness, provided rigorous selection criteria are applied.
SDR leads to weight gain in most patients.
Abbreviations
GMFCS - Gross Motor Function Classification System
GMFM - Gross Motor Function Measure
SDR - selective dorsal rhizotomy
Footnotes
Competing interests: None.
All the work was carried out in the above institution, and the same contact details apply to all authors (Dr Gaynor Cole, Mrs Sybil Farmer, Mr Andrew Roberts, Dr Caroline Stewart, Mr John Patrick).
References
- 1.Goldberg M J. Measuring outcomes in cerebral palsy. J Paediatr Orthop 199111682–685. [PubMed] [Google Scholar]
- 2.Arens L J, Peacock W J, Peter J. Selective posterior rhizotomy: a long‐term follow‐up study. Childs Nerv Syst 198951148–152. [DOI] [PubMed] [Google Scholar]
- 3.Ablot R, Johann‐Murphy M, Siminiski‐Maher, et al Selective dorsal rhizotomy: outcome and complications. Neurosurgery 199333851–857. [DOI] [PubMed] [Google Scholar]
- 4.Cohen A R, Webster M C. How selective is selective posterior rhizotomy? Surg Neurol 199135267–272. [DOI] [PubMed] [Google Scholar]
- 5.Steinbok P, Reiner A M, Beauchamp R.et al A randomised clinical trial to compare selective posterior rhizotomy plus physiotherapy with physiotherapy alone in children with spastic diplegic cerebral palsy. Dev Med Child Neurol 199739178–184. [DOI] [PubMed] [Google Scholar]
- 6.McLaughlin J F, Bjornson K F, Astley S J.et al Selective dorsal rhizotomy: efficacy and safety in an investigation masked randomised clinical trial. Dev Med Child Neurol 199840220–232. [DOI] [PubMed] [Google Scholar]
- 7.Wright F V, Sheil E M H, Drake J M.et al Evaluation of selective dorsal rhizotomy for the reduction of spasticity in cerebral palsy: a randomised controlled trial. Dev Med Child Neurol 199840239–247. [DOI] [PubMed] [Google Scholar]
- 8.Russell D, Rosenbaum P, Cadman D.et al The gross motor function measure: a means to evaluate the effects of physical therapy. Dev Med Child Neurol 198931341–352. [DOI] [PubMed] [Google Scholar]
- 9.McLaughlin J, Bjornson K, Tenikein N.et al Selective dorsal rhizotomy: meta‐analysis of three randomised controlled trials. Dev Med Child Neurol 20024417–25. [DOI] [PubMed] [Google Scholar]
- 10.Cahan L D, Adams J M, Perry J. Instrumental gait analysis after selective dorsal rhizotomy. Dev Med Child Neurol 1990321037–1043. [DOI] [PubMed] [Google Scholar]
- 11.Vaughan C L, Berman B, Peacock W J. Cerebral palsy and rhizotomy. A 3‐year follow‐up evaluation with gait analysis. J Neurosurg 199174178–184. [DOI] [PubMed] [Google Scholar]
- 12.Boscarino L F, Ounpauu S, Davis R B. Effects of selective dorsal rhizotomy on gait in children with cerebral palsy. J Pediatr Orthop 199313174–179. [PubMed] [Google Scholar]
- 13.Palisano R, Rosenbaum P, Walter S.et al Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol 199739214–223. [DOI] [PubMed] [Google Scholar]
- 14.Haley S, Coster W, Ludlow L H.et alPediatric Evaluation of Disability Inventory (PEDI). Version 1.0. Boston: New England Medical Centre Hospital, 1992
- 15.Msall M E, DiGaudio K, Duffy L C. Normative sample of an instrument for tracking functional independence in children. Clin Pediatr (Phila) 199433431–438. [DOI] [PubMed] [Google Scholar]
- 16.Morris C, Galuppi B, Rosenbaum P L. Reliability of family report for the Gross Motor Function Classification System. Dev Med Child Neurol 200446455–460. [DOI] [PubMed] [Google Scholar]
- 17.Van de Wiele B M, Staudt L A, Rubinstein E M.et al Perioperative complications in children undergoing selective posterior rhizotomy: a review of 105 cases. Paediatr Anaesth 19966479–486. [DOI] [PubMed] [Google Scholar]
- 18.Yasuoka S, Peterson H A, MacCarty C S. Incidence of spinal column deformity after multilevel laminectomy in children and adults. J Neurosurg 198257441–445. [DOI] [PubMed] [Google Scholar]
- 19.Greene W B, Dietz F R, Goldberg M J.et al Rapid progression of hip subluxation in cerebral palsy after selective posterior rhizotomy. J Pediatr Orthop 199111494–497. [DOI] [PubMed] [Google Scholar]
- 20.Roberts A P, Stewart C, Cole G F.et al Spasticity consumes energy. Dev Med Child Neurol 200244284. [DOI] [PubMed] [Google Scholar]
- 21.Patrick J H, Roberts A P, Cole G F. Therapeutic choices in the locomotor movement of the child with cerebral palsy – more luck than judgement? Arch Dis Child 200185275–279. [DOI] [PMC free article] [PubMed] [Google Scholar]