Abstract
The osteopathic profession has been challenged over the past decade to provide clinically relevant research. The conduct of evidence-based osteopathic research is imperative not only for scientific, economic, and professional reasons, but also to drive health care policy and clinical practice guidelines. This paper summarizes recent studies in response to the osteopathic research challenge, including clinical trials registered with ClinicalTrials.gov and a systematic review and meta-analysis of osteopathic manipulative treatment (OMT) for low back pain. The concept of the OMT responder is introduced and supported with preliminary data. Within the context of a pain processing model, consideration is given to genomic (e.g., the catechol-O-methyltransferase gene) and psychological (e.g., depression and somatization) factors that are associated with pain sensitivity and pain progression, and to the role that such factors may play in screening for OMT responders. While substantial progress has been made in osteopathic research, much more needs to be done.
Keywords: osteopathy, osteopathic medicine, osteopathic manipulative treatment (OMT), low back pain, genomics, catechol-O-methyltransferase (COMT), pain processing
1. The osteopathic research challenge
In a recent Journal editorial, Lucas and Moran raised critical questions regarding the relevancy of contemporary osteopathy research in the evolving healthcare environment.1 Their ultimate challenge to the osteopathic profession was to provide the “numbers” supporting the clinical effectiveness of osteopathy, or osteopathic manipulative treatment (OMT) as it is better known in the United States. They aptly identified important consumers of the numbers, including researchers, universities, health insurers, government agencies, and the media, with the latter noted to exert considerable influence over public opinion and, potentially, policy. The recent media attention afforded the Ernst and Canter2 systematic review of systematic reviews of spinal manipulation and its conclusion that “data do not demonstrate that spinal manipulation is an effective intervention for any condition,” illustrates their point.
The osteopathic profession in the United States has been similarly challenged in the past decade. In 1997, Goldstein editorialized that the profession should foster evidence-based osteopathic medicine.3 In 1999, following publication of a major randomized controlled trial of OMT in the New England Journal of Medicine,4 Howell commented that “the long-term survival of osteopathic medicine will depend on its ability to define itself as distinct from and yet still equivalent to allopathic medicine. That argument may best be articulated not in theoretical terms, but by demonstrating treatment outcomes.”5 Apparently, Howell’s “outcomes” relate to osteopathic medicine as Lucus and Moran’s numbers relate to osteopathy.
1.1 Ongoing osteopathic clinical trials
The American response to such osteopathic research challenges has grown considerably over time. Just 10 years ago, the evidence base for OMT was virtually non-existent. In contrast, at the end of 2006, a search of the ClinicalTrials.gov database maintained by the National Institutes of Health identified six ongoing randomized controlled trials involving OMT and five other trials that had been completed or were no longer recruiting subjects. These clinical trials are summarized in Table 1, which reveals the wide variety of conditions being evaluated for OMT efficacy. Most trials involve comparisons of OMT with placebo or active treatments, and a majority of trials report using a double-blind methodology. A flurry of OMT research reports should be forthcoming in the near future based on these recently completed trials and those that are scheduled to stop recruiting subjects in 2007.
Table 1.
Osteopathic clinical trials registered with ClinicalTrials.gov according to size.*
| Target Disorder or Condition | ClinicalTrials.gov Identifier | Status | NIH Funded | Phase | N | Start Date | End Date† | Blinding | Control | Assignment |
|---|---|---|---|---|---|---|---|---|---|---|
| Chronic low back pain | NCT00315120 | Ongoing | Yes | III | 488 | Aug 2006 | Oct 2010 | Double | Placebo | Factorial |
| Pneumonia | NCT00258661 | Ongoing | No | NS | 360 | Mar 2004 | Jul 2007 | Double | Placebo | Factorial |
| Carpal tunnel syndrome | NCT00394043 | Ongoing | Yes | II | 138 | Oct 2006 | Jan 2010 | Double | Placebo | Parallel |
| Low back pain | NCT00394264 | Ongoing | No | II | 100 | Oct 2006 | Dec 2007 | Single | Active | Parallel |
| Muscle tension | NCT00403936 | Completed | No | NS | 100 | Apr 2005 | May 2005 | Single | Active | Cross-over |
| Pregnancy | NCT00298935 | Completed | No | NS | 99 | Jul 2003 | May 2006 | Double | Placebo | Parallel |
| Postoperative nausea and vomiting | NCT00387361 | Ongoing | No | II | 60 | Oct 2006 | Mar 2007 | Double | Placebo | Parallel |
| Low back and hip pain | NCT00410397 | Ongoing | No | NS | 20 | Dec 2006 | Feb 2007 | Single | Placebo | Parallel |
| Emphysema | NCT00034112 | Completed | Yes | II | NS | Apr 2001 | Mar 2002 | NS | Placebo | Parallel |
| Spastic cerebral palsy | NCT00011024 | Completed | Yes | II | NS | Sep 1998 | Jun 2004 | NS | NS | NS |
| Otitis media | NCT00010465 | Completed | Yes | II | NS | NS | NS | Double | Placebo | NS |
Information as reported in December 2006; NIH denotes National Institutes of Health; NS, not specified.
Based on data entry closure date.
1.2 Systematic review and meta-analysis of osteopathic manipulative treatment outcomes
As suggested by Lucas and Moran,1 the numbers are particularly germane to systematic reviews and meta-analyses (SRMAs), which lie at the top of the evidence pyramid and often drive healthcare policy decisions, such as payment for various clinical services. In that regard, the Ernst and Cantor study2 noted above pre-dated the first and only known SRMA specifically addressing OMT, published by Licciardone and colleagues in 2005.6 The overall findings of the latter study, which assessed the efficacy of OMT in treating low back pain, are presented as a forest plot in Figure 1. The subjects who received OMT experienced a greater reduction in low back pain compared with those subjects who did not receive OMT (mean effect size, −0.30; 95% confidence interval, −0.47 – −0.13; P=.001). Thus, OMT provided clinically relevant and statistically significant reductions in low back pain.
Figure 1.
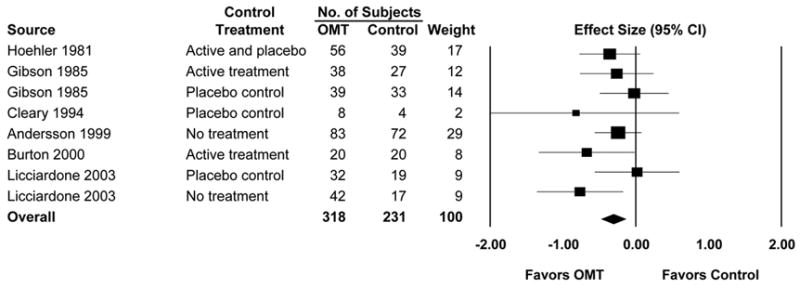
Osteopathic manipulative treatment (OMT) of low back pain. CI denotes confidence interval. (Reproduced with permission from Licciardone and colleagues.6 Source citations for meta-analysis are provided therein.)
Beyond these overall findings, the SRMA subgroup analyses yielded three important results. First, in those randomized controlled trials in which OMT was compared with other active or placebo treatments, the OMT group continued to demonstrate clinically relevant and statistically significant reductions in low back pain (mean effect size, −0.26; 95% confidence interval, −0.48 – −0.05; P=.02). This finding supports the conclusion that benefits of OMT in treating low back pain cannot be attributed merely to the placebo effect. Second, comparable OMT effects were observed in randomized controlled trials conducted in the United States (mean effect size, −0.31; 95% confidence interval, −0.52 – −0.10; P=.004) and in the United Kingdom (mean effect size, −0.29; 95% confidence interval, −0.58 – −0.00; P=.05). Because osteopathic physicians in the United States and osteopaths in the United Kingdom have disparate clinical practice rights by law, these comparable findings support the validity of the SRMA in isolating the treatment effects attributable to OMT, exclusive of other co-treatments such as prescription drugs. Finally, OMT effects increased over time (mean effect size, −0.28; 95% confidence interval, −0.51 – −0.06; P=.01 for short-term outcomes; mean effect size, −0.33; 95% confidence interval, −0.51 – −0.15; P<.001 for intermediate-term outcomes; and mean effect size, −0.40; 95% confidence interval, −0.74 – −0.05; P=.03 for long-term outcomes). These findings suggest the possibility that OMT benefits are cumulative, yielding augmented analgesic responses over time. Licciardone and colleagues are presently conducting a comprehensive SRMA project aimed at assessing the efficacy of OMT for a variety of clinical conditions. More than 2000 bibliographic citations have been identified and evaluated for potential inclusion in that study.
2. The osteopathic manipulative treatment responder
The variability among similar patients in response to a given pain treatment has perplexed health care providers and payers alike for many years.7 At present there is no consensus on what constitutes a favorable response to a given treatment for low back pain. However, Assendelft and colleagues in collaboration with the Cochrane Collaboration’s Back Editorial Board, recommended that a mean pain reduction of 10 mm on a 100-mm visual analogue scale (VAS) be considered clinically relevant when comparing spinal manipulation with control treatments.8 Using their assumption of a 25-mm standard deviation on such scales, the clinically relevant effect size in comparison with a “perfect” placebo that provides no therapeutic effect is (10 mm – 0 mm)/25 mm, or 0.40. However, perfect placebos are rarely encountered in pain studies. In fact, a review of clinical trials involving placebo treatment vs. no treatment arms found an effect size of 0.27 associated with placebo treatment in pain studies.9 Thus, an effect size of 0.67 (0.40 + 0.27) or a 17-mm (0.67 effect size × 25-mm SD) pain reduction on a 100-mm VAS may be used to identify responders to OMT.
As there is little published work on the response to OMT, data from the North Texas clinical trial10 involving OMT in subjects with chronic low back pain were re-analyzed to determine if OMT responders could be identified. This re-analysis focused on the 48 subjects who were randomly allocated to the OMT + usual care (UC) group, as opposed to the sham manipulative treatment + UC group or the UC only group. The OMT + UC subjects included 33 women and 15 men having a mean age (SD) of 49.0 (11.6) years. A total of 32 (67%) subjects completed the entire 6-month protocol. Using the 17-mm pain reduction criterion established above, 13 OMT subjects (27% of randomized subjects and 41% of trial completers) were identified as OMT responders at the 6-month exit visit. A comparison of these OMT responders and 19 non-responders with regard to VAS pain scores over time is depicted in Figure 2. This provides graphical evidence to support the existence of the OMT responder. Further, statistical analysis confirmed significantly different pain outcomes over time in OMT responders and non-responders (P<.001 based on group × time interaction in repeated measures analysis of variance).
Figure 2.
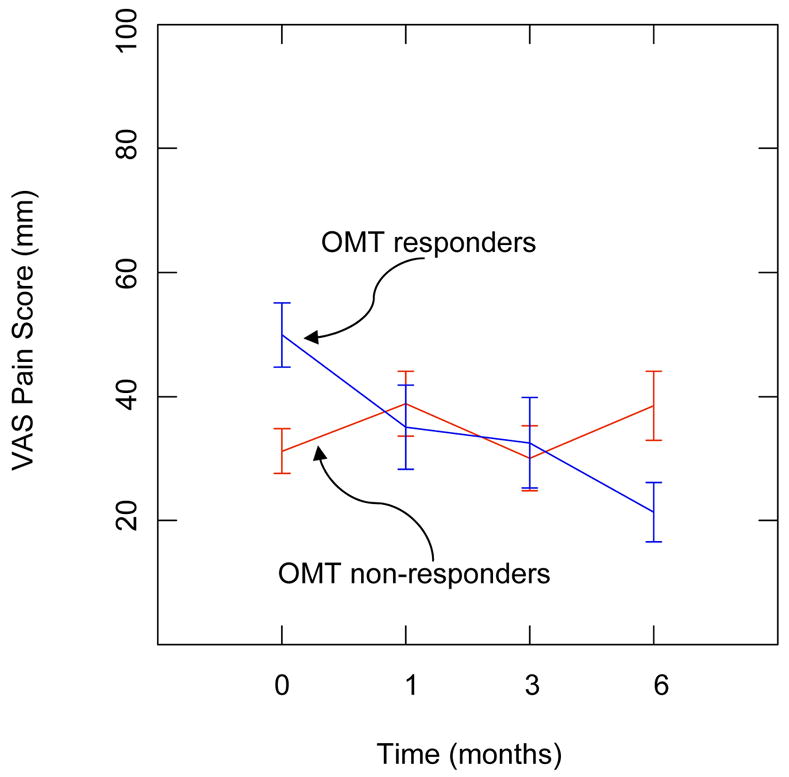
Pain outcomes over time in osteopathic manipulative treatment (OMT) responders and non-responders. VAS denotes visual analogue scale.
3. Genomic considerations in osteopathic manipulative treatment response
With the advent of genomic medicine and greater recognition of host-environment interactions in the etiology of and response to disease,11 another challenge for osteopathic research will be to use emerging technologies to help identify OMT responders. This osteopathic research challenge is currently foreshadowed by analogous developments in pharmacogenomics.12 Responses to drug therapy are quite variable, ranging from complete disease remission to fatal adverse events. Such pharmacologic variation may be attributed to many factors, including disease susceptibility, drug metabolism, drug transport, and target-organ response. Genomics offers the promise of individualizing drugs and dosages for a given patient. Similarly, genomics may help identify OMT responders and better guide their individualized treatment.
With regard to pain sensitivity, recent studies have shown a link between gene polymorphisms (often referred to as single nucleotide polymorphisms, or SNPs) and pain phenotypes. In 2003, Zubieta and colleagues found that the gene encoding for catechol-O-methyltransferase (COMT) modulates pain sensitivity.13 Subjects homozygous for the met158 allele of the COMT polymorphism (val158met) showed diminished regional μ-opioid system responses to pain compared with heterozygotes who, correspondingly, showed diminished responses compared with val158homozygotes. Importantly, they found that diminished μ-upload system responses to pain were associated with higher sensory and affective ratings of pain, thereby suggesting that the COMT val158met polymorphism influences the human experience of pain and may underlie interindividual differences in response to pain.
In 2005, these findings were extended by Diatchenko and colleagues,14 who studied haplotypes of the COMP gene. Haplotypes are sets of SNPs on a single chromatid that are statistically associated. They reported that three haplotypes of the COMT gene were associated with the risk of developing myogenous temperomandibular joint (TMJ) disorder. These haplotypes, which comprise 96% of the human population, were designated as low (LPS), average (APS), or high pain sensitivity (HPS). The relative risk of developing TMJ disorder was 2.3 (95% confidence interval, 1.1 – 4.8) in subjects having only HPS and/or APS haplotypes compared with subjects having at least one LPS haplotype. In addition to COMT, other high priority candidate genes for human neuropathic pain (and the molecules that they encode) include: IL1B (interleukin 1β), IL6 (interleukin 6), NOS1 (neuronal nitric oxide synthase), NOS2A (inducible nitric oxide synthase), OPRM1 (μ-opioid receptor), SLC6A4 (serotonin transporter), BDKRB2 (bradykinin receptor 2), 2TNFα (tumor necrosis factor α), GDNF (glial-derived nerve factor), and PDYN (prodynorphin).15 Additional research is needed to elucidate the role that such genes and molecules may play in the etiology of somatic dysfunction and pain, and in the response to OMT.
4. Psychological considerations in osteopathic manipulative treatment response
With regard to pain treatment, third-party payers often require pretreatment psychological screenings to help identify patients at risk for poor outcomes.7 Thus, another challenge for osteopathic research is to develop a psychological profile of OMT responders, particularly with regard to chronic pain disorders. The stages of pain processing model developed by Wade and colleagues,16, 17 as schematically represented in Figure 3, may be used to glean some insight into the multiple factors associated with low back pain. Intensity, the initial stage of pain, is dependent upon sensory-discriminative sensitivity to noxious stimuli and, as indicated above, may be related to host genetic and molecular factors. The next stage of pain processing involves unpleasantness, which reflects the immediate affective response to the painful sensation. Chronic pain results in suffering, which is strongly related to higher cognitive processes, including negative beliefs and emotions. Several prospective studies have assessed psychological factors thought to play a role in the progression of low back pain 18–23; however, not until Pincus and colleagues conducted a systematic review was there a critical appraisal of the relevant scientific evidence.24 Depression (often labeled as “distress,” and comprised of depressive symptoms, depressive mood, or psychological distress) and somatization were the primary psychological factors implicated in the transition to chronic low back pain. The last stage of pain processing involves overt behavioral expressions of pain, such as functional disability. Studies of psychological factors, including those associated with pain processing, may not only help disentangle the specific effects (i.e, those attributable to OMT) from the non-specific effects (i.e., those attributable to placebo), but may also be useful in addressing the potential interaction between OMT and placebo responses.25 Thus, this represents a crucial area of osteopathic research.
Figure 3.
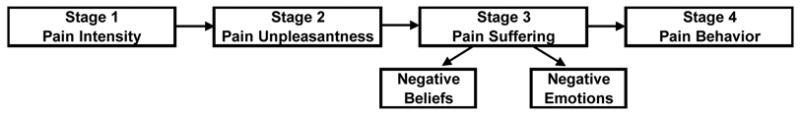
Stages of pain processing. (Adapted from Wade and colleagues.16, 17.)
5. Conclusion
A pain processing model such as that presented above provides a useful framework for considering the multifactorial etiology and progression of chronic pain disorders, such as those often treated with OMT. Consequently, in studying the response to OMT, this framework enables integration of both emerging scientific disciplines such as genomics and more traditional disciplines such as psychology. For example, the COMT haplotypes described above serve as key regulators of pain perception, cognitive function, and affective mood by altering the mRNA secondary structure that modulates protein expression.26 Thus, genetic factors may help explain multisomatoform pain disorders that share neural abnormalities that amplify sensory stimuli and increase the risk of mood disorders.27 While substantial progress has been made in osteopathic research during the past few years, particularly involving clinical trials to assess OMT efficacy, much more basic and translational research is needed to move beyond the status quo in osteopathic research.
Acknowledgments
This work was supported in part by a grant (No. K24AT002422) from the National Institutes of Health.
Funding support: National Institutes of Health (No. K24AT002422)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lucas NP, Moran RW. Is osteopathy research relevant? A challenge has been made. Int J Osteopath Med. 2006;9:75–6. [Google Scholar]
- 2.Ernst E, Canter PH. A systematic review of systematic reviews of spinal manipulation. J R Soc Med. 2006;99:192–6. doi: 10.1258/jrsm.99.4.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldstein M. A challenge to the profession: initiate evidence-based osteopathic medicine now. J Am Osteopath Assoc. 1997;97:448–451. doi: 10.7556/jaoa.1997.97.8.448. [DOI] [PubMed] [Google Scholar]
- 4.Andersson GB, Lucente T, Davis AM, Kappler RE, Lipton JA, Leurgans S. A comparison of osteopathic spinal manipulation with standard care for patients with low back pain. New Engl J Medicine. 1999;341:1426–31. doi: 10.1056/NEJM199911043411903. [DOI] [PubMed] [Google Scholar]
- 5.Howell JD. The paradox of osteopathy. New Engl J Medicine. 1999;341:1465–8. doi: 10.1056/NEJM199911043411910. [DOI] [PubMed] [Google Scholar]
- 6.Licciardone JC, Brimhall AK, King LN. Osteopathic manipulative treatment for low back pain: a systematic review and meta-analysis of randomized controlled trials. BMC Musculoskelet Disord. 2005;6:43. doi: 10.1186/1471-2474-1186-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turk DC. Understanding pain sufferers: the role of cognitive processes. Spine J. 2004;4:1–7. doi: 10.1016/s1529-9430(03)00068-8. [DOI] [PubMed] [Google Scholar]
- 8.Assendelft WJJ, Morton SC, Yu EI, Suttorp MJ, Shekelle PG. Spinal manipulative therapy for low back pain: a meta-analysis of effectiveness relative to other therapies. Ann Intern Med. 2003;138:871–81. doi: 10.7326/0003-4819-138-11-200306030-00008. [DOI] [PubMed] [Google Scholar]
- 9.Hróbjartsson A, Gøtzsche PC. Is the placebo powerless? An analysis of clinical trials comparing placebo with no treatment. New Engl J Med. 2001;344:1594–1602. doi: 10.1056/NEJM200105243442106. [DOI] [PubMed] [Google Scholar]
- 10.Licciardone JC, Stoll ST, Fulda KG, Russo DP, Siu J, Winn W, et al. Osteopathic manipulative treatment for chronic low back pain: a randomized controlled trial. Spine. 2003;28:1355–62. doi: 10.1097/01.BRS.0000067110.61471.7D. [DOI] [PubMed] [Google Scholar]
- 11.Rabinowitz PM, Poljak A. Host-environment medicine: a primary care model for the age of genomics. J Gen Intern Med Mar. 2003;18:222–7. doi: 10.1046/j.1525-1497.2003.11101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roden DM, Altman RB, Benowitz NL, Flockhart DA, Giacomini KM, Johnson JA, et al. Pharmacogenomics: challenges and opportunities. Ann Intern Med. 2006;145:749–57. doi: 10.7326/0003-4819-145-10-200611210-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zubieta JK, Heitzeg MM, Smith YR, Bueller JA, Xu K, Xu Y, et al. COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science. 2003;299:1240–3. doi: 10.1126/science.1078546. [DOI] [PubMed] [Google Scholar]
- 14.Diatchenko L, Slade GD, Nackley AG, Bhalang K, Sigurdsson A, Belfer I, et al. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum Mol Genet. 2005;14:135–43. doi: 10.1093/hmg/ddi013. [DOI] [PubMed] [Google Scholar]
- 15.Belfer I, Wu T, Kingman A, Krishnaraju RK, Goldman D, Max MB. Candidate gene studies of human pain mechanisms: methods for optimizing choice of polymorphisms and sample size. Anesthesiology. 2004;100:1562–72. doi: 10.1097/00000542-200406000-00032. [DOI] [PubMed] [Google Scholar]
- 16.Wade JB, Dougherty LM, Archer CR, Price DD. Assessing the stages of pain processing: a multivariate analytical approach. Pain. 1996;68:157–67. doi: 10.1016/S0304-3959(96)03162-4. [DOI] [PubMed] [Google Scholar]
- 17.Wade JB, Hart RP. Attention and the stages of pain processing. Pain Med. 2002;3:30–8. doi: 10.1046/j.1526-4637.2002.02008.x. [DOI] [PubMed] [Google Scholar]
- 18.Klenerman L, Slade PD, Stanley IM, Pennie B, Reilly JP, Atchison LE, et al. The prediction of chronicity in patients with an acute attack of low back pain in a general practice setting. Spine. 1995;20:478–84. doi: 10.1097/00007632-199502001-00012. [DOI] [PubMed] [Google Scholar]
- 19.Linton SJ, Hallden K. Can we screen for problematic back pain? A screening questionnaire for predicting outcome in acute and subacute back pain. Clin J Pain. 1998;14:209–15. doi: 10.1097/00002508-199809000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Thomas E, Silman AJ, Croft PR, Papageorgiou AC, Jayson MI, Macfarlane GJ. Predicting who develops chronic low back pain in primary care: a prospective study. BMJ. 1999;318:1662–7. doi: 10.1136/bmj.318.7199.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cherkin DC, Deyo RA, Street JH, Barlow W. Predicting poor outcomes for back pain seen in primary care using patients’ own criteria. Spine. 1996;21:2900–07. doi: 10.1097/00007632-199612150-00023. [DOI] [PubMed] [Google Scholar]
- 22.van den Hoogen HJ, Koes BW, Deville W, van Eijk JT, Bouter LM. The prognosis of low back pain in general practice. Spine. 1997;22:1515–21. doi: 10.1097/00007632-199707010-00019. [DOI] [PubMed] [Google Scholar]
- 23.Carey TS, Garrett JM, Jackman AM. Beyond the good prognosis. Examination of an inception cohort of patients with chronic low back pain. Spine. 2000;25:115–20. doi: 10.1097/00007632-200001010-00019. [DOI] [PubMed] [Google Scholar]
- 24.Pincus T, Burton AK, Vogel S, Field AP. A systematic review of psychological factors as predictors of chronicity/disability in prospective cohorts of low back pain. Spine. 2002;27:E109–20. doi: 10.1097/00007632-200203010-00017. [DOI] [PubMed] [Google Scholar]
- 25.Lucas N. To what should we attribute the effects of OMT? Int J Osteopath Med. 2005;8:121–23. [Google Scholar]
- 26.Diatchenko L, Nackley AG, Slade GD, Bhalang K, Belfer I, Max MB, et al. Catechol-O-methyltransferase gene polymorphisms are associated with multiple pain-evoking stimuli. Pain. 2006;125:216–24. doi: 10.1016/j.pain.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 27.Mogil JS, Max MB. The genetics of pain. In: McMahon SB, Koltzenburg M, editors. Wall and Melzack’s textbook of pain. 5. Philadelphia: Elsevier Churchill Livingstone; 2006. pp. 159–74. [Google Scholar]


