Abstract
Objective
To determine the prevalence of vitamin D deficiency in newborn infants of mothers at risk of vitamin D deficiency because of dark skin or the wearing of concealing clothes (such as a veil) compared with a group presumed not to be at risk. A second aim was to correlate these newborn infants' vitamin D concentrations with biochemical parameters of vitamin D metabolism and bone turnover at birth.
Design
A prospective study conducted between April 2004 and February 2006 including women delivering during this period and their newborn infants.
Setting
The outpatient clinic of the obstetrics department, Sint Franciscus Gasthuis, Rotterdam, the Netherlands.
Patients
Eighty seven newborn infants of healthy mothers with either dark skin and/or concealing clothing (risk group) or light skin (control group).
Results
We found a significant difference in the prevalence of vitamin D deficiency (25‐hydroxyvitamin D3 <25 nmol/l) between newborn infants of mothers at risk and those of mothers in the control group (63.3% vs 15.8%; p<0.001). Mean alkaline phosphatase concentrations were significantly higher in the at risk group.
Conclusions
Newborn infants of mothers with dark skin or wearing concealing clothes are at great risk of vitamin D deficiency at birth. The clinical implications are unknown. Further research is necessary to determine the long‐term consequences of maternal and neonatal vitamin D deficiency so that guidelines on vitamin D supplementation during pregnancy can be issued.
Keywords: Vitamin D, deficiency, newborns, pregnancy
Vitamin D is transported to the liver and hydroxylated to 25‐hydroxyvitamin D3. Regulated by parathyroid hormone, additional hydroxylation to 1,25‐dihydroxyvitamin D3 takes place in the kidney. 1,25‐Dihydroxyvitamin D3 is the active metabolite of vitamin D that increases intra‐ and extracellular calcium concentrations by several mechanisms: it absorbs intestinal calcium, diminishes renal calcium excretion and, in conjunction with parathyroid hormone, mobilises calcium from bone. Of late, the focus of vitamin D deficiency research has shifted from breastfeeding without vitamin D supplementation to the newborn infants of mothers at risk of vitamin D deficiency. Several studies have reported a higher risk of vitamin D deficiency in pregnant women due to ethnocultural factors; dark skin or concealing clothing may lead to limited sun exposure.9,10
We conducted a study among a group of pregnant women and their newborn infants in a general hospital in Rotterdam, the Netherlands. This is a multi‐cultural city in which residents of Surinamese origin constitute the largest immigrant group (20%), those of Turkish origin the second largest (17%) and those of Moroccan origin the third largest (13%).11
The prevalence rates of vitamin D deficiency in these immigrant groups are unknown, and few data are available concerning newborn infants of mothers at risk.
Our first aim in this study was to determine prevalences of vitamin D deficiency in newborn infants born to mothers in different risk groups in the Netherlands. A second aim was to correlate these newborn infants' vitamin D concentrations with biochemical parameters of vitamin D metabolism and bone turnover at birth.
Methods
The study was reviewed and approved by the medical ethics review board of MCRZ Hospital, Rotterdam, the Netherlands.
All pregnant women visiting the obstetrics outpatient department at Sint Franciscus Gasthuis, Rotterdam, between April 2004 and February 2006 were invited to participate in our study. Those who gave informed consent completed a questionnaire. Hospital interpreters were available. The questionnaire asked about degree of skin pigmentation, country of origin, hours of sun exposure per day, clothing style, use of vitamins, diet (especially dairy products and fish) and medical history including clinical features suggestive of vitamin D deficiency. We aimed to include a “risk group” of 50 women with dark skin or veiled clothing and a control group of 50 women with light skin. Colour of skin was determined through self‐assessment by the women who participated. The self‐assigned skin colour was light, dark or intermediate. Women with intermediate skin colour were assigned to the group with dark skin. Newborn infants born to mothers in the risk group were also considered at risk. Newborn infants of mothers with light skin served as controls. The risk group consisted of 17 newborn infants of mothers with intermediate skin, five infants of mothers with dark skin and 20 infants with veiled mothers. Exclusion criteria were illnesses during pregnancy, chronic diseases, use of glucocorticoids and vitamin D deficiency secondary to hereditary illnesses of vitamin D metabolism. Maternal blood and urine samples were collected at the end of the third trimester. Maternal umbilical cord blood values at birth were taken to represent the newborn infant's values. The infants' birth weight, gestational age, sex and season of birth were documented. Infants presenting with symptomatic hypocalcaemia were admitted to the hospital and treated by the paediatrician on call. Blood samples were taken to evaluate therapy.
Biochemical analysis
Umbilical cord blood was tested for 25‐hydroxyvitamin D3 in 87 newborn infants using the 25‐hydroxyvitamin D3 kit (Advantage, Nichols Institute Diagnostics, San Juan Capistrano, CA). Alkaline phosphatase, parathyroid hormone, ionised calcium and phosphorus were tested in 85, 80, 74 and 86 newborn infants, respectively.
Calcium, phosphorus and alkaline phosphatase levels were measured by standard methods (Synchron LX20, Beckman‐Coulter, Fullerton, CA). Ionised calcium was determined by ion selective electrodes (Phox Plus, Nova Biomedical, Waltham, MA). Intact parathyroid hormone levels were determined by LIEMA (Immulite analyser, DPC, Los Angeles, CA) at IJsselland Hospital in Capelle a/d IJssel, the Netherlands. Serum levels of 25‐hydroxyvitamin D3 <25 nmol/l in newborn infants were considered to reflect vitamin D deficiency.
Sample size calculation and statistical analysis
Sample size was calculated by a priori power analysis, aiming at a power of 0.80 to allow for determination of a difference in the prevalence of vitamin D deficiency between the risk group and controls. As prevalences reported in the literature vary widely, we aimed at a 20% minimal difference in prevalence. Thus, a minimal sample size of 102 with a power of 0.80 and α = 0.05 was calculated. Statistical analysis was conducted using SPSS (version 12.0). Because some of the biochemical parameters showed skewed distribution, the results are presented with medians and quartiles (25th and 75th percentile). In addition, we used logarithmically transformed data for statistical analysis of comparison of means.
p Values, for comparison of log‐transformed means for the risk group compared to controls, were tested by one‐way ANOVA.
Results
Between April 2004 and February 2006, 166 pregnant women were recruited, and these gave birth to 182 children. Multiple pregnancies (ie, 16 twins) were excluded to prevent a bias. No data were available for 25 newborn infants, either because the mother was transferred in pregnancy to another hospital or because of intra‐uterine death (two cases). Of the 125 newborn infants for whom data were available, 25‐hydroxyvitamin D3 was not measured in 38 for various reasons (eg, insufficient material, not requested for testing or loss of samples). Thus, 25‐hydroxyvitamin D3 levels of 87 newborn infants were available for analysis. Paired measurements of both the mother and the newborn infant were available in 73 cases. Data on the use of vitamin D supplements were available for 70 women: five of the 40 of these women (12.5%) in the risk group, and 11 of the 30 in the control group (36.7%) had used vitamin supplements. Table 1 shows the baseline characteristics and prevalences of vitamin D deficiency for both the risk and control groups. Mean gestational age, birth weight and season of birth did not differ between the two groups. The control group included 17 (45%) boys and the risk group 33 (67%) boys.
Table 1 Baseline characteristics of newborn infants and prevalence rates in the control and risk groups.
| Control group | Risk group | p Value | |
|---|---|---|---|
| Gestational age | |||
| n | 38 | 48 | 0.400 |
| Mean (weeks) | 39.7 | 39.4 | |
| (min–max) (weeks) | (34–42) | (34–42) | |
| Birth weight | |||
| n | 37 | 47 | 0.790 |
| Mean (g) | 3382 | 3416 | |
| (min–max) (g) | (1775–4250) | (2250–4750) | |
| Season of birth | 0.560 | ||
| Spring, n (%) | 10 (26.3) | 13 (26.5) | |
| Summer, n (%) | 9 (23.7) | 18 (36.7) | |
| Autumn, n (%) | 14 (36.8) | 13 (26.5) | |
| Winter, n (%) | 5 (13.2) | 5 (10.2) | <0.001 |
| Vitamin D deficient, n (%) | 6 (15.8) | 31 (63.3) |
25‐hydroxyvitamin D3 and the seasons
The mean serum 25‐hydroxyvitamin D3 values did not differ significantly between the seasons of birth in both groups.
25‐hydroxyvitamin D3 prevalence
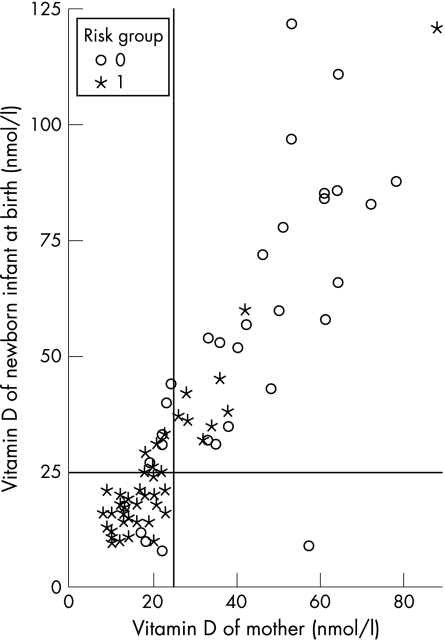
We found a higher prevalence of vitamin D deficiency in the newborn infants of mothers at risk (63.3%) compared with the control group (15.8%). Within the risk group, we identified the newborn infants of mothers wearing veils to be at highest risk of vitamin D deficiency with a prevalence of 90.9%. 25‐Hydroxyvitamin D3 values in pregnant women and their newborn infants showed a positive correlation (r = 0.88), as shown in fig 1. The Pearson correlations of neonatal 25‐hydroxyvitamin D3 with ionised calcium, phosphorus, alkaline phosphatase and parathyroid hormone were non‐significant at −0.172 (p = 0.14), 0.17 (p = 0.28), −0.12 (p = 0.28) and −0.18 (p = 0.12), respectively.
Figure 1 Positive correlation between 25‐hydroxyvitamin D3 values in mothers and newborn infants.
Mean cord blood ionised calcium concentrations were not significantly lower in the risk group. However, mean alkaline phosphatase concentrations were significantly higher in the risk group (table 2).
Table 2 Newborn (cord blood) laboratory values in the control and risk groups.
| Control group | Risk group | p Value | |||
|---|---|---|---|---|---|
| n | Median (25th‐75th percentile) | n | Median (25th–75th percentile) | ||
| 25(OH)D3 (nmol/l) | 38 | 52.5 (31.8–79.0) | 49 | 20.0 (15.5–30.0) | <0.001 |
| iCa (mmol/l) | 34 | 1.29 (1.21–1.38) | 40 | 1.34 (1.28–1.43) | 0.061 |
| P (mmol/l) | 38 | 1.74 (1.58–1.91) | 48 | 1.66 (1.55–1.88) | 0.453 |
| AP (U/l) | 38 | 161 (124–213) | 47 | 177 (156–217) | 0.050 |
| PTH (pmol/l) | 35 | 0.10 (0.10–0.40) | 45 | 0.20 (0.10–0.80) | 0.143 |
AP, alkaline phosphatase; iCa, ionised calcium; 25 (OH)D3, 25‐hydroxyvitamin D3; P, phosphorus; PTH, parathyroid hormone.
Discussion
Our study provides data on the prevalence of vitamin D deficiency in newborn infants born to mothers from risk groups in the Netherlands. The most important finding was a higher prevalence of vitamin D deficiency in newborn infants of mothers at risk of vitamin D deficiency (63.3%) as compared with a control group (15.8%). The prevalence of vitamin D deficiency in the total group (with vitamin D deficiency defined as 25‐hydroxyvitamin D3 levels <25 nmol/l) was 42.5%. Cut‐off values for vitamin D deficiency in newborn infants are still being debated. However, 25‐hydroxyvitamin D3 concentrations below 25 nmol/l are considered deficient in children.12,13
Vitamin D deficiency in pregnant women at risk because of ethnocultural factors has been widely reported.9,10,14,15,16,17,18,19 Prevalences in the various study groups range from 50% to 84%. Vitamin D deficiency as described is becoming a major problem and although the prevalence rates for newborn infants are unknown, they may be high. Very few studies have reported the prevalence of vitamin D deficiency in newborn infants. One report, however, found a high prevalence of vitamin D deficiency in newborn infants and pregnant women from India, a country with abundant sunlight.15 The newborn infants' mean cord blood 25‐hydroxyvitamin D3 level was low (8.4±5.7 ng/ml) and most of the newborn infants (95.7%) were vitamin D deficient (serum 25‐hydroxyvitamin D3 <20 ng/ml≈50 nmol/l). However, the cut‐off value was lower than that used in our study.
In a Dutch study, severe vitamin D deficiency (25‐hydroxyvitamin D3 <13 nmol/l) was found in 54% of newborn infants of non‐European origin compared with 6% of Dutch/West European newborn infants.20 This study did not report data on pigmentation or clothing habits and their possible associations with vitamin D deficiency. Our study found a comparable prevalence (63.3%) of vitamin D‐deficient newborn infants from the risk group defined by intermediate/dark skin or veiling. In addition, the newborn infants of the veiled mothers showed an extremely high prevalence of vitamin D deficiency (90.9%).
In our study, mean alkaline phosphatase concentrations in the risk group were higher than in controls, which may indicate increased bone turnover. Nevertheless, as values remained within the normal range, this finding may not be clinically relevant.
Few studies have reported on the consequences of maternal and neonatal vitamin D deficiency for fetal growth and bone development. Data from randomised trials of oral supplementation during pregnancy in women with vitamin D deficiency showed inconsistent results regarding offspring size at birth.4 A recent study showed that low maternal 25‐hydroxyvitamin D3 values in late pregnancy were associated with decreased knee–heel length, which is a measure of intrauterine long bone growth at birth.21 Javaid et al showed an association between reduced concentration of 25‐hydroxyvitamin D3 in white mothers during late pregnancy and reduced whole‐body and lumbar‐spine bone mineral content in their children at the age of 9 years.22
Further studies are needed to provide more conclusive evidence of the long‐term consequences of fetal and neonatal vitamin D deficiency. The lack of evidence so far has led to contradictory recommendations on vitamin D supplementation during pregnancy in many countries. In the Netherlands the Health Council advises that pregnant women at risk should receive vitamin D supplements; however, this is not common practice.12,23,24,25,26,27
In conclusion, we found a high prevalence of vitamin D deficiency in newborn infants of mothers at risk. The higher mean alkaline phosphatase levels found in the newborn children of these women might reflect an effect on bone mass. Further research, preferably by randomised controlled trials, is needed not only to establish the effects of vitamin D supplementation during pregnancy, but also the long‐term consequences of neonatal vitamin D deficiency for long bone growth and health. The results of such trials would enable clear‐cut guidelines on vitamin D supplementation to be issued.
What is already known on this topic
25‐Hydroxyvitamin D3 values in the mother's blood and cord blood are strongly correlated.
High prevalence rates of vitamin D deficiency in dark skinned and veiled pregnant women have been described in countries at the same latitude as the Netherlands.
What this study adds
High prevalence rates of vitamin D deficiency are found in newborn infants of dark skinned or veiled mothers from a Northern European population.
Newborn infants of mothers at risk of vitamin D deficiency showed higher mean alkaline phosphatase concentrations than controls, which suggests increased bone turnover. However, as values were still within the normal range, this finding may not be clinically relevant.
Acknowledgements
We thank GA Christiaan and G Arpaci for collecting laboratory data, A Bowier for setting up our database and helping us with the study, M de Ridder, medical statistician, Department of Epidemiology, Erasmus MC, Rotterdam, the Netherlands, for help with the statistical analysis, and J Hagoort for editing.
Footnotes
Competing interests: None.
References
- 1.Paunier L, Lacourt G, Pilloud P.et al 25‐Hydroxyvitamin D and calcium levels in maternal, cord and infant serum in relation to maternal vitamin D intake. Helv Paediatr Acta 19783395–103. [PubMed] [Google Scholar]
- 2.Salle B L, Delvin E E, Lapillonne A.et al Perinatal metabolism of vitamin D. Am J Clin Nutr 200071(Suppl)1317S–24S. [DOI] [PubMed] [Google Scholar]
- 3.Prentice A. Micronutrients and the bone mineral content of the mother, fetus and newborn. J Nutr 20031331693S–9S. [DOI] [PubMed] [Google Scholar]
- 4.Specker B. Vitamin D requirements during pregnancy. Am J Clin Nutr 200480(Suppl)1740–47S. [DOI] [PubMed] [Google Scholar]
- 5.Namgung R, Tsang R C. Factors affecting newborn bone mineral content: in utero effects on newborn bone mineralization. Proc Nutr Soc 20005955–63. [DOI] [PubMed] [Google Scholar]
- 6.Pawley N, Bishop N J. Prenatal and infant predictors of bone health: the influence of vitamin D. Am J Clin Nutr 200480(Suppl)1748S–51S. [DOI] [PubMed] [Google Scholar]
- 7.Clemens T L, Adams J S, Henderson S L.et al Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet 1982174–76. [DOI] [PubMed] [Google Scholar]
- 8.Norman A W. Sunlight, season, skin pigmentation, vitamin D, and 25‐hydroxyvitamin D: integral components of the vitamin D endocrine system. Am J Clin Nutr 1998671108–1110. [DOI] [PubMed] [Google Scholar]
- 9.Grover S R, Morley R. Vitamin D deficiency in veiled or dark‐skinned pregnant women. Med J Aust 2001175251–252. [DOI] [PubMed] [Google Scholar]
- 10.Nozza J M, Rodda C P. Vitamin D deficiency in mothers of infants with rickets. Med J Aust 2001175253–255. [DOI] [PubMed] [Google Scholar]
- 11.Statistics Netherlands Population. Available at http://www.cbs.nl/en‐GB/default.htm (accessed 30 June 2006)
- 12.Greer F R. Issues in establishing vitamin D recommendations for infants and children. Am J Clin Nutr 200480(Suppl)1759S–62S. [DOI] [PubMed] [Google Scholar]
- 13.Wharton B, Bishop N. Rickets. Lancet 20033621389–1400. [DOI] [PubMed] [Google Scholar]
- 14.Okonofua F, Menon R K, Houlder S.et al Calcium, vitamin D and parathyroid hormone relationships in pregnant Caucasian and Asian women and their neonates. Ann Clin Biochem 19872422–28. [DOI] [PubMed] [Google Scholar]
- 15.Sachan A, Gupta R, Das V.et al High prevalence of vitamin D deficiency among pregnant women and their newborns in northern India. Am J Clin Nutr 2005821060–1064. [DOI] [PubMed] [Google Scholar]
- 16.Datta S, Alfaham M, Davies D P.et al Vitamin D deficiency in pregnant women from a non‐European ethnic minority population‐‐an interventional study. BJOG 2002109905–908. [DOI] [PubMed] [Google Scholar]
- 17.Goswami R, Gupta N, Goswami D.et al Prevalence and significance of low 25‐hydroxyvitamin D concentrations in healthy subjects in Delhi. Am J Clin Nutr 200072472–475. [DOI] [PubMed] [Google Scholar]
- 18.Van der Meer I M, Karamali N S, Boeke A J.et al High prevalence of vitamin D deficiency in pregnant non‐Western women in The Hague, Netherlands. Am J Clin Nutr 200684350–353. [DOI] [PubMed] [Google Scholar]
- 19.Dawodu A, Agarwal M, Sankarankutty M.et al Higher prevalence of vitamin D deficiency in mothers of rachitic than nonrachitic children. J Pediatr 2005147109–111. [DOI] [PubMed] [Google Scholar]
- 20.Wielders J P, van Dormael P D, Eskes P F.et al Severe vitamin‐D deficiency in more than half of the immigrant pregnant women of non‐western origin and their newborns [in Dutch]. Ned Tijdschr Geneeskd 2006150495–499. [PubMed] [Google Scholar]
- 21.Morley R, Carlin J B, Pasco J A.et al Maternal 25‐hydroxyvitamin D and parathyroid hormone concentrations and offspring birth size. J Clin Endocrinol Metab 200691906–912. [DOI] [PubMed] [Google Scholar]
- 22.Javaid M K, Crozier S R, Harvey N C.et al Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet 200637636–43. [DOI] [PubMed] [Google Scholar]
- 23.Hollis B W, Wagner C L. Assessment of dietary Vitamin D requirements during pregnancy and lactation. Am J Clin Nutr 200479717–726. [DOI] [PubMed] [Google Scholar]
- 24.Mahomed K, Gulmezoglu A M. Vitamin D supplementation in pregnancy. Cochrane Database Syst Rev 2000(2)CD000228. [DOI] [PubMed]
- 25.Moy R, Shaw N, Mather I. Vitamin D supplementation in pregnancy. Lancet 2004363574. [DOI] [PubMed] [Google Scholar]
- 26.Shenoy S D, Swift P, Cody D.et al Maternal vitamin D deficiency, refractory neonatal hypocalcaemia and nutritional rickets. Arch Dis Child 200590437–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wiersma T J, Daemers D O, Steegers E A.et al Unfounded recommendations for vitamin D supplementation in pregnant and breastfeeding women [in Dutch]. Ned Tijdschr Geneeskd 20011451700–1701. [PubMed] [Google Scholar]