Abstract
Background
Repetitive deformation stimulates proliferation in human Caco-2 intestinal epithelial cells via an ERK1/2-dependent pathway. We examined the effects of cytoskeletal perturbation on deformation-induced signaling in Caco-2 cells.
Methods
The Caco-2 cell cytoskeleton was disrupted with either cytochalasin D, phalloidin, colchicine, or paclitaxel. α-Actinin-1, -4, and paxillin were reduced by specific siRNA. Cells on collagen I-precoated membranes were subjected to 10% repetitive deformation at 10cycles/min. After 1h, cells were lysed for western blot analysis.
Results
Strain activated ERK1/2, FAK, and Src phosphorylation in DMSO-and/or NT siRNA-treated control cell populations. Cytochalasin D and paclitaxel, but not phalloidin and colchicine, blocked ERK1/2 phosphorylation. Reduction of α-actinin-1, but not α-actinin-4 and paxillin, inhibited ERK1/2 and FAK phosphorylation, whereas Src activation appears to be independent of these effects.
Conclusions
The intestinal epithelial cell cytoskeleton may transduce mechanical signals via α-actinin-1 into the focal adhesion complex, culminating in ERK1/2 activation and proliferation.
Keywords: Strain, Mechanotransduction, Proliferation, Intestinal epithelial cell
INTRODUCTION
Physical forces including strain, pressure, and shear can initiate intracellular signals that regulate diverse aspects of cell biology1-3. Intestinal epithelial cells are subject to repetitive physical deformation during peristalsis and villous motility4. Such cyclic deformation stimulates signals within intestinal epithelial cells that cause cell differentiation and proliferation in vitro4-7 and in vivo8. The mucosa atrophies during sepsis or ileus when such stimuli are absent9, even if the patient is adequately nourished parenterally. Strain-induced deformation has been shown to activate focal adhesion kinase (FAK), Src, and extracellular signal regulated kinases (ERK1/2) in intestinal epithelial cells10, 11 and tyrosine kinase signaling within the gut mucosa in vivo12. Although kinases activated by mechanical forces are now well known, both in intestinal and other cells, the specific manner by which cells sense mechanical stimuli and convert them into kinase-driven chemical signals remains poorly understood.
Stretch-activated ion channels, mechanosensitive membrane-associated enzymes, distortion of cytoskeletal filaments, and integrin-ECM interactions have all been shown to contribute to the relay of mechanical signals in various cell models13-17. More specifically, it has been theorized that the mechanical strain imposed by repetitive cell deformation may be transferred to load-bearing filaments in the cytoskeleton and thence to cytoskeleton-associated molecules in the focal adhesion complex18. We therefore sought to determine whether the cell cytoskeleton and associated linking molecules α-actinin and paxillin play a role in the mechanotransduction of strain-induced signals.
We used the actin polymerization inhibitor cytochalasin D, the actin stabilizer, phalloidin, the microtubule polymerization inhibitor, colchicine, and the microtubule stabilizer, paclitaxel, to examine the requirement for cytoskeletal integrity on strain-induced ERK1/2 phosphorylation in Caco-2 cells. We also examined the effect of α-actinin-1, -4, and paxillin reduction by siRNA. Due the higher specificity of siRNA-mediated perturbation of this phenomenon, the effects of α-actinin-1, -4, and paxillin reduction on FAK and Src phosphorylation were also studied.
MATERIALS AND METHODS
Cell culture
Human Caco-2 intestinal epithelial cells were isolated and maintained as previously described19. Cells were grown to confluence on collagen I matrix-precoated Flexwell I plates (Flexcell International Corp, Hillsborough, NC). All studies were performed on cells within 8 passages.
Transfection and inhibitor treatments
Cells were transfected with double-stranded small interfering RNA (siRNA) directed toward the mRNA target 5′- CACAGAUCGAGAACAUCGAAG-3′ for α-actinin-1, toward 5′-CCACAUCAGCUGGAAGGAUGGUCUU-3′ for α-actinin-4, and toward 5′-GTGTGGAGCCTTCTTTGGT-3′ to inhibit paxillin expression. SiRNA duplexes were synthesized by Dharmacon (Lafayette, CO). A Dharmacon siCONTROL Non-Targeting siRNA #1 (siNT) sequence was used as a control. SiRNAs were introduced with Oligofectamine according to the manufacturer's protocol (Invitrogen, Carlsbad, CA). Cell transfectants were used for strain experiments after 48 hours. Alternate cell populations were pretreated for 1 hour with either 5μM cytochalasin D, 10μM phalloidin, 10μM colchicine, or 10μM paclitaxel prior to strain application.
Strain application
Once cell monolayers reached confluency, Flexwell plates were placed in a cell culture incubator (5% CO2, 37°C) and the membranes were repetitively deformed, via a computer-controlled vacuum manifold (FX3000; Flexcel, McKeesport, PA), by ∼20 kPa vacuum at 10 cycles/min, producing an average 10% strain on the adherent cells during deformation. Cells were exposed to either static or strain conditions for 1 hour. Non-uniformity of strain in the center of the flexible wells was addressed by placing a Plexiglass ring in the center, so that cells could be plated peripheral to the ring where strain is relatively uniform. The cells remain adherent under these conditions and experience parallel elongation and relaxation6.
Western blotting
Following strain, Caco-2 cells were lysed in lysis buffer containing 50 mM Tris pH 7.4, 150 mM NaCl, 1% Triton X-100, 10% glycerol, 1% deoxycholic acid, 0.1% SDS, 1 mM EDTA, 1 mM EGTA, 1 mM PMSF, 1 mM sodium vanadate, 50 mM NaF, 10 mM sodium pyrophosphate, 2 μg/mL aprotinin and 2 μg/mL leupeptin. Cell lysate protein concentrations were determined using a BCA protein assay kit (Pierce, Rockford, IL). Equal amounts of protein were resolved by SDS–PAGE and transferred to Hybond ECL nitrocellulose membrane (Amersham Pharmacia Biotech, Piscataway, NJ). Antibodies to phosphorylated ERK1/2 Y202/T204, total ERK1/2 (Cell Signaling, Beverly, MA), phosphorylated FAK Y397 and Y576 (BD Transduction Laboratories, San Diego, CA), total FAK, clone 4.47 (Upstate, Temecula, CA), phosphorylated Src (Y416), total Src, clone L4A1 (Cell Signaling), GAPDH (Biodesign International, Saco, MN), α-actinin-1 (US Biological, Swampscott, MA), α-actinin-4 (ALEXIS Corp., Lausen, Switzerland), and paxillin (BD Transduction Laboratories) coupled with appropriate horseradish peroxidase-conjugated secondary antibodies (Cell Signaling) were used for immunodetection of blotted proteins. Bands were detected with enhanced chemiluminescence (Amersham) and analyzed with a Kodak Image Station 440CF (Perkin Elmer, Boston, MA). All exposures were within the linear range of the system.
Statistical analysis
Statistical analysis was done using SigmaStat software (SPSS, Inc., Chicago, IL). Student t tests or paired t tests were employed as appropriate. A 95% confidence interval was set a priori as the desired level of statistical significance.
RESULTS
Strain-induced ERK1/2 phosphorylation requires both an intact cytoskeleton and the capacity for microtubule rearrangement
To investigate the role of the actin cytoskeleton and microtubule network in conveying strain-induced intracellular signals, human Caco-2 intestinal epithelial cell monolayers were pretreated with either DMSO, cytochalasin D, phalloidin, colchicine, or paclitaxel, then subjected to an average 10% repetitive deformation at 10 cycles per minute for 1 hour (Fig. 1). Lysates were analyzed by western blot for relative ERK1/2 phosphorylation. DMSO-treated control cells displayed a 29±10% increase (n=7; p<0.02) in ERK1/2 phosphorylation following strain application compared with cells under static conditions. Inhibition of actin polymerization by cytochalasin D significantly reduced basal ERK1/2 phosphorylation and prevented any strain-induced effect (n=7; p<0.01). However, actin filament stabilization by phalloidin, an inhibitor of actin depolymerization, did not inhibit strain-induced ERK1/2 phosphorylation (n=7; p<0.01). Cells treated with colchicine also still exhibited a 26±8% increase in deformation-induced ERK1/2 activation (n=7; p<0.04), while microtubule stabilization by paclitaxel inhibited this effect (n=7; p<0.01).
Figure 1. Effect of pharmacologic cytoskeletal perturbation on strain-induced ERK1/2 phosphorylation.
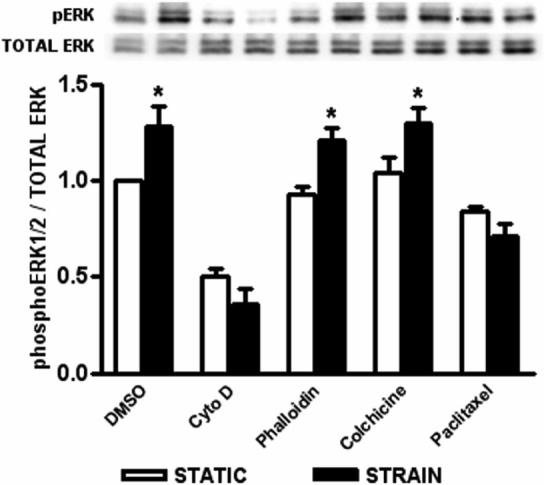
Caco-2 cells grown on collagen I and subjected to 1 hour of cyclic strain show increased ERK1/2 phosphorylation. Cells treated with either DMSO, cytochalasin D (5μM), phalloidin (10μM), colchicine (10μM), or paclitaxel (10μM), were assessed for ERK1/2 phosphorylation under static (open bars) and strain (closed bars) conditions. Western blots were probed for phosphorylated ERK1/2, then stripped and reprobed for total ERK1/2. All bars are normalized against the DMSO-treated static control and expressed as mean ± SEM. (*P<0.05; n=7)
Strain-induced ERK1/2 phosphorylation requires α-actinin-1, but not α-actinin-4 or paxillin
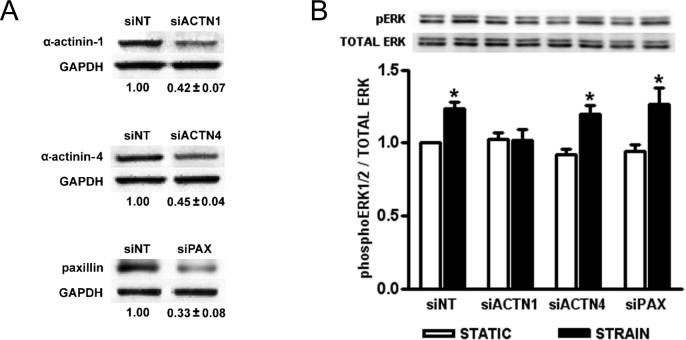
We next investigated whether the cytoskeleton-associated molecules α-actinin and paxillin were necessary for strain-induced mechanotransduction. α-Actinin facilitates focal adhesion formation and physically links integrin-associated focal adhesion complexes with the cytoskeleton20, whereas paxillin is a focal adhesion-associated protein that functions as a multi-domain adapter molecule21. α-Actinin isoforms, α-actinin-1 and -4 are found in colonic epithelial cells22, 23. We therefore sought to assess whether strain-induced signaling involved a specific α-actinin isoform. Individual isoform-specific siRNA sequences and transfection conditions were optimized to achieve a 50−70% knockdown of α-actinin-1 and -4, and paxillin protein in Caco-2 cells (Fig. 2A). Cells transfected with siNT served as a control group for each cell population. Following repetitive deformation (Fig. 2B), siNT transfectants displayed a 23±5% increase (n=5; p<0.01) in ERK1/2 phosphorylation. α-Actinin-1 reduction blocked strain induced ERK1/2 phosphorylation (n=5; p<0.02), whereas neither α-actinin-4 nor paxillin reduction had any effect (n=5; p<0.03).
Figure 2. Effect of siRNA-mediated reduction of α-actinin-1, -4, and paxillin on strain-induced ERK1/2 phosphorylation.
(A) Typical reduction of total α-actinin-1, -4, and paxillin protein in Caco-2 cells transfected with siRNA targeted to α-actinin-1 (siACTN1), α-actinin-4 (siACTN4), and paxillin (siPAX) as measured by western blot. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control and target protein expression is normalized against that of non-target siRNA (siNT) transfectant controls (P<0.01; n=5). (B) Caco-2 cell siRNA transfectants were grown on collagen I and subjected to 1 hour of cyclic strain, then assessed for ERK1/2 phosphorylation. Western blots were probed for phosphorylated ERK1/2, then stripped and reprobed for total ERK1/2. All bars are normalized against the siNT-transfected static control and expressed as mean ± SEM. (*P<0.05; n=5)
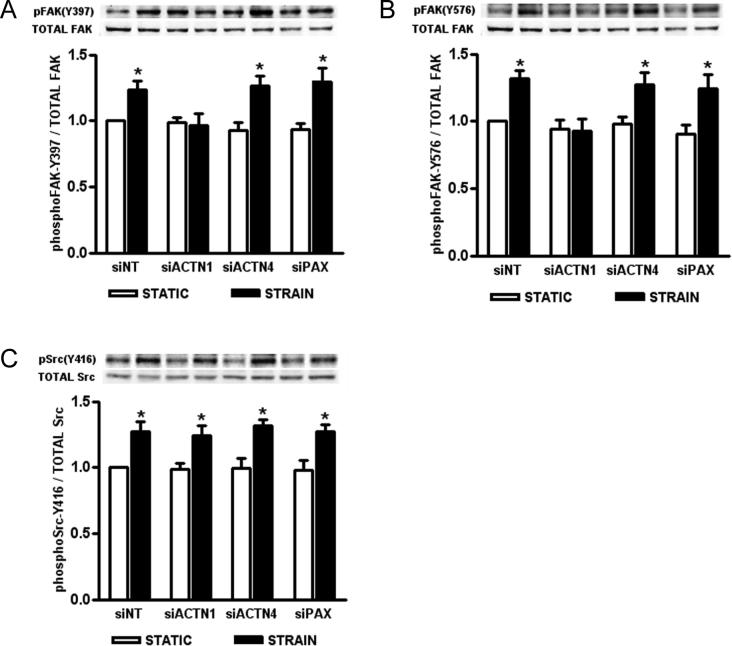
SiRNA-mediated reduction of α-actinin-1 blocks strain-induced FAK phosphorylation, but not Src
We have previously reported that Caco-2 strain-induced ERK1/2 mitogenic signaling requires activation of both FAK and Src11. Because siRNA-mediated perturbation of cytoskeletal signaling is more specific, we used this technique rather than pharmacologic manipulation to further evaluate the role of the cytoskeleton in strain-induced FAK and Src phosphorylation, again reducing α-actinin-1, -4, and paxillin by siRNA (Fig. 3). Following strain, siNT-transfected cells displayed 23±7% (Fig. 3A; n=5; p<0.02) and 31±6% (Fig. 3B; n=5; p<0.01) increased FAK Y397 and Y576 phosphorylation, respectively. SiNT transfectants also exhibited a 28±7% increase in Src Y416 phosphorylation (Fig. 3B; n=5; p<0.01), consistent with previous results11. SiRNA-mediated reduction of α-actinin-1 blocked strain-induced phosphorylation of FAK Y397 (Fig. 3A; n=5; p<0.02) and Y576 (Fig. 3B; n=5; p<0.01), but did not affect Src (Fig. 3C; n=5; p<0.02). Similar to the siNT controls, cells with reduced α-actinin-4 and paxillin expression displayed a 33±8% (Fig. 3A; n=5; p<0.01) and 37±10% (Fig. 3A; n=5; p<0.02) increase in strain-induced FAK Y397 phosphorylation and a 29±9% (Fig. 3B; n=5; p<0.02) and 34±10% (Fig. 3B; n=5; p<0.03) increase in phospho-FAK Y576. Reducing α-actinin-4 or paxillin also did not alter strain-mediated Src Y416 phosphorylation (Fig. 3C; n=5; p<0.02).
Figure 3. Effect of siRNA-mediated reduction of α-actinin-1, -4, and paxillin on strain-induced FAK and Src phosphorylation.
Following reduction of α-actinin-1, -4, and paxillin protein, Caco-2 cell transfectants were grown on collagen I and subjected to 1 hour of cyclic strain, then lysed for western blot analysis. (A-C) Western blots were probed for phosphorylated FAK Y397, FAK Y576, and Src Y416, then stripped and reprobed for total FAK and Src. All bars are normalized against their respective siNT-transfected static control and expressed as mean ± SEM. (*P<0.05; n=5)
DISCUSSION
Mechanical forces in the gut contribute to intestinal homeostasis by influencing intestinal epithelial cell proliferation and differentiation. We have previously reported repetitive deformation stimulates proliferation in human Caco-2 intestinal epithelial cells in an integrin- and matrix-dependent manner via an ERK1/2-dependent pathway that requires activation of both FAK and Src11, 24. In our current investigation, actin disruption with cytochalasin D, but not phalloidin, blocked strain-induced ERK1/2 phosphorylation, whereas microtubule perturbation by paclitaxel, but not colchicine, also inhibited this effect. These opposing results suggest that while an intact actin cytoskeleton is required for strain-induced signaling, the capacity for microtubule reorganization is also necessary. Further, reduction of the actin-associated molecule, α-actinin-1, inhibited deformation-induced ERK1/2 phosphorylation and activation of FAK, while α-actinin-4 and paxillin silencing had no effect. The failure of α-actinin-1, -4, and paxillin reduction to influence strain-induced Src phosphorylation suggests that Src and FAK activation may be independently regulated.
Transmembrane integrin adhesion receptors provide a structural bridge between the extracellular matrix (ECM) and the interior cell cytoskeleton. They are well-positioned to mediate and convey bi-directional mechanical signals between the ECM and intracellular signaling molecules25. The necessity of α-actinin-1 in strain-induced mechanotransduction may reflect its function as a physical link between the actin cytoskeleton and integrins18, 26. This idea is consistent with Ingber's “cellular tensegrity” model, in which physical distortion of the cell cytoskeleton is proposed to transfer mechanical loads through actin-associated molecules and initiate intracellular signaling. This concept is further supported by our previous observation that pharmacologic perturbation of the cell cytoskeleton inhibits pressure-stimulated cell adhesion and FAK activation, but not Src27.
Apart from the structural role of α-actinin-1 as a tethering molecule, its ability to function as a scaffolding protein and directly interact with ERK1/2 may potentially be of similar importance in this phenomenon28. However, α-actinin-1 strain-induced protein-protein interactions were deemed outside of the scope of the current investigation. The focal adhesion adapter protein paxillin has also been implicated in mitogenic signaling pathways29 and previously was found to be necessary for pressure-stimulated cell adhesion30, 31. Interestingly, in contrast to pressure-induced signaling, paxillin does not appear to play a seminal role in strain-activated mechanotransduction. This difference may be due to a lesser dependence on focal adhesion formation and turnover in the strain-induced signaling of already adherent cells as opposed to pressure-activated signaling in suspended cells.
In summary, our data suggest that the intestinal epithelial cytoskeleton may sense and transduce mechanical signals via α-actinin-1 into the focal adhesion complex, culminating in ERK1/2 activation and proliferation. The orchestrated response of these signaling elements to mechanical stimuli is crucial for the maintenance of intestinal homeostasis. Aberrations in this signaling pathway during ileus or fasting may contribute to mucosal atrophy and offer potential targets for therapy.
Acknowledgements
Supported in part by Department of Veterans Affairs Merit Review (MDB) and NIH RO1 DK60771 (MDB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Summary to appear in table of contents: Repetitive mechanical deformation stimulates proliferation in human Caco-2 intestinal epithelial cells via an ERK1/2-dependent pathway requiring both an intact cytoskeleton and α-actinin-1. Aberrations in this signaling pathway contributing to mucosal atrophy offer potential targets for therapy.
REFERENCES
- 1.Ashida N, Takechi H, Kita T, Arai H. Vortex-mediated mechanical stress induces integrin-dependent cell adhesion mediated by inositol 1,4,5-trisphosphate-sensitive Ca2+ release in THP-1 cells. J Biol Chem. 2003;278(11):9327–31. doi: 10.1074/jbc.M212316200. [DOI] [PubMed] [Google Scholar]
- 2.Haier J, Nicolson GL. Tumor cell adhesion under hydrodynamic conditions of fluid flow. Apmis. 2001;109(4):241–62. doi: 10.1034/j.1600-0463.2001.d01-118.x. [DOI] [PubMed] [Google Scholar]
- 3.Shin HY, Gerritsen ME, Bizios R. Regulation of endothelial cell proliferation and apoptosis by cyclic pressure. Ann Biomed Eng. 2002;30(3):297–304. doi: 10.1114/1.1458595. [DOI] [PubMed] [Google Scholar]
- 4.Basson MD. Paradigms for mechanical signal transduction in the intestinal epithelium. Category: molecular, cell, and developmental biology. Digestion. 2003;68(4):217–25. doi: 10.1159/000076385. [DOI] [PubMed] [Google Scholar]
- 5.Zhang J, Li W, Sanders MA, et al. Regulation of the intestinal epithelial response to cyclic strain by extracellular matrix proteins. Faseb J. 2003;17(8):926–8. doi: 10.1096/fj.02-0663fje. [DOI] [PubMed] [Google Scholar]
- 6.Basson MD, Li GD, Hong F, et al. Amplitude-dependent modulation of brush border enzymes and proliferation by cyclic strain in human intestinal Caco-2 monolayers. J Cell Physiol. 1996;168(2):476–88. doi: 10.1002/(SICI)1097-4652(199608)168:2<476::AID-JCP26>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 7.Basson MD, Turowski G, Emenaker NJ. Regulation of human (Caco-2) intestinal epithelial cell differentiation by extracellular matrix proteins. Exp Cell Res. 1996;225(2):301–5. doi: 10.1006/excr.1996.0180. [DOI] [PubMed] [Google Scholar]
- 8.Spencer AU, Sun X, El-Sawaf M, et al. Enterogenesis in a clinically feasible model of mechanical small-bowel lengthening. Surgery. 2006;140(2):212–20. doi: 10.1016/j.surg.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zittel TT, Meile T, Huge A, et al. Preoperative intraluminal application of capsaicin increases postoperative gastric and colonic motility in rats. J Gastrointest Surg. 2001;5(5):503–13. doi: 10.1016/s1091-255x(01)80088-3. [DOI] [PubMed] [Google Scholar]
- 10.Li W, Duzgun A, Sumpio BE, Basson MD. Integrin and FAK-mediated MAPK activation is required for cyclic strain mitogenic effects in Caco-2 cells. Am J Physiol Gastrointest Liver Physiol. 2001;280(1):G75–87. doi: 10.1152/ajpgi.2001.280.1.G75. [DOI] [PubMed] [Google Scholar]
- 11.Chaturvedi LS, Marsh HM, Shang X, et al. Repetitive deformation activates focal adhesion kinase and ERK mitogenic signals in human Caco-2 intestinal epithelial cells through Src and Rac1. J Biol Chem. 2007;282(1):14–28. doi: 10.1074/jbc.M605817200. [DOI] [PubMed] [Google Scholar]
- 12.Basson MD, Coppola CP. Repetitive deformation and pressure activate small bowel and colonic mucosal tyrosine kinase activity in vivo. Metabolism. 2002;51(12):1525–7. doi: 10.1053/meta.2002.36303. [DOI] [PubMed] [Google Scholar]
- 13.Atance J, Yost MJ, Carver W. Influence of the extracellular matrix on the regulation of cardiac fibroblast behavior by mechanical stretch. J Cell Physiol. 2004;200(3):377–86. doi: 10.1002/jcp.20034. [DOI] [PubMed] [Google Scholar]
- 14.Yang F, Li YH. [Roles of integrins and cytoskeleton in cellular mechanotransduction]. Space Med Med Eng (Beijing) 2002;15(4):309–12. [PubMed] [Google Scholar]
- 15.Huynh TT, Iaccarino G, Davies MG, et al. External support modulates G protein expression and receptor coupling in experimental vein grafts. Surgery. 1999;126(2):127–34. [PubMed] [Google Scholar]
- 16.Ghazi A, Berrier C, Ajouz B, Besnard M. Mechanosensitive ion channels and their mode of activation. Biochimie. 1998;80(56):357–62. doi: 10.1016/s0300-9084(00)80003-6. [DOI] [PubMed] [Google Scholar]
- 17.Katsumi A, Orr AW, Tzima E, Schwartz MA. Integrins in mechanotransduction. J Biol Chem. 2004;279(13):12001–4. doi: 10.1074/jbc.R300038200. [DOI] [PubMed] [Google Scholar]
- 18.Ingber DE. Tensegrity: the architectural basis of cellular mechanotransduction. Annu Rev Physiol. 1997;59:575–99. doi: 10.1146/annurev.physiol.59.1.575. [DOI] [PubMed] [Google Scholar]
- 19.Basson MD, Modlin IM, Madri JA. Human enterocyte (Caco-2) migration is modulated in vitro by extracellular matrix composition and epidermal growth factor. J Clin Invest. 1992;90(1):15–23. doi: 10.1172/JCI115828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rajfur Z, Roy P, Otey C, et al. Dissecting the link between stress fibres and focal adhesions by CALI with EGFP fusion proteins. Nat Cell Biol. 2002;4(4):286–93. doi: 10.1038/ncb772. [DOI] [PubMed] [Google Scholar]
- 21.Herreros L, Rodriguez-Fernandez JL, Brown MC, et al. Paxillin localizes to the lymphocyte microtubule organizing center and associates with the microtubule cytoskeleton. J Biol Chem. 2000;275(34):26436–40. doi: 10.1074/jbc.M003970200. [DOI] [PubMed] [Google Scholar]
- 22.Pavalko FM, Otey CA, Simon KO, Burridge K. Alpha-actinin: a direct link between actin and integrins. Biochem Soc Trans. 1991;19(4):1065–9. doi: 10.1042/bst0191065. [DOI] [PubMed] [Google Scholar]
- 23.Honda K, Yamada T, Endo R, et al. Actinin-4, a novel actin-bundling protein associated with cell motility and cancer invasion. J Cell Biol. 1998;140(6):1383–93. doi: 10.1083/jcb.140.6.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang J, Owen CR, Sanders MA, et al. The motogenic effects of cyclic mechanical strain on intestinal epithelial monolayer wound closure are matrix dependent. Gastroenterology. 2006;131(4):1179–89. doi: 10.1053/j.gastro.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 25.Coppolino MG, Dedhar S. Bi-directional signal transduction by integrin receptors. Int J Biochem Cell Biol. 2000;32(2):171–88. doi: 10.1016/s1357-2725(99)00043-6. [DOI] [PubMed] [Google Scholar]
- 26.Ingber DE. Tensegrity II. How structural networks influence cellular information processing networks. J Cell Sci. 2003;116(Pt 8):1397–408. doi: 10.1242/jcs.00360. [DOI] [PubMed] [Google Scholar]
- 27.Thamilselvan V, Basson MD. The role of the cytoskeleton in differentially regulating pressure-mediated effects on malignant colonocyte focal adhesion signaling and cell adhesion. Carcinogenesis. 2005;26(10):1687–97. doi: 10.1093/carcin/bgi135. [DOI] [PubMed] [Google Scholar]
- 28.Leinweber BD, Leavis PC, Grabarek Z, et al. Extracellular regulated kinase (ERK) interaction with actin and the calponin homology (CH) domain of actin-binding proteins. Biochem J. 1999;344(Pt 1):117–23. [PMC free article] [PubMed] [Google Scholar]
- 29.Tatsumi Y, Cho YY, He Z, et al. Involvement of the paxillin pathway in JB6 Cl41 cell transformation. Cancer Res. 2006;66(11):5968–74. doi: 10.1158/0008-5472.CAN-05-4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conway WC, Van der Voort van Zyp J, Thamilselvan V, et al. Paxillin modulates squamous cancer cell adhesion and is important in pressure-augmented adhesion. J Cell Biochem. 2006;98(6):1507–16. doi: 10.1002/jcb.20819. [DOI] [PubMed] [Google Scholar]
- 31.van Zyp JV, Conway WC, Craig DH, et al. Extracellular Pressure Stimulates Tumor Cell Adhesion In Vitro by Paxillin Activation. Cancer Biol Ther. 2006;5(9) doi: 10.4161/cbt.5.9.3002. [DOI] [PubMed] [Google Scholar]