Abstract
Cocaine’s (COC) direct interaction with the dopamine (DA) transporter is usually considered the most important action underlying the psychomotor stimulant and reinforcing effects of this drug. However, some physiological, behavioral and psycho-emotional effects of COC are very rapid and brief and they remain intact during DA receptor blockade, suggesting possible involvement of peripheral non-DA neural mechanisms.
To assess this issue, single-unit recording with microiontophoresis was used to examine changes in impulse activity of dorsal and ventral striatal neurons to intravenous (iv) COC (0.25–0.5 mg/kg) in the same rats under two conditions: awake with dopamine (DA) receptor blockade and anesthetized with urethane. In the awake preparation ~70% striatal neurons showed rapid and transient (latency ~ 6 s, duration ~15 s) COC-induced excitations. These effects were stronger in ventral than dorsal striatum. During anesthesia, these phasic effects were fully blocked and COC slowly decreased neuronal discharge rate. COC-methiodide (COC-M), a derivative that cannot cross the blood-brain barrier, also caused phasic excitations in the awake, but not anesthetized condition. In contrast to regular COC, COC-M had no tonic effect on discharge rate in either preparation. Most striatal neurons that were phasically excited by both COC forms also showed short-latency excitations during tail-touch and tail-pinch in the awake preparation, an effect strongly attenuated during anesthesia. Finally, most striatal neurons that in awake conditions were phasically excited by somato-sensory stimuli and COC salts were also excited by iontophoretic glutamate (GLU). Although striatal neurons were sensitive to GLU in both preparations, the response magnitude at the same GLU current was higher in awake than anesthetized conditions.
These data suggest that in awake animals iv COC, like somato-sensory stimuli, transiently excites striatal neurons via its action on peripheral neural elements and rapid neural transmission. While the nature of these neuronal elements needs to be clarified using other analytical techniques, they might involve voltage-gated K+ and Na+ channels, which have a high affinity for COC and are located on terminals of visceral sensory nerves that densely innervate peripheral vessels. Therefore, along with direct action on specific brain substrates, central excitatory effects of COC may occur via indirect action, involving afferents of visceral sensory nerves and rapid neural transmission. By providing a rapid sensory signal and triggering transient neural activation, such a peripherally triggered action might play a crucial role in the sensory effects of COC, thus contributing to learning and development of drug-taking behavior.
Keywords: dopamine, cocaine methiodide, somato-sensory stimuli, glutamate, visceral sensory nerves, sodium and potassium channels, drug-taking behavior, reinforcement
Peripheral blood vessels, especially veins, are densely innervated by sensory nerves (Goder et al., 1993; Michaelis et al., 2005), which are activated by “pathological” shifts in plasma chemicals and can be experimentally activated by noxious stimuli, heat, mechanical stimuli, and chemical substances (Lee et al., 2005). Activation here results in rapid neural transmission to the cortex through thalamo-cortical pathways similar to those engaged in the transmission of cutaneous somato-sensory stimuli (Ploner et al., 2002). Amongst the multiple receptors and channels on the thinly myelinated and unmyelinated afferents of these sensory nerves are voltage-gated Na+ and K+ channels (Lee et al., 2005), targets upon which cocaine (COC) acts (Catterall and Mackie 1996; Premkumar, 2005; Wu et al., 2006). Undoubtedly, these neural substrates on vascular sensory terminals should be directly affected by COC following intravenous (iv) administration, thereby contributing to this drug’s sensory, behavioral and physiological effects.
Abundant evidence suggests that the dopamine (DA) system mediates the reinforcing effects of COC (Wise and Bozarth, 1987) and interaction with central monoamine transporters, especially the DA transporter, is considered the most important central action of COC. However, it is also known that several effects of this drug, including euphoria (Gawin, 1986; Sherer et al., 1989), sympathoexcitation, and acute vasoconstriction (Kiritsy-Roy et al., 1990; Poon and van der Boose, 1992) are resistant to DA receptor blockade, suggesting the involvement of non-DA mechanisms. In drug-experienced individuals, COC euphoria is mimicked by iv procaine (Adinoff et al., 1998), a structurally similar local anesthetic with negligible effects on monoamine uptake (1:140–2000 affinity to monoamine transporters vs. COC; Ritz et al. 1987). Like COC, iv procaine induces strong sensory and subjective effects in drug-naive individuals (Servan-Shreiber et al., 1998), sympathoexcitation (Pitts et al., 1987a), rapid EEG desynchronization (Adamec and Stark-Adamec, 1987; Parekh et al., 1995) and brain hyperthermia (Brown and Kiyatkin, 2006). COC-methiodide (COC-M), which cannot cross the blood-brain barrier (Shriver and Long, 1971) also mimics COC in its sympathoexcitatory (Dickerson et al., 1999) and brain hyperthermic effects (Brown and Kiyatkin, 2006), indicating the involvement of peripheral mechanisms.
The rapid and transient nature of some subjective and physiological effects of iv COC also point toward possible involvement of peripheral neural elements and rapid neural transmission. Cocaine-induced euphoria (Fischman and Shuster, 1983; Zernig et al., 2002), EEG desynchronization (Lukas et al., 1990; Matsuzaki et al., 1978), acute increase in arterial blood pressure (Poon and van den Buuse, 1992) and peripheral vasoconstriction (Kiyatkin and Brown, 2005) all occur within seconds after iv COC injection. However, more time is presumably necessary for COC to reach the brain, cross the blood-brain barrier, passively diffuse within brain tissue, and interact with central receptor sites. While the exact time-course of changes in brain COC levels remains unknown, PET studies with radio-labeled COC in baboons reveal its appearance in the brain at 30 s and a peak at 90–120 s after a single iv injection (Fowler et al., 1998).
Since procaine can in some ways mimic the physiological effects of COC without the benefit of monoaminergic action, COC-M can be excitatory even though it does not cross the blood-brain barrier, and since iv COC can rapidly induce central effects before the drug has circulated throughout the body, interaction with peripherally located neural elements may be a secondary but important pathway by which COC may act. The present study was designed to test this hypothesis using traditional electrophysiological techniques. Impulse activity of dorsal and ventral striatal neurons was monitored following iv COC, iv COC-M, somato-sensory stimuli, and GLU iontophoresis in rats during two conditions: urethane anesthesia and awake with DA receptor blockade.
The striatum was chosen because of its unique role in sensomotor integration (Mogenson et al., 1993). Striatal neurons are a homogeneous population of mostly GABA-containing spiny neurons, which have slow irregular activity both in anesthetized and awake preparations (Kiyatkin and Rebec, 1996, 1999a; Wilson, 1993). These neurons are preferentially activated by various somato-sensory stimuli (Nagy et al., 2006; West, 1998; Rebec, 2006) and iontophoretic glutamate (GLU) (Kiyatkin and Rebec 1996, 1999a), which appears to be the primary factor responsible for their excitatory responses (Parent et al., 1995; Rebec, 2006; Wilson, 1993). Additionally, the ventral striatum is heavily implicated in drug reinforcement and addiction (Wise and Bozarth, 1987).
Although an awake, freely moving animal is best for studying impulse activity and responsiveness of central neurons during COC delivery, single-unit recordings with high-impedance, fine-tip glass electrodes under this condition are virtually impossible because of robust locomotor activation and muscular activity. While the development of multi-wire bundle technology has made it possible to provide long-term single-unit recording in freely moving rats (Nicolelis et al., 1993), this method provides a much weaker signal-to-noise ratio of unit recording and does not allow for iontophoresis, which was important for examining the pattern of neuronal activity following direct activation by GLU. Because of the ability of DA antagonists to largely eliminate COC-induced motor activity (Kiyatkin and Brown, 2005) without evident changes in sensory responsiveness of striatal neurons (Kiyatkin and Rebec, 1999b), an awake animal with DA receptor blockade was used as the primary preparation in this study. In addition to providing artifact-free recording, pre-treatment with DA antagonists allows one to study neural effects of COC that are independent of DA.
As an important control, striatal neuronal activity and responsiveness was also examined during urethane anesthesia. General anesthesia provides recording stability, but by altering the activity state of central neurons and inhibiting their responses to somato-sensory stimuli (see Windels, 2006 for review), it may drastically modulate the effects of systemic COC delivery. Similar to somato-sensory stimuli, general anesthesia not only blocks sensory and locomotor effects of iv COC, it also attenuates or blocks its centrally mediated sympathoexcitatory effects (Abrahams et al., 1996; Knuepfer and Branch, 1992; Wilkerson, 1988) as well as brain activation as demonstrated by Fos expression (Kreuter et al., 2004). With the exception of the monoamine-containing cells (Einhorn et al., 1988; Pitts and Marwah, 1987), single-unit studies with iv COC delivery in anesthetized animals are limited and focused on slow drug-induced changes in discharge rate or neuronal responses to afferent inputs (Jimenes-Rivera and Waterhouse, 1991; Waterhouse et al., 1991). Urethane was chosen because this drug, compared to other general anesthetics, minimally inhibits brain metabolism, neuronal activity and responses to sensory stimuli (Buzsaki et al. 1983; Friedberg et al. 1999). Therefore, by comparing data obtained in the same animals in two preparations we were able to clarify the role of an animal’s activity state in determining striatal neuronal responses to both somato-sensory stimuli and COC.
Materials and Methods
Animals and Surgery
Data were obtained from 11 male Long-Evans rats (400±50 g) supplied by Charles River Laboratories (Greensboro, NC). All animals were housed individually under standard laboratory conditions (12-hr light cycle beginning at 07:00) with free access to food and water. Protocols were performed in compliance with the Guide for the Care and Use of Laboratory Animals (NIH, Publication 865-23) and were approved by the NIDA-IRP Animal Care and Use Committee. Maximal care was taken to minimize the number of animals used and their suffering.
Single-unit recording combined with microiontophoresis in all experiments was performed with the use of a microdriver (Rebec et al., 1993) that allowed manual fine movement of four-barrel glass microelectrodes within the recording track. The surgical procedures used have been described previously (Kiyatkin and Rebec, 2001). Briefly, under general anesthesia (Equithesin 3.3 ml/kg i.p.; dose of sodium pentobarbital 32.5 mg/kg and chloral hydrate 145 mg/kg), rats were implanted with a plastic, cylindrical hub, designed to mate with a microelectrode during recording. This hub was centered over a hole drilled above the striatum (1.2 mm anterior and 1.2 mm lateral to bregma). During the same session, each rat was implanted with a chronic jugular catheter, which was run subcutaneously to the head mount and secured with dental cement. After a 3–4 day period of recovery and two-three days of habituation to the experimental chamber, recording sessions were held once daily over the next 2–3 days.
Experimental Protocol
Each rat was used for electrophysiological recordings under two conditions. During all but the last session, recordings were performed in awake, non-anesthetized conditions after systemic administration of a mixture of D1-like (SCH23390 1 mg/kg) and D2-like (eticlopride 1 mg/kg) DA antagonists. Both drugs were obtained from Sigma (St. Louis, MO), freshly dissolved, mixed, and injected subcutaneously. Neuronal data obtained between 20 min and 3 h following the injection of DA antagonists were accepted as occurring during full DA receptor blockade, as confirmed in our previous study (Kiyatkin and Rebec, 1999b) by the antagonism of striatal neuronal responses to iontophoretic DA. DA blockade was maintained by additional injections of DA antagonists at half the dose, three hours after the initial administration. During the last session each rat was anesthetized with urethane (1.25 g/kg, ip) and recording was continued for 6–8 hours after drug administration.
Single-unit recording and iontophoresis
Four-barrel, microfilament-filled, glass pipettes (Omega Dot 50744, Stoelting, Wood Dale, IL), pulled and broken to a diameter of 4±1 μm, were used for single-unit recording and iontophoresis. The recording barrel contained 2% Pontamine Sky Blue (BDH Chemicals Ltd, Poole, England) in 3 M NaCl; the balance barrel contained 0.25 M NaCl; one of the remaining barrels was filled with 0.25 M solution l-GLU monosodium salt dissolved in distilled water (pH 7.5). The resistance, measured at 100 Hz, was 3–5 MΩ for the recording barrel and 10–35 MΩ for the iontophoretic barrels. GLU was ejected by anionic currents (5–50 nA for 10 s), with a continuous retaining current of opposite polarity (8 to 10 nA), using constant current generators (Ion 100 and Ion 100T, Dagan, Minneapolis, MN). Each multibarrel pipette was filled with fresh solution less than one hour before use and fixed in a microdrive assembly that later was inserted into the skull-mounted hub. The electrode was then advanced 4.0 mm below the skull surface to the starting point of unit recording.
Neuronal discharge signals were sent to a head-mounted preamplifier (OPA 404KP, Burr Brown, Tucson, AZ) and then additionally amplified and filtered (band pass: 300–3,000 Hz) with a Neurolog System (Digitimer, Hertfordshire, UK). The filtered signal was then recorded using a Micro 1401 MK2 interface (Cambridge Electronic Design, Cambridge, UK). Spike activity was monitored with a digital oscilloscope and audio amplifier and analyzed using a Spike2 interface (Cambridge Electronic Design).
After isolating a signal-unit discharge and recording basal impulse activity, several stimuli were administered. These stimuli included: a 5-s touch of the tail by a wooden clothespin, a 5-s tail-pinch with a clothespin, and several 10-s GLU applications (5–50 nA). All stimuli were presented with at least a 60-s interval. In addition to these stimuli, rats were injected iv with either COC HCl (0.25–0.50 mg/kg in 0.1–0.2 ml saline), COC-methiodide (0.33–0.67 mg/kg in 0.1–0.2 ml saline), or saline. In each session only one drug was used and individual injections occurred with at least 7-min intervals. Typically, the rat received 2–6 drug injections within a session with much longer inter-injection intervals (a total cumulative dose of 0.6–2 mg/kg), except the cases when individual neurons were tested with several repeated drug injections. At these dose ranges, iv delivered COC is detected and self-administered by both rats and humans, and induces acute behavioral activation and cardio-vascular effects (Poon and van der Boose, 1998). A 7-min post-injection interval was chosen because this is an interval at which rats self-administer COC at 1 mg/kg (Kiyatkin and Stein, 1995).
Histology
After the last recording session, animals were anesthetized and Pontamine sky blue was deposited by current ejection (−20 μA for 20 min) at the last recording site. Rats were sacrificed and the brains placed in a formalin solution. Coronal 30μm tissue sections were prepared at −20°C using a microtome cryostat. The Paxinos and Watson atlas (Paxinos and Watson, 1998) served as the basis for histological analyses.
Data analysis
Impulse activity of individual striatal neurons was characterized by mean rate (X), standard deviation (SD) and coefficient of variation (CV) based on ten 1-s values of discharge rate preceding each drug injection accepted for analysis. These values were grouped together and further analyzed by using standard statistical procedures (i.e., mean and modal group values, variability, distributions). Since discharge rates of individual striatal units are distributed according to the ln-normal law (Kiyatkin and Rebec 1999a), ln-derivatives of X, SD and CV were used for statistical comparisons of impulse activity in the two recording conditions.
Responses to somato-sensory and drug stimuli were statistically analyzed both for each presentation and in a group. Individual analysis was based on differences in discharge rates between pre-stimulus and post-stimulus conditions. In the case of sensory stimuli, discharge rate was analyzed at 0.5 bins with 10 bins (i.e. 5 s) preceding and 60 bins (i.e. 30 s) following stimulus presentation. In the case of drugs, the analysis bin was 1 s with 10 s preceding and 60 s following each drug presentation. Because individual neurons tested with sensory and especially drug stimuli showed changes in activity that widely varied in latency, duration, amplitude and even in direction, a sign criterion (z, two or more consecutive values higher or lower than each of 10 values in pre-stimulus baseline) was used to recognize a unit response. Since this approach provides an approximate evaluation of response following a single presentation, one-way ANOVA with repeated measures (followed by Fisher post-hoc test) as well as two-way ANOVA were used for quantitative evaluation of the effects of stimuli and drugs in groups as well as between-group differences in effects. All neurons, independent of presence or absence of the response and the response direction (excitation or inhibition) were included in group analyses. To provide an equal contribution of each recorded unit in group analyses, only one test was included for evaluating the effects of each sensory stimuli and, if several drug injections were performed on individual neurons, changes in activity were averaged and then included as one data point to evaluate mean changes in activity.
Along with detecting phasic fluctuations in impulse activity, we also analyzed slow, tonic alterations in discharge rate of striatal neurons following iv drug injections. In this case, discharge rate was averaged for each subsequent 30-s interval with two data points before and 12 data points after the injection. This duration of analysis generally covers the time interval found between individual COC self-injections in behaving animals (Kiyatkin and Stein, 1995).
While most units were exposed to several GLU applications at the same or different currents (5–50 nA), statistical analysis of GLU effects was performed only at 20 nA and only one response per unit was used for group analyses. Discharge rates before (10 0.5-s values) and 11 s (22 0.5-s values) after the start of 10-s GLU applications were analyzed using one-way ANOVA with repeated measures.
Although dorsal and ventral striatum differ in their afferent inputs and efferent projections (Gerfen, 2004; Parent and Hazrati, 1995), for most analyses units recorded within both striatal sub-divisions were combined in one group for several reasons. They are both comprised primarily of morphologically similar medium spiny GABA-containing neurons, which have similar organization of impulse flow, similar responsiveness to iontophoretic GLU, GABA and DA, and similar responses to somato-sensory stimuli (Kiyakin and Rebec, 1996, 1999a). Although in an awake, unrestrained preparation, more ventral striatal neurons are spontaneously active and their discharge rate is slightly higher than in dorsal striatal neurons, most of them show similar phasic movement-related modulations in impulse activity, which were mimicked by brief GLU applications (Kiyatkin and Rebec, 1996; Rebec, 2006). Finally, neurons located in both striatal subdivisions showed similar modulations in activity during DA receptor blockade in awake animals and both have kept high sensitivity to somato-sensory stimuli and iontophoretic GLU in this condition compared to drug-free control (Kiyatkin and Rebec, 1999b).
Results
1. Unit sample Impulse activity of striatal neurons in two recording conditions
Data were obtained from 102 single striatal neurons, which were recorded in 11 animals over 2–3 daily sessions and histologically verified to be located within the striatum. Forty-three units were recorded in awake conditions (23 in dorsal and 20 in ventral striatum) and 59 units (34 in dorsal and 25 in ventral striatum) were recorded during anesthesia. The duration of recording varied from 9 to 115 min, with a similar mean time in awake and anesthetized conditions (34.6 and 35.5 min). Consistent with our previous work (Kiyatkin and Rebec, 1996, 1999b), striatal neurons had biphasic, short-duration (<2.0 ms) single spikes and slow, irregular impulse activity. Although many striatal neurons, especially within the caudo-putamen, appeared during pulsative GLU applications and these silent neurons could be excited by somato-sensory stimuli, our sample included only spontaneously active neurons, which maintained activity (mean rate >1 imp/s) during the entirety of recording.
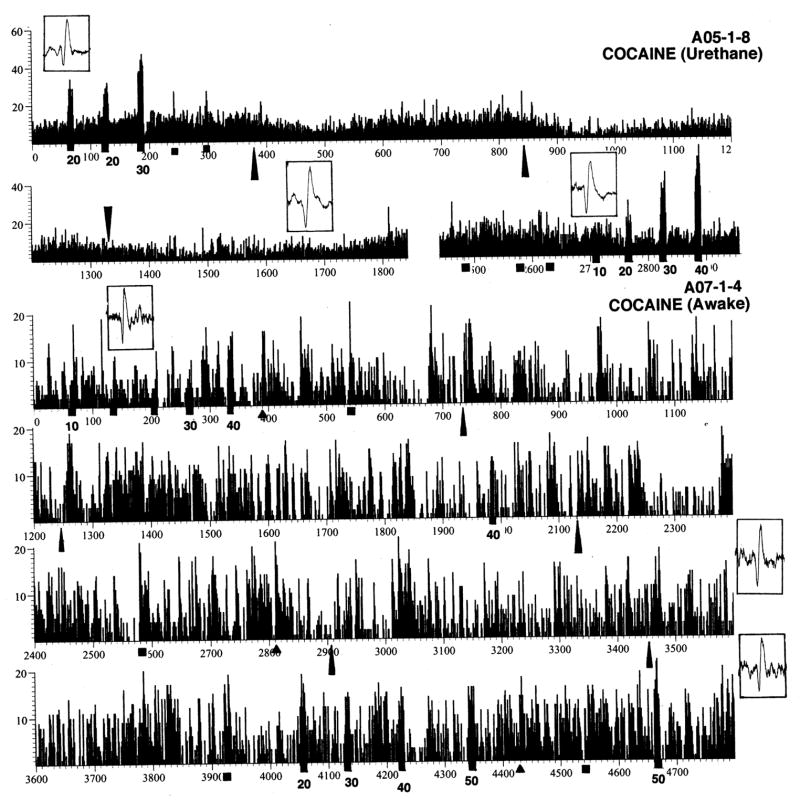
Figure 1 shows rate histograms of impulse activity of two ventral striatal neurons recorded for a relatively long time in awake (A07-1-4) and anesthetized conditions (A05-1-8). Each cell was exposed to a number of somato-sensory stimuli, several injections of COC, and GLU applications at different ejection currents. Each histogram also contains several pictures of single spikes, which show the stability and quality of recording during the session. While the activity of the first cell was unusually tonic, the second cell, like most striatal cells recorded in awake conditions, had a highly irregular activity with fluctuations in sec-to-sec rates from 0 to 20 imp/s. While this cell showed strong phasic excitatory responses to GLU, tail-touch, tail-pinch and several injections of COC, these responses were within the upper limits of spontaneous fluctuations in discharge rate and did not exceed 20 imp/s. GLU dose-dependently increased discharge rate of this cell, but even for the largest current used (50 nA at 4300 and 4670 s), the rate of GLU-induced activity was less ~20 imp/s. This record also demonstrates that, despite wide fluctuations in sec-to-sec values of discharge rate, the cell maintains a relatively stable but tonically fluctuating discharge rate during long-term recording despite periodic phasic excitations induced by sensory stimuli and COC.
Fig. 1.
Original examples of impulse activity of two ventral striatal neurons recorded in rats in anesthetized (A05-1-8) and awake (A07-1-4) conditions. These cells were exposed to somato-sensory stimuli (triangle=tail-touch, square=tail-pinch), iontophoretic ejections of GLU at different currents (black squares with numbers of iontophoretic current in nA) and iv injections of COC (long triangles). Insets show single spike waveforms (window is 5 ms) at different recording times.
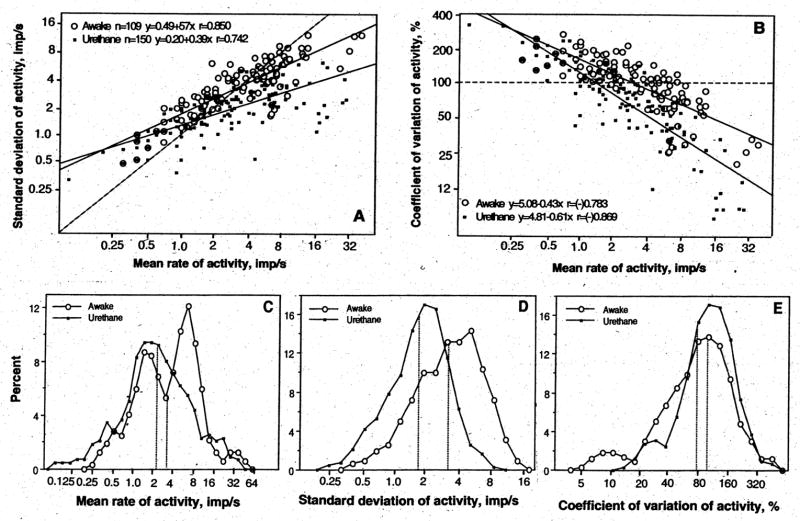
In both awake and anesthetized conditions, striatal neurons had slow, irregular impulse activity, with most cells discharging within 1–8 imp/s (Fig. 2A, C). As shown in Fig. 2A and B, major parameters of impulse activity of individual units (X, SD and CV) were distributed according to ln-normal law and closely interrelated in each condition. A higher discharge rate (X) was related to higher absolute variability (SD) and lower relative variability (CV) of impulse flow. There were, however, between-state differences in these parameters (Fig. 2C–E). In awake conditions, striatal cells as a group had significantly higher X (mean 5.35 imp/s, mode 3.32 imp/s, mean of ln[Xi]) 1.20±0.10) than during anesthesia (mean 4.10 imp/s, mode 2.25 imp/s, mean of ln[Xi] 0.81±0.10, p<0.05). Distribution analyses also revealed that striatal neurons in non-anesthetized conditions with DA receptor blockade represent a more heterogeneous population by discharge rate than in anesthetized condition (Fig. 2C). In addition to slow-firing neurons (mode 1–2 imp/s), which dominated during anesthesia, in awake rats there was a sub-group of cells with a higher discharge rate (mode ~7.4 imp/s). Maximal between-group differences were found by SD (Fig. 2D), with much higher discharge variability in awake (mean 3.91 imp/s, mode 3.22 imp/s, mean of ln[SDi] 1.17±0.17) than anesthetized conditions (mean 1.91 imp/s, mode 1.68 imp/s, ln[SDi] 0.52±0.05, p<0.001). Discharge rate in awake conditions was more irregular by CV (Fig. 1E), which is an index of relative discharge variability. These relative differences, however, were quantitatively smaller (mode 98.49% vs. 74.19%).
Fig. 2.
Impulse activity of striatal neurons in rats during urethane anesthesia and in awake state with DA receptor blockade. A and B show the relationships between major parameters of impulse activity. Each graph has a line of no effect (hatched), two regression lines that correspond to each condition, regression equations, and coefficients of correlations. C, D and E show distributions of major parameters of impulse activity in two conditions. Vertical hatched lines show mean values. For explanations see the text.
2. Phasic responses of striatal neurons to somato-sensory stimuli
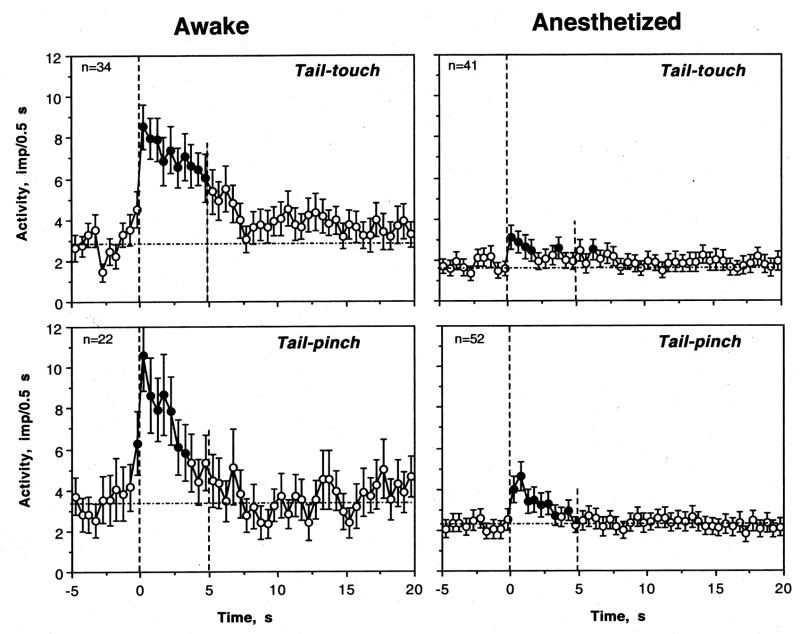
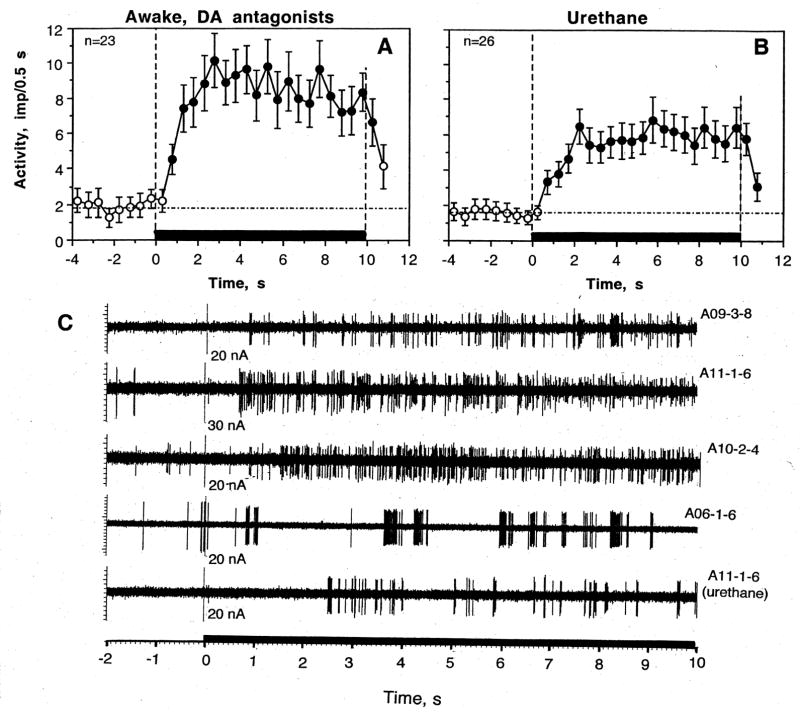
Most striatal neurons tested in awake conditions showed excitatory responses to somato-sensory stimuli (Fig. 3 and Table 1). Since the number of tests with the same stimulus varied for each neuron, there were differences in percent response. For example, tail-touch was used in 34 cells (88 tests) and 25 cells (or 73.5%) showed excitation based on evaluation of the first test. Among all tests, excitations were seen in 61 cases or in 69.3%. Other tests produced inhibitions (n=2) or no responses (n=25). Tail-pinch induced excitations in 17 of 22 tested cells (77.3%), or in 33 cases of all 46 tests (71.7%). No significant changes and inhibitions were seen in 11 and two tests, respectively. When changes in activity preceding and following stimuli presentation were superimposed for all tested cells (one, first test for each unit) and evaluated with one-way ANOVA with repeated measures, there was a highly significant effect of time for both stimuli (tail-touch: F32,1022=7.31 and tail-pinch: F20,650=6.52; p<0.001 for both cases, see Fig. 4). In both cases, the pattern of excitation was also similar with a rapid increase immediately following stimulus onset and a duration of about 4–5 s. In terms of magnitude, the excitations were also comparable, albeit slightly stronger and more phasic for tail-pinch. Interestingly, in awake conditions impulse activity also slightly increased for the last second before the stimulus onset, which reflects the animal’s response to sensory stimulation (hand movement of the experimenter) immediately preceding these tests. Following individual analysis of neurons with several stimulus presentations, we found that some cells showed a consistent excitation with several tests but in other cells significant excitation following one test showed no response (or not significant change) during the next test.
Fig. 3.
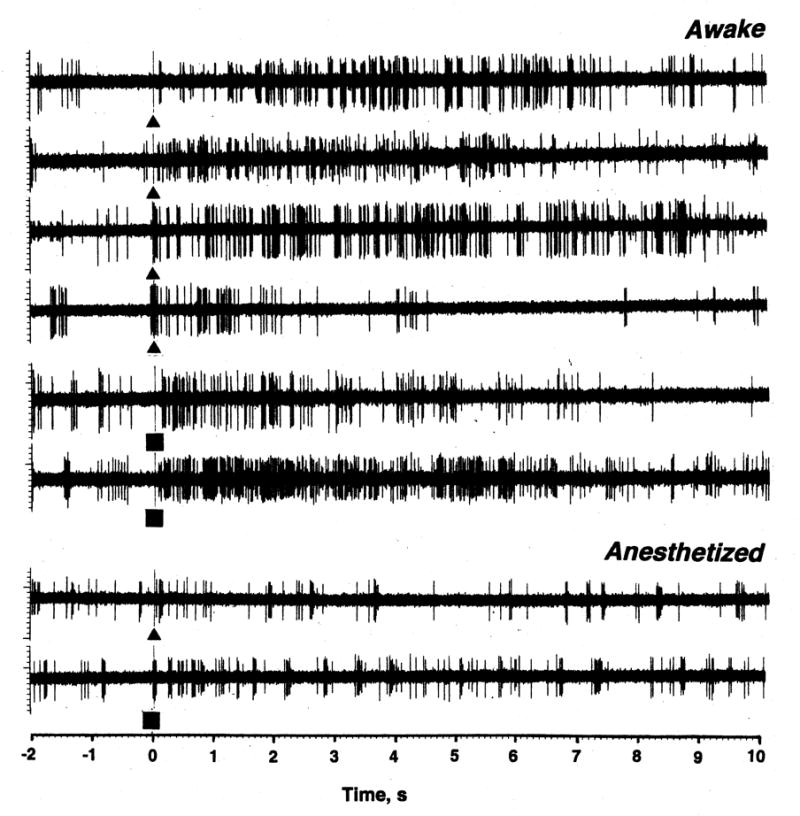
Original examples of changes in impulse activity of single striatal neurons following presentation of somato-sensory stimuli (triangle= tail-touch; and square=tail-pinch) in rats during urethane anesthesia and in awake state with DA receptor blockade. While the effects of stimuli were analyzed on a larger time scale, examples show activity for 2 s before and 10 s after the stimulus onset.
Fig. 4.
Changes in discharge rate (mean±standard error, imp/0.5 s) induced by tail-touch and tail-pinch in rats in rats during urethane anesthesia and in awake conditions with DA receptor blockade. In awake conditions, each stimulus had a significant, strong effect on discharge rate (touch: F32,1022=7.31, pinch: F 20,650=6.52; p<0.001 for each case). During anesthesia, the effect of time was also significant in each case but quite different in its strength (touch: F40,819=1.61, p=0.043, pinch: F51,1091=6.60; p<0.001). Filled symbols show values significantly higher (Fisher F test) than baseline. Because discharge rate increased immediately preceding stimulus onset, mean basal rate was calculated based on 5-s before stimulus presentation (shown as a hatched horizontal line). n is the number of tested units.
Dorsal and ventral striatal neurons showed virtually identical responses to both stimuli. With respect to tail-touch, no differences were found both in the number of excitations (15/21 and 10/13 for dorsal and ventral cells, respectively) and the strength of the effect (F19,619=4.84 and F12,402=3.74; both p<0.001). Similar responses were also found with respect to tail-pinch. Both in dorsal and ventral striatum most cells showed excitations (9/12 and 8/10, respectively) and the effect on discharge rate was equally strong (F11,371=4.50 and F9,309=3.81; p<0.001). These cells also had similar mean values of basal, pre-stimulus activity (3.57±0.27 and 2.89±0.26 imp/s for dorsal and ventral cells, respectively).
Responses to somato-sensory stimuli were strongly inhibited during urethane anesthesia (Table and Fig. 3 and 4). Although some cells showed weak responses to each stimulus, the numbers of excitations after both tail-touch and tail-pinch were significantly lower than those in awake conditions. Excitations following tail-touch during anesthesia were seen in 11/41 cells (26.8%, p<0.001 vs. control) or in 42/100 tests (42.0%, p<0.001 vs. control). Tail-pinch induced excitatory responses more often than tail-touch. These responses were seen in 24/52 cells (46.2%), or in 63/125 individual tests (50.4%). The differences with respect to awake conditions were significant by both measures. Similar differences were found for mean changes in discharge rate (Fig. 4). While the effect of time was significant with respect to both stimuli, for tail-pinch it was stronger (F51,1091=6.60, p<0.001) than that for tail-touch (F40,819=1.61; p=0.04). While significant, both these effects were lower than those seen in awake conditions. Despite a powerful decrease in response magnitude during anesthesia, the response pattern was similar, and neuronal activity significantly increased for 2–3 s after stimulus presentation.
Table.
Responses of striatal neurons to sensory stimuli and iv drug injections in rats in anesthetized and awake conditions during DA receptor blockade
| Awake, DA blockade | Urethane Anesthesia | |||||||
|---|---|---|---|---|---|---|---|---|
| Stimuli | + | NS | − | n | + | NS | − | n |
| Tail-touch | ||||||||
| cells (%) | 25 (73.5±7.6%) | 8 | 1 | 34 | 11 (26.8±6.9***) | 30 | 0 | 41 |
| tests (%) | 61 (69.3±4.9%) | 25 | 2 | 88 | 42 (42.0±4.9%***) | 57 | 1 | 100 |
| Tail-pinch | ||||||||
| cells (%) | 17 (77.3±8.9%) | 4 | 1 | 22 | 24 (46.1±6.9*) | 25 | 3 | 52 |
| tests (%) | 33 (71.7±6.6%) | 11 | 2 | 46 | 63 (50.4±4.5%**) | 56 | 6 | 125 |
| Cocaine HCl | ||||||||
| cells (%) | 14 (73.7±10.1%) | 5 | 0 | 19 | 5 (13.2±5.5***) | 33 | 0 | 38 |
| tests (%) | 32 (66.7±6.8%) | 15 | 1 | 48 | 24 (23.8±4.2%***) | 76 | 1 | 101 |
| Cocaine-M | ||||||||
| cells (%) | 9 (90.9±9.5%) | 1 | 0 | 10 | 4 (28.6±12.1**) | 9 | 1 | 14 |
| tests (%) | 24 (80.0±7.3%) | 6 | 0 | 30 | 5 (16.7±6.8%***) | 35 | 0 | 30 |
| Saline | ||||||||
| cells (%) | 3 (25.0±12.5%) | 9 | 0 | 12 | 3 (42.9±18.7) | 4 | 0 | 7 |
| tests (%) | 10 (31.3±8.2%) | 20 | 2 | 32 | 7 (36.8±11.1%) | 12 | 0 | 19 |
n, number of units and tests. +, − and NS are excitations, inhibitions and no responses. Asterisks show between-group differences in numbers of excitatory responses
p<0.01 and
p<0.001).
3. Phasic responses of striatal neurons to drug injections in awake conditions
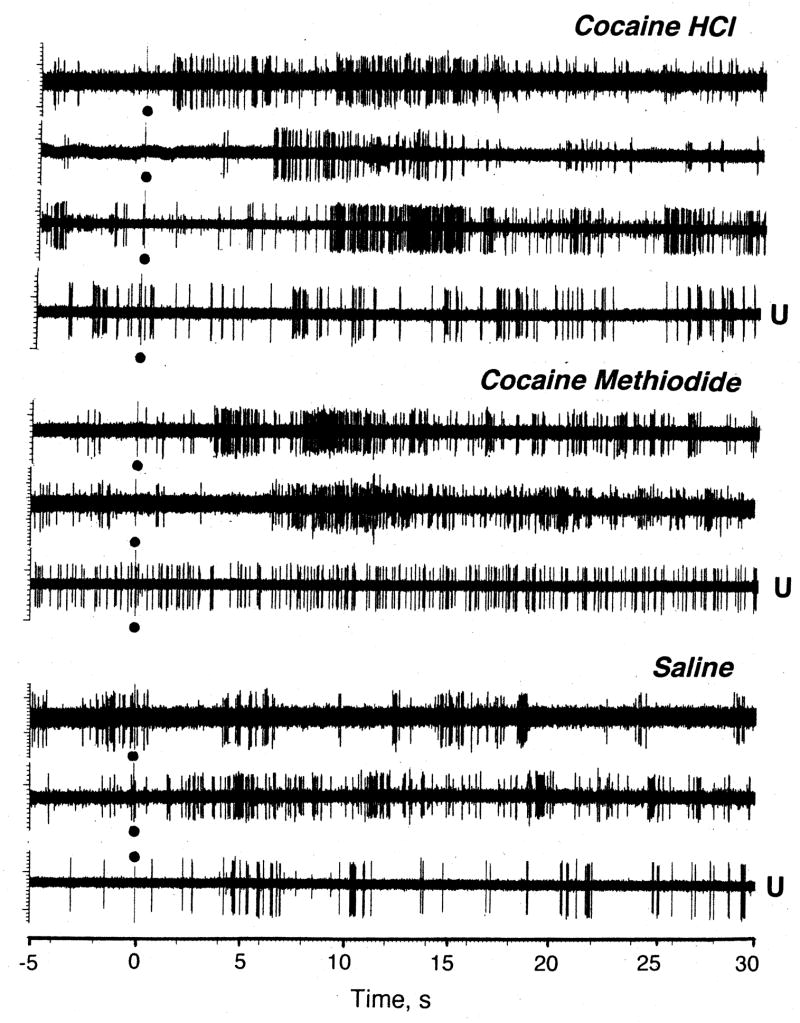
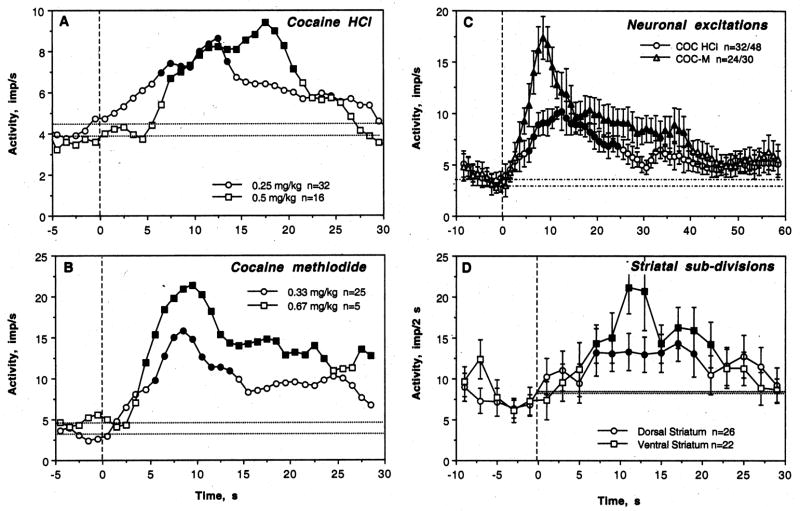
As shown in the Table and Fig. 5, COC induced significant neuronal excitations in most tested cells (14/19, or 73.7%) and in most tests (32/48, or 66.7%). These phasic excitations occurred with different latencies (2–12 s) but consistently within the duration of the injection. While some cells showed no phasic responses with one or several injections (5/22), phasic inhibition was seen only in one cell in one test. When changes in discharge rate preceding and following cocaine injection were averaged for all tested cells independent of the presence or absence of the response, its amplitude, or dose (0.25 and 0.5 mg/kg), the effect of time evaluated with ANOVA with repeated measures was significant (F18,588=2.24; p<0.001), with significant rate increases from 7 to 21 s after the injection start (Fig. 6).
Fig. 5.
Original examples of changes in impulse activity of single striatal neurons following iv injections of COC HCl, COC-methiodide and saline in rats during urethane anesthesia and in awake conditions with DA receptor blockade. While the effects of stimuli were analyzed on larger time scale, examples show activity for 5 s before and 30 s after the start of iv injection (black dots).
Fig. 6.
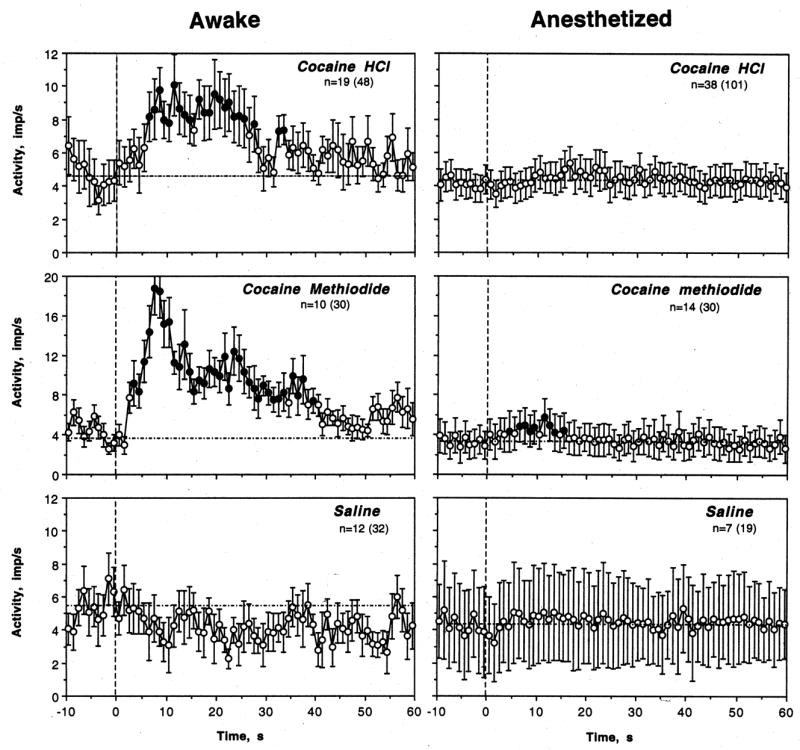
Changes in discharge rate (mean±standard error, imp/s) of striatal neurons following iv injections of cocaine HCl, cocaine methiodide and saline in rats during urethane anesthesia and in awake conditions with DA receptor blockade. n is the number of tested units (number of tests is shown in brackets). In awake conditions, the effect of time on discharge rate was significant for both COC HCl (F18,588=2.24; p<0.001) and COC-M (F9,309=4.21; p<0.001) but absent for saline (F11,371=0.83, p=0.72). In anesthetized conditions, the effect of time was significant for COC HCl (F37,1177=1.63, p<0.02) and COC-M (F13,433=2.39, p<0.01) but much smaller than in awake conditions. The effect was not significant for saline (F8,278=1.11, p=0.16). Filled symbols show values significantly higher than pre-injection baseline (mean for 10-s before the injection; hatched line).
COC-M also induced significant increases in discharge rate in most tested units (Table, Fig. 5 and 6). These effects were seen in 9/10 cells (90.0%) or in 24/30 (80.0%) tests. While some tests revealed no response, phasic inhibitions were never seen. The effect of this drug was stronger than that of COC HCl both by number of excitations (see Table 1), the strength of the effect (F9,309=4.21, p<0.0001 vs. F18,588=2.24 for cocaine HCl), its latency (3 vs. 7 s), and mean change in discharge rate (Fig. 6). While COC doubled neuronal discharge rate, it was nearly quadrupled by COC-M.
The effects of both COC and COC-M were dose-dependent, with stronger and more prolonged excitations at double vs. single doses (Fig. 7A and B). Despite a smaller sample tested with COC HCl at twice the normal dose, the effect of time evaluated with ANOVA was stronger (F15,495=3.06, p<0.001) than at a normal dose (F31,991=1.63, p<0.03). Similar dose-dependence was also seen with COC-M (Fig. 7B). Although an averaged response was stronger for COC-M than COC HCl, the differences were lower when only excitatory responses to each drug were averaged (Fig. 7C). In both cases discharge rate rapidly increased during the injection, peaking at 10 and 12 s. In the case of COC-M, the increase was more rapid, stronger in amplitude and longer in duration.
Fig. 7.
A and B. Dose-response relationships for the effects of cocaine HCl and COC-methiodide on discharge rate in awake rats during DA receptor blockade. Each graph shows mean changes in discharge rate (imp/s) after injection of either COC (A) or COC-M (B) at a single (0.25 and 0.33 mg/kg) or double dose (0.5 and 0.67 mg/kg). For clarity, data are shown without standard errors. n depicts the numbers of tests in each group. The effect of COC HCl was significant for both doses, but stronger at higher (F15,495=3.06, p<0.001) than lower dose (F31,991=1.63, p <0.03). The effect of COC-M was significant for both doses (0.33 mg/kg: F24,774=4.72, p<0.01; 0.67 mg/kg: F4,309=2.49, p<0.001), but the amplitude of rate increase was higher at larger dose. Values statistically different from baseline are shown as filled symbols. C. Time-course of neuronal excitations induced by COC and COC-M in awake rats during DA receptor blockade. In this case, only excitations were averaged and shown as mean changes for 10 s before and 60 s after the injection start. Filled symbols show values significantly different from baseline. D. Differences in the pattern of phasic excitation induced by COC in dorsal and ventral striatal neurons in awake rats during DA receptor blockade. To decrease variability, data are shown for each subsequent 2-s intervals. While the effect of cocaine on discharge rate was significant in both striatal sub-divisions, it was quantitatively stronger in the NAcc (F21,351=3.12, p<0.001) than in the caudo-putamen (F25,415=1.91, p=0.02).
While saline injections usually have no effects on striatal neurons and the effect of saline injection on discharge rate was not significant as a group mean (Fig. 5 and 6; F11,371=0.83, p=0.72), some tests revealed significant excitations (see original examples in Fig. 4). Such responses were seen in 3/12 (25.0%) of cells and in 10/32 tests (31.3%). The difference with respect to both drugs was significant for both conditions (see Table). In contrast to drug-induced changes, these phasic excitations were much shorter and more variable than those induced by drugs and they did not result in any changes in activity rate in averaged group means.
There was a propensity for single striatal neurons activated by somato-sensory stimuli to also be activated by drugs. Nine of 10 cells excited by tail-touch and/or tail-pinch were also excited by COC-M. Eleven of 16 cells excited by these sensory stimuli were also excited by COC HCl; the other five units showed no responses during individual analyses.
To evaluate possible structural specificity of phasic neuronal effects of COC, analysis was performed separately for dorsal and ventral striatal neurons. Although the number of neuronal excitations was similar in dorsal (16/26 or 61.5%) and ventral striatum (16/22 or 72.7%), ventral striatal neurons showed a much stronger excitatory effect to COC (F21,351=3.12, p<0.001) than dorsal striatal neurons (F25,415=1.91, p=0.02). Despite a larger increase in discharge rate, the time-course of change was similar in both groups (Fig. 7D).
4. Phasic responses of striatal neurons to drug injections during urethane anesthesia
Although some neurons showed small increases in discharge rate following injections of both COC salts during urethane anesthesia (see original examples in Fig. 5), the effects were marginal and drastically weaker than in awake conditions (see Table 2, Fig. 6). Significant excitations following COC injections during anesthesia were found in 11/41 cells (26.8%) and 42/100 tests (42.0%), i.e. significantly more rare than in awake conditions (p<0.001 for both number of cells and units). Although the effect of COC on discharge rate during anesthesia was significant (F37,1177=1.63, p<0.02), it was much weaker than that for awake conditions and there were no post-COC values that were higher than baseline. Even stronger differences were found for COC-M, which induced weak excitatory responses in 4/14 cells (28.6%; p<0.01 vs. control) or in 5 of 30 tests (16.7%; p<0.001 vs. awake conditions). Although the effect was significant in the group (F13,433=2.39, p<0.001), it was much weaker than that in awake conditions. Between-state differences were significant for both COC salts as evaluated by the number of excitatory responses (see Table) and mean change in discharge rate (see Fig. 6). Similar to awake conditions, transient and variable excitations were seen in some cells (3/7 or 42.9%) and some individual tests (7/19 tests or 36.8%) following saline injections. Similar to awake conditions, these effects, if they occurred, were transient and did not result in significant change in discharge rate during group analysis (F8,278=1.11, p=0.16; see Fig. 6).
Tonic responses of striatal neurons to drug challenges
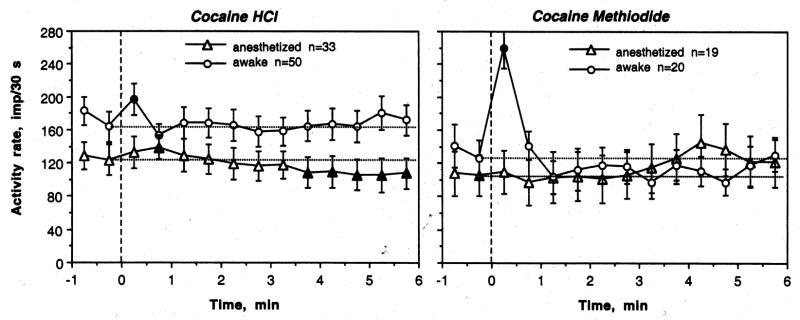
Fig. 8 shows tonic changes in striatal discharge rate induced by COC and COC-M in two conditions. In this case, discharge rates were calculated for each subsequent 30-s interval and shown for 1 min before and 6-min after drug administration. As can be seen, COC had a transient excitatory effect with no subsequent changes in awake conditions (F31,543=1.31, p=0.19), but during anesthesia induced a biphasic effect (F24,574=5.44, p<0.001), with a weak increase in discharge rate followed by its prolonged decrease (see also cell A05-1-8 in Fig. 1). COC-M in awake conditions only had a transient excitatory effect with no subsequent changes in discharge rate (F18,246=6.77, p<0.001). During anesthesia, this drug did not affect striatal discharge rate at all (F23,311=0.91, p=0.53).
Fig. 8.
Tonic (slow) changes in impulse activity (mean for 30 s ± standard errors) of striatal neurons following iv administration of COC and COC-M in rats during urethane anesthesia and in awake conditions with DA receptor blockade awake. n is number of units analyzed. The effect of time on discharge rate for COC: urethane: F49,549=4.36, p<0.001; awake: F31,543=1.31, p=0.19; for COC-M: urethane: F23,311=0.91, p=0.53; awake: F18,246=6.77, p<0.001.
When the effects of COC on tonic discharge rate were compared for single (0.25 mg/kg) and double (0.5 mg/kg) doses, the effect was stronger for the higher (F15,207=3.25; p<0.001) than lower (F32,428=1.87, p<0.05) dose. At the higher dose, both the initial excitation and especially subsequent inhibition were greater than those at the lower dose (data not shown).
5. Responses of striatal neurons to iontophoretic GLU
Most cells tested with somato-sensory stimuli and either COC form, were also tested with GLU, typically showing excitatory responses (Fig. 9). Consistent with our previous studies (Kiyatkin and Rebec 1996, 1999b), GLU-induced excitations both in awake and anesthetized conditions occurred with short latencies, had equally short after-effects and were current- (dose-) dependent; these responses were similar to those induced by stimuli and both forms of COC (Fig. 9C). Fig. 9A and B shows mean changes in discharge rate induced by GLU at 20 nA in both preparations (one response in 26 and 23 units recorded in anesthetized and awake preparations, respectively). In both cases, excitation had 1–2 s onset latency, grew over the next several seconds, remained stable following iontophoresis, and rapidly disappeared after the ejection current was switched off. Although in unit samples basal discharge rates were comparable in both conditions (3.70 vs. 3.14 imp/s), the magnitude of GLU-induced excitation in awake conditions (16.37±0.70 imp/s) was significantly larger than that during anesthesia (11.17±0.51 imp/s; p<0.05)
Fig. 9.
Changes in discharge rate (A and B- mean values for 0.5 s±standard errors and C – original examples) induced by iontophoretic application of glutamate (20 nA) in rats during urethane anesthesia and in awake conditions with DA receptor blockade. n is the number of cells (=applications). The effect of time was significant and similar for both conditions (F24,574=5.44 and F22,528=4.55, p<0.001), but its amplitude was significantly larger in awake than anesthetized conditions (see text). As shown in C, GLU in each neuron induced excitation, which developed with 1–2 s latencies. This excitation manifested as increased discharge rate, often accompanied by increased bursting.
Discussion
COC self-administration has been the focus of many multi-wire electrophysiological studies (Carelli and Deadwyler, 1994; Chang et al., 1994; Peoples and West, 1996; Peoples et al., 2007), but none have detailed neuronal effects induced by passive drug delivery to naive animals. Though important from a behavioral point of view, the self-administration paradigm, without necessary controls in drug-naive and trained animals, makes it difficult to separate pharmacological influences from behavioral and learning contributions. Such differing contributions are likely to exist. For example, although phasic modulations in activity that are tightly linked to individual COC-reinforced lever-presses are evident in most ventral striatal neurons, and these units showed phasic excitations following sensory stimuli paired with drug self-injections, passive COC injections made in trained animals do not significantly affect impulse activity of these cells (Carelli, 2000). Although the effects of single COC injections in drug-naive conditions were studied in anesthetized animals, this work was usually focused on slow, tonic modulations of impulse activity, specific structures (i.e., monoamine-containing cells), or modulating effects of this drug on neuronal responses to sensory stimuli or monoamines.
To our knowledge, no studies have detailed immediate neuronal effects of acute iv COC injections either in awake or anesthetized conditions and none were focused on striatal neurons, which appear to play an essential role in somato-sensory integration, psychomotor stimulant effects of COC, and regulation of drug-taking behavior. This study appears to be the first in which the effects of iv COC on striatal neurons were compared in an awake state and during general anesthesia. To evaluate the role of peripheral neural mechanisms in triggering central effects of COC, we also tested the effects of COC-M, a derivative that is retained by the blood-brain barrier and cannot reach brain receptor sites that are normally affected by COC. Although both COC forms appear to have similar effects on monoamine transporters and ionic channels, detailed studies of their quantitative differences are currently absent. COC-M in this study was used in equimolar doses to COC, but it remains unknown whether this dosage accurately mimics the action of traditional COC HCl on various neural elements. The effects of both forms of COC were compared in this study with striatal neuronal responses elicited by putative somato-sensory stimuli. Although detailed evaluation of neuronal responsiveness in awake and anesthetized states has its own value, this aspect had a special importance for the goals of this study, allowing a comparison of responses that are triggered via activation of cutaneous sensory nerves, involving somatic sensory pathways, with those that are triggered by drugs, presumably involving venous terminals of visceral sensory nerves. Finally, we complemented our study with GLU iontophoresis, allowing a comparison of responsiveness of striatal neurons resulting from peripheral triggering of sensory afferents and local GLU application. Indirect evidence suggests that phasic excitations of striatal neurons induced by somato-sensory stimuli and occurring during movement results from phasic GLU release from cortico-striatal and thamo-striatal afferents (see Rebec, 2006 for review), and iontophoresis allowed us to model this process by direct activation of this final pathway.
State-dependency of neuronal activity and responsiveness
The present study revealed that iv COC injection induces rapid (latency of 2–12 s) and transient (~20 s) excitations in the majority of striatal neurons in awake conditions. This effect was virtually absent during urethane anesthesia. This state-dependent effect of COC was mimicked by peripherally acting COC-M and somato-sensory stimuli, which induced phasic excitations of striatal neurons more often and of much stronger magnitude in awake than anesthetized conditions (see Fig. 3 and 5). Unexpectedly, neuronal excitations induced by iontophoretic GLU at the same small currents were also stronger in awake than anesthetized animals. Importantly, these profound between-state differences in neuronal responsiveness were not related to basal discharge rates. Most striatal neurons in awake and anesthetized animals had low and irregular activity, though it was much more irregular in awake than anesthetized conditions. Mean discharge rate during anesthesia was slightly lower than in the awake state, but this subtle difference cannot explain the robust between-state differences in neuronal responsiveness. Therefore, it appears that the awake animal preparation is better for studying natural activity and responsiveness of central neurons to somato-sensory and drug challenges than the anesthetized preparation.
Although urethane, compared to other general anesthetics, has weaker inhibiting effects on neuronal responsiveness and most striatal neurons in our sample still showed weak excitations following tail-touch and tail-pinch during urethane anesthesia, the phasic responses to both forms of COC, which were clearly evident in awake conditions could not be detected during anesthesia. Both chloral hydrate and pentobarbital anesthesia also significantly attenuated or blocked cortical EEG desynchronization (Chang et al. 1996) and transient increases in sympathetic nerve discharges (Abrahams et al. 1996), other rapid, centrally mediated effects of iv COC seen in awake rats. The latter effect in splanchnic nerve discharges, suggesting transient sympathoexcitation, matched temporally the COC-induced activation of striatal neurons in this study, suggesting a possible common cause.
To eliminate the possible contribution of DA mechanisms to neuronal responses and provide better conditions for artifact-free recording of striatal neuronal activity following somato-sensory stimuli and COC, we used an awake rat treated with DA antagonists. Although rats administered a mixture of D1- and D2-selective DA antagonists were hypoactive and showed greatly diminished motor responses to sensory stimuli and COC, they were awake and usually vocalized to tail-touch and, especially, tail-pinch. These data are generally consistent with our early work that showed haloperidol, a mixed D1- and D-2 antagonist, greatly reduces motor responses to noxious stimuli, while slightly potentiating affective (vocalization) responses to the same stimuli (Kiyatkin, 1989). Despite hypoactivity, striatal neurons during DA receptor blockade in awake, unrestrained rats had impulse activity similar to (but with slightly higher rates) drug-free animals and showed virtually identical neuronal responses to somato-sensory stimuli and iontophoretic GLU (Kiyatkin and Rebec, 1999b). Therefore, although DA receptor blockade diminishes behavioral responses to arousing stimuli and COC, it keeps neuronal responses to these stimuli and major afferent inputs generally intact, making it unlikely to influence significantly our primary results. In contrast, motor activity elicited by stimuli may provide additional contributions to striatal activity because most striatal neurons show phasic motor-related excitations either preceding or following the initiation of motor acts (see Rebec, 2006 for review).
Mechanisms mediating phasic excitations of striatal neurons induced by iv cocaine
COC-induced excitations of striatal neurons seen in awake conditions occurred too quickly to reflect direct central action. Although COC may enter brain arterial vessels 10–20 s after the start of iv injection, more time is necessary to cross the blood-brain barrier, passively diffuse to specific neural substrates and interact with them. Neuronal excitations, moreover, were not only rapid, but brief, which is inconsistent with the slower and more prolonged increases in brain cocaine levels evaluated by PET (Fowler et al. 1998). These phasic neuronal excitations do not involve DAergic transmission, since they were observed during full DA receptor blockade, and occurred too fast to reflect cocaine’s action on other monoamine systems. Moreover, transient neuronal excitations are inconsistent with the time-course of monoamine uptake inhibition, which is much slower, more prolonged, and may lead to monoamine accumulation, likely resulting in prolonged neuronal inhibition. Such an effect was seen here after COC injection during anesthesia (see Fig. 8).
Although the short onset of neuronal excitations implies a peripheral trigger, this mechanism was confirmed by COC-M. While this COC derivative cannot cross the blood-brain barrier, like regular COC, it resulted in rapid, transient neuronal excitations in most tested cells. At equimolar doses, the COC-M-induced phasic excitations were even at higher amplidues and frequencies than those induced by COC HCl. Therefore, it appears that rapid excitations of striatal neurons induced by iv COC are triggered via its interaction with peripheral neural elements.
Although tail-touch and tail-pinch activate partially different receptor pools, information from the somato-sensory periphery is rapidly transmitted via the same neural substrates, involving sensory nerves, dorsal root ganglions and the spinothalamocortical system (Hendry et al. 1999). In each case, stimulation results in phasic activation of GLU-containing thalamic and cortical cells, which provide the major excitatory input to striatal neurons. The similarity of neuronal responses induced by somato-sensory stimuli and both forms of COC suggests that they share a common mechanism involving rapid neural transmission and phasic GLU release as a final event underlying striatal excitations. While voltage-gated Na+ and K+ channels are densely expressed on terminals of sensory nerves that innervate peripheral vessels (Lee et al. 2005) and these channels are directly affected by COC (Catterall and Mackie 1996; Gifford and Johnson 1992), the precise mechanisms of how iv COC interacts with vascular receptors and triggers the ascending excitatory drive remain unclear. It is known, however, that direct activation of these vascular receptors induces rapid excitatory cortical responses that occur via the thalamocortical pathways similar to those involved in the transmission of cutaneous sensations (Ploner et al. 2002). Although the ability of procaine to mimic central excitatory effects of COC, as evaluated by brain temperature increases (Kiyatkin and Brown, 2006), supports the involvement of Na+ channels, its acute transient inhibition cannot trigger excitatation. Both COC and procaine also bind to various subtypes of K+ channels (Premkumar, 2005; Wu et al., 2006), and direct interaction with these channels may be responsible for ascending excitatory drive. A recent study, moreover, revealed that COC in fact elicits action potential bursts in snail neurons via the blocking action on K+ channels, which is independent of drug action on neurotransmitters but involves Na+ channels (Chen et al., 2006). In contrast to the relatively slowly developing and prolonged action on Na+ channels, blockade of K+ channels appeared very quickly, at very low COC concentrations (ED50=5–7 μM) and had a rapid recovery, suggesting ultra-rapid kinetics (Ferreira et al., 2001; Zhang et al., 2001).
While K+ channels on venous sensory afferents may be a primary candidate for triggering ascending excitatory signals to the CNS, because of the multiple channels (i.e., Na+, K+, TRP, Ca++) and subtypes that are expressed on terminals of visceral sensory nerves and may be directly affected by COC, special analytical studies using other techniques are necessary to delineate the role of individual channel subtypes.
While the exact mechanisms of how COC may activate vascular afferents of sensory nerves remain unknown, this action manifests as unusual sensory feelings in humans and transient arousal in animals following administration of COC and other local anesthetic drugs. Both humans and animals detect COC as a sensory stimulus following subcutaneous and iv injections and, although the subjective effects of iv procaine in drug-naive individuals differ from those of cocaine, they are equally rapid and powerful, combining euphoria, anxiety, depression, and fear, as well as strong sensory and somatic sensations (Servan-Shreiber et al. 1998). In contrast to drug-naive conditions, procaine it is not differentiated from COC in experienced drug users (Adinoff et al. 1998) and is self-injected in COC-experienced animals (Johanson 1980; Kiyatkin and Stein 1995). While our present study was limited to striatal neurons, it is reasonable to assume that iv COC, by involving the same spinothalamocortical activation mechanism, would mimic somato-sensory stimuli in their ability to induce generalized neuronal activation, which can be detected in different central neurons (i.e., spinal, thalamic, cortical, etc.). Therefore, it is likely that other central neurons that are phasically activated by somato-sensory stimuli will also be activated by iv COC.
Rapid peripheral action of COC that involves K+, Na+ and possibly TRP channels resulting in activation of visceral sensory nerves is not limited to iv COC administration only. These channels are present on terminals of sensory nerves that innervate the nasal (Domann et al., 2006) and intraperitoneal (Lee et al., 2005) cavities—other common sites for COC administration in humans and animals, respectively.
Functional implications
By providing a rapid, neurally mediated signal and triggering transient neural activation, iv COC may display its acute sensory properties. While experience and conditioning may alter the acute sensory and emotional effects of any addictive drug (Bozarth and Wise 1987), rapid, transient neural activation appears to be related to acute COC euphoria (crash, rush)—the rapid, powerful, but transient psycho-emotional experience (or sensation) occurring immediately after iv drug intake or inhalation (Zernig et al. 2003). Although DA antagonists greatly attenuate COC-induced locomotion (Kiyatkin and Brown 2005) and block COC self-administration (Bozarth and Wise 1987; Ettenberg et al. 1982), suggesting the importance of DA in drug-taking behavior, DA receptor blockade fails to block these acute subjective effects of COC in experienced drug users (Gawin 1986; Sherer et al. 1989). Although procaine has only one-hundredth the affinity that COC has for the DA transporter (Ritz et al. 1987), in experienced users its iv administration fully mimics the initial subjective effects of iv COC (Adinoff et al. 1998; Fischman and Schuster 1983). In addition to sensory and affective actions, iv procaine also induces an acute, transient hypertensive response (Pitts et al., 1987) associated with peripheral vasoconstriction (Brown and Kiyatkin, 2006), although acting topically it has an opposite, weak vasodilatative action (Lindoft, 1979; Willats and Reynolds, 1985).
By affecting various central neurons involved in the processing of somato-sensory information and organization of brain activational processes, this rapid and brief “indirect” action of COC can modulate its slow and prolonged “direct” actions on monoamine uptake. Although in vitro studies suggest that uptake inhibition is the primary action of COC (Heikkela et al. 1975), after iv administration COC may affect monoamine release via indirect, sensory mechanisms before it reaches the brain. It is known that the activity of central neurons containing DA, norephinephrine and serotonin are modulated by sensory stimuli, producing in most cases phasic neuronal excitations (Aston-Jones and Bloom, 1981; Dahan et al., 2007; Heym et al., 1982; Steinfels et al., 1983). For example, most presumed DA VTA neurons recorded in awake rats change their activity rate or pattern (preferentially increase) following presentation of both simple sensory (light, sound) and aversive (tail-pinch and noxious tail-prick) stimuli, showing a tight correlation with stimuli-induced motor (EMG) and autonomic (arterial blood pressure) responses (Kiyatkin, 1988). If, like other salient sensory stimuli, iv COC phasically excites DA neurons, thus providing an impulse-dependent DA release, then this release may be potentiated by a later, prolonged drug action on DA uptake, altering synaptic DA levels. As evaluated by cyclic voltammetry coupled with iontophoretic DA delivery in awake rats, inhibition of DA uptake in the striatum occurs with some latency (~1–1.5 min), peaks at 7–8 min, and disappears at ~20 min after a single 1 mg/kg COC injection (Kiyatkin et al., 2000; see, however, Mateo et al., 2004 for alternative data). While rapid compared to other uptake inhibitors, reliable interaction with DA uptake sites (evaluated by displacement of [3H]WIN32524 binding) was only found starting two minutes after iv COC injection in mice despite a much higher drug dose (7 mg/kg; Pogun et al., 1991). Therefore, COC’s effects on DA transmission may be determined by at least two different and independent actions, which may interact with each other.
Although such a transient excitatory effect of iv cocaine on DA cell activity has never been reported, there were no studies in which the immediate effects of this drug were tested in awake animals. This factor appears to be very important because general anesthesia may dramatically alter neuronal responses to the drug. For example, amphetamine, a related psychostimulant drug, consistently inhibited striatal neurons during anesthesia, but typically excited them after administration to awake, unrestrained animals (see Rebec, 2006 for review). Our current data, showing a strong reduction or disappearance of rapid striatal responses to COC and sensory stimuli under urethane anesthesia, strongly support this view. In anesthetized animals, iv COC preferentially decreases discharge rate of presumed DA VTA neurons, with the effect appearing within the first one-two min and continuing up to 10–20 min (Einhorn et al., 1988; Pitts and Marwah, 1987). This effect may reflect COC-induced DA uptake inhibition, accumulation of synaptic DA, and its action on DA autoreceptors. In fact, DA levels in ventral striatum rapidly (~20–30 s) increase after iv COC delivery in awake, drug-naive rats (Heien et al., 2005). Because DA dose-dependently decreases striatal neuronal activity (Kiyatkin and Rebec, 1996), a slow, moderate and prolonged inhibition of striatal neurons induced by iv COC in anesthetized animals (Fig. 8), in which DA antagonists were not used, may result from its direct action on DA reuptake. This mechanism is supported by the absence of this effect in awake animals during DA receptor blockade and with peripherally acting COC-M in both preparations.
The interaction of two independent and temporally separated actions converging on the same neural substrates may play an important role in COC reinforcement. While usually envisioned as an interaction between the drug’s pharmacological actions and associated sensory stimuli or voluntary movement acts, our data suggest that COC itself can provide its own pharmacologically mediated sensory signal, which may interact with other drug actions. At neuronal and neurochemical levels, this is an interaction between rapid, generalized, but transient neuronal activation and subsequent slow, more prolonged pharmacological actions mediated via direct drug action on brain substrates. While cellular mechanisms underlying these interactions following repeated drug use remain unclear, they may play important roles in the development of drug-seeking behavior, the enhancement of both the psycho-physiological experience, and behavioral effects induced by repeated COC administration. This does not exclude the role external stimuli play in development of self-administration behavior. Our data, however, point out the crucial role that COC’s sensory effects might play in reinforcement and their importance in the development and maintenance of drug-seeking and drug-taking behavior.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, NIDA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrahams TP, Cuntapay M, Varner KJ. Sympathetic nerve responses elicited by cocaine in anesthetized and conscious rats. Physiol Behav. 1996;59:109–115. doi: 10.1016/0031-9384(95)02075-6. [DOI] [PubMed] [Google Scholar]
- Adamec RE, Stark-Adamec C. The effects of procaine HCl on population cellular and evoked response activity within the limbic system of the cat. Evidence for differential excitatory action of procaine in a variety of limbic circuits. Prog Neuropsychoparmacol Biol Psychiatry. 1987;11:345–364. doi: 10.1016/0278-5846(87)90012-1. [DOI] [PubMed] [Google Scholar]
- Adinoff B, Brady K, Sonne S, Mirabella RF, Kellner CH. Cocaine-like effects of intravenous procaine in cocaine addicts. Addiction Biol. 1998;3:189–196. doi: 10.1080/13556219872245. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE. Norepinephrine-containing locus coeruleus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. J Neurosci. 1981;1:887–900. doi: 10.1523/JNEUROSCI.01-08-00887.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PL, Kiyatkin EA. The role of peripheral Na+ channels in triggering the central excitatory effects of intravenous cocaine. Eur J Neurosci. 2006;24:1182–1192. doi: 10.1111/j.1460-9568.2006.05001.x. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Leung LW, Vanderwolf CH. Cellular bases of hippocampal EEG in the behaving rat. Brain Res. 1983;287:139–171. doi: 10.1016/0165-0173(83)90037-1. [DOI] [PubMed] [Google Scholar]
- Carelli RM. Activation of accumbens cell firing by stimuli associated with cocaine delivery during self-administration. Synapse. 2000;25:238–242. doi: 10.1002/(SICI)1098-2396(20000301)35:3<238::AID-SYN10>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Carelli RM, Deadwyler SA. A comparison of nucleus accumbens neuronal firing patterns during cocaine self-administration and water reinforcement in rats. J Neurosci. 1994;14:7735–7746. doi: 10.1523/JNEUROSCI.14-12-07735.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W, Mackie K. Local anesthetics. In: Hardman JC, Limbird LE, editors. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. Vol. 9. NY: McGraw; 1996. pp. 331–347. [Google Scholar]
- Chang JY, Sawyer SF, Lee RS, Woodward DJ. Electrophysiological and pharmacological evidence for the role of the nucleus accumbens in cocaine self-asdministration in freely moving rats. J Neurosci. 1994;14:1224–1244. doi: 10.1523/JNEUROSCI.14-03-01224.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang AY, Kuo TB, Chan JY, Chan SH. Concurrent elicitation of electroencephalographic desynchronization and penile erection by cocaine in the rat. Synapse. 1996;24:233–239. doi: 10.1002/(SICI)1098-2396(199611)24:3<233::AID-SYN5>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Chen YH, Lin CH, Lin PL, Tsai MC. Cocaine elicits action potential burst in a central snail neuron: the role of delayed rectifying K+ channels. Neuroscience. 2006;138:257–280. doi: 10.1016/j.neuroscience.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Dahan L, Astier B, Vautrelle N, Urbain N, Kocsis B, Chouvet G. Prominent burst firing of dopaminergic neurins in the ventral tegmental are during paradoxical sleep. Neuropsychopharmacology. 2007;32:1232–1241. doi: 10.1038/sj.npp.1301251. [DOI] [PubMed] [Google Scholar]
- Damann N, Rothermel M, Klupp BG, Mettenleiter TC, Hatt H, Wetzel CH. Chemosensory properties of murine nasal and cutaneous trigemical neurons identified by viral tracing. MBC Neurosci. 2006;7:7–46. doi: 10.1186/1471-2202-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson LW, Rodak DJ, Kuhn FE, Wahlstrom SK, Tessel RE, Visner MS, Schaer GL, Gillis RA. Cocaine-induced cardiovascular effects: Lack of evidence for a central nervous system site of action based on hemodynamic studies with cocaine methiodide. J Cardiovasc Pharmacol. 1999;33:36–42. doi: 10.1097/00005344-199901000-00006. [DOI] [PubMed] [Google Scholar]
- Einhorn LC, Johansen PA, White FJ. Electrophysiological effects of cocaine in the mesoaccumbens dopamine system: studies in the ventral tegmental area. J Neurosci. 1988;8:100–112. doi: 10.1523/JNEUROSCI.08-01-00100.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenberg A, Pettit HO, Bloom FE, Koob GF. Heroin and cocaine intravenous self-administration in rats: mediation by separate neural systems. Psychopharmacology. 1982;78:204–209. doi: 10.1007/BF00428151. [DOI] [PubMed] [Google Scholar]
- Ferreira S, Crumb WJ, Carlton CG, Clarkson CW. Effects of cocaine and its major metabolites on the HERG-encoded potassium channel. J Pharmacol Exp Ther. 2001;299:220–226. [PubMed] [Google Scholar]
- Fischman MW, Schuster CR. A comparison of the subjective and cardiovascular effects of cocaine and procaine in humans. Pharmacol Biochem Behav. 1983;18:711–716. doi: 10.1016/0091-3057(83)90011-4. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Logan J, Gatley SJ, Pappas N, King P, Ding Y-S, Wang G-J. Measuring dopamine transporter occupancy by cocaine in vitro: radiotracer considerations. Synapse. 1998;28:111–116. doi: 10.1002/(SICI)1098-2396(199802)28:2<111::AID-SYN1>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Friedberg MH, Lee SM, Ebner FF. Modulation of receptive field properties of thalamic somatosensory neurons by the depth of anesthesia. J Neurophysiol. 1999;81:2243–2252. doi: 10.1152/jn.1999.81.5.2243. [DOI] [PubMed] [Google Scholar]
- Gawin F. Neuroleptic reduction of cocaine-induced paranoia but not euphoria? Psychopharmacology. 1986;90:142–143. doi: 10.1007/BF00172886. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. Basal ganglia. In: Paxinos G, editor. The Rat Nervous System. Amsterdam: Elsevier; 2004. pp. 455–508. [Google Scholar]
- Gifford AN, Johnson KM. Comparison of the role of local anesthetic properties with dopamine uptake blockade in the inhibition of striatal and nucleus accumbens [3H]acetylcholine release by cocaine. J Pharmacol Exp Ther. 1992;263:757–761. [PubMed] [Google Scholar]
- Goder R, Habler HJ, Janig W, Michaelis M. Receptor properties of afferent nerve fibers associated with the rat saphenous vein. Neurosci Lett. 1993;164:175–178. doi: 10.1016/0304-3940(93)90885-o. [DOI] [PubMed] [Google Scholar]
- Heien MLAV, Khan AS, Ariansen JL, Cheer JF, Phillips PEM, Wassum KM, Weightman RM. Real-time measurements of dopamine fluctuations after cocaine in the brain of behaving rats. Proc Natnl Acad Sci USA. 2005;102:10023–10028. doi: 10.1073/pnas.0504657102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkila RE, Orlanski H, Cohen G. Studies on the distinction between uptake inhibition and release of [3H]-dopamine in rat brain tissue slices. Biochem Pharmacol. 1975;24:847–852. doi: 10.1016/0006-2952(75)90152-5. [DOI] [PubMed] [Google Scholar]
- Hendry SHC, Hsiao SS, Bushell MC. Somatic sensation. In: Zigmong MJ, Bloom FE, Landis SC, Roberts JL, Squire LR, editors. Fundamental Neuroscience. San-Diego: Academic Press; 1999. pp. 761–789. [Google Scholar]
- Heym J, Trulson ME, Jacobs BL. Raphe unit activity in freely moving cats: effect of phasic auditory and vcisceral stimuli. Brain Res. 1982;232:29–39. doi: 10.1016/0006-8993(82)90608-4. [DOI] [PubMed] [Google Scholar]
- Jimenez-Rivera CA, Waterhouse BD. Effects of systemically and locally applied cocaine on cerebrocortical neuron responsiveness to afferent synaptic inputs and glutamate. Brain Res. 1991;546:287–296. doi: 10.1016/0006-8993(91)91493-k. [DOI] [PubMed] [Google Scholar]
- Johanson CE. The reinforcing properties of procaine, chlorprocaine and proparacaine in rhesus monkeys. Psychopharmacology. 1980;67:189–194. doi: 10.1007/BF00431976. [DOI] [PubMed] [Google Scholar]
- Kiritsy-Roy JA, Halter JB, Gordon SM, Smith MJ, Terry LC. Role of the central nervous system in hemodynamic and sympathoadrenal responses to cocaine in rats. J Pharmacol Exp Ther. 1990;256:154–160. [PubMed] [Google Scholar]
- Kiyatkin EA. Functional properties of presumed dopamine-containing and other ventral tegmental area neurons in conscious rats. Int J Neurosci. 1988;42:21–43. doi: 10.3109/00207458808985756. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA. Dopaminergic involvement in nociceptive sensitivity/behavioral reactivity regulation during aversive states of different nature in the rat. Int J Neurosci. 1989;44:111–133. doi: 10.3109/00207458908986188. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA. Dopamine in the nucleus accumbens: cellular actions, drug- and behavior-associated fluctuations, and a possible role in an organism’s adaptive activity. Behav Brain Res. 2002;137:27–46. doi: 10.1016/s0166-4328(02)00283-8. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Brown PL. Dopamine-dependent and dopamine-independent actions of cocaine as revealed by brain thermorecording in freely moving rats. Eur J Neurosci. 2005;22:930–938. doi: 10.1111/j.1460-9568.2005.04269.x. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Kiyatkin DE, Rebec GV. Phasic inhibition of dopamine uptake in nucleus accumbens induced by intravenous cocaine in freely behaving rats. Neuroscience. 2000;98:729–741. doi: 10.1016/s0306-4522(00)00168-8. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Rebec GV. Dopaminergic modulation of glutamate-induced excitations of neurons in the neostriatum and nucleus accumbens of awake, unrestrained rats. J Neurophysiol. 1996;75:142–153. doi: 10.1152/jn.1996.75.1.142. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Rebec GV. Modulation of striatal neuronal activity by glutamate and GABA: iontophoresis in awake, unrestrained rats. Brain Res. 1999a;822:88–106. doi: 10.1016/s0006-8993(99)01093-8. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Rebec GV. Striatal neuronal activity and responsiveness to dopamine and glutamate after selective blockade of D1 and D2 dopamine receptors in freely moving rats. J Neurosci. 1999b;19:3594–3609. doi: 10.1523/JNEUROSCI.19-09-03594.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin EA, Rebec GV. Impulse activity of ventral tegmental area neurons during heroin self-administration in rats. Neuroscience. 2001;102:565–580. doi: 10.1016/s0306-4522(00)00492-9. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Stein EA. Fluctuations in nucleus accumbens dopamine during cocaine self-administration behavior: an in vivo electrochemical study. Neuroscience. 1995;64:599–617. doi: 10.1016/0306-4522(94)00436-9. [DOI] [PubMed] [Google Scholar]
- Knuepfer MM, Branch CA. Cardiovascular responses to cocaine are initially mediated by the central nervous system in rats. J Pharmacol Exp Ther. 1992;263:734–741. [PubMed] [Google Scholar]
- Lee Y, Lee C-H, Oh U. Painful channels in sensory neurons. Mol Cells. 2005;20:315–324. [PubMed] [Google Scholar]
- Lindoft HH. Investigation of the vascular effect of newer local anesthetics and vasoconstrictors. Oral Surg Oral Med Oral Pathol. 1979;48:2192–297. doi: 10.1016/0030-4220(79)90026-4. [DOI] [PubMed] [Google Scholar]
- Lukas SE, Mendelson JH, Amass L, Benedikt R. Behavioral and EEG studies of acute cocaine administration: comparisons with morphine, amphetamine, pentobarbital, nicotine, ethanol and marijuana. NIDA Res Monogr. 1990;95:146–152. [PubMed] [Google Scholar]
- Mateo Y, Budygin EA, Morgan D, Roberts DCS, Jones SR. Fast onset of dopamine uptake inhibition by intravenous cocaine. Eur J Neurosci. 2004;20:2838–2842. doi: 10.1111/j.1460-9568.2004.03736.x. [DOI] [PubMed] [Google Scholar]
- Matsuzaki M, Spingler PJ, Whitlock EG, Misra AL, Mule SJ. Comparative effects of cocaine and pseudococaine on EEG activities, cardiorespiratory functions, and self-administration behavior in the rhesus monkey. Psychopharmacology (Berlin) 1978;57:12–20. doi: 10.1007/BF00426951. [DOI] [PubMed] [Google Scholar]
- Michaelis M, Goder R, Habler HJ, Janig W. Properties of afferent nerve fibers supplying the saphenous vein in the cat. J Physiol (London) 1994;474:233–243. doi: 10.1113/jphysiol.1994.sp020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogenson GJ, Jones D, Yim CY. From motivation to action: Functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Nagy A, Eordegh G, Paroczy Z, Markus Z, Benedek G. Multisensory integration in the basal ganglia. Eur J Neurosci. 2006;24:917–924. doi: 10.1111/j.1460-9568.2006.04942.x. [DOI] [PubMed] [Google Scholar]
- Nicolelis MA, Lin RC, Woodward DJ, Chapin JK. Dynamic and distributed properties of many-neuron ensembles in the ventral posterior medial thalamus of awake rats. Proc Natl Acad Sci USA. 1993;90:2212–2216. doi: 10.1073/pnas.90.6.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh PI, Spencer JW, George MS, Gill DS, Ketter TA, Andreason P, Herscovitch P, Post RM. Procaine-induced increases in limbic rCBF correlate positively with increases in occipital and temporal EEG fast activity. Brain Topogr. 1995;7:209–218. doi: 10.1007/BF01202380. [DOI] [PubMed] [Google Scholar]
- Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Rev. 1995;20:91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- Paxinos J, Watson C. The Rat Brain in Stereotaxic Coordinates. Sydney: Academic Press; 1998. [Google Scholar]
- Peoples LL, Kravitz AV, Lynch KG, Cavanaugh DJ. Accumbal neurons that are activated during cocaine self-administration are spared from inhibitory effects of repeated cocaine self-administration. Neurophychopharmacology. 2007;32:1141–1158. doi: 10.1038/sj.npp.1301203. [DOI] [PubMed] [Google Scholar]
- Peoples LL, West MO. Phasic firing of single neurons in the rat nucleus accumbens correlated with the timing of intravenous cocaine self-administration. J Neurosci. 1996;16:3459–3473. doi: 10.1523/JNEUROSCI.16-10-03459.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts DK, Udom CE, Marwah J. Cardiovascular effects of cocaine in anesthetized and conscious rats. Life Sci. 1987a;40:1099–1111. doi: 10.1016/0024-3205(87)90573-x. [DOI] [PubMed] [Google Scholar]
- Pitts DK, Marwah J. Cocaine modulation of central monoaminergic transmission. Pharmacol Biochem Behav. 1987b;26:453–461. doi: 10.1016/0091-3057(87)90147-x. [DOI] [PubMed] [Google Scholar]
- Ploner M, Holthusen H, Noetges P, Schnitzler A. Cortical representation of venous nociception in humans. J Neurophysiol. 2002;88:300–305. doi: 10.1152/jn.2002.88.1.300. [DOI] [PubMed] [Google Scholar]
- Pogun S, Scheffel U, Kuhar MJ. Cocaine displaces [3H]WIN 35,428 binding to dopamine uptake sites in vivo more rapidly than mazindol or GBR 12909. Eur J Pharmacol. 1991;198:203–205. doi: 10.1016/0014-2999(91)90622-w. [DOI] [PubMed] [Google Scholar]
- Poon J, van den Buuse M. Autonomic mechanisms in the acute cardiovascular effects of cocaine in conscious rats. Eur J Pharmacol. 1998;363:147–152. doi: 10.1016/s0014-2999(98)00804-8. [DOI] [PubMed] [Google Scholar]
- Premkumar LS. Block of a Ca++-activated potassium channel by cocaine. J Membr Biol. 2005;204:129–136. doi: 10.1007/s00232-005-0755-6. [DOI] [PubMed] [Google Scholar]
- Rebec GV. Behavioral electrophysiology of psychostimulants. Neuropsychopharmacology. 2006;31:2341–2348. doi: 10.1038/sj.npp.1301160. [DOI] [PubMed] [Google Scholar]
- Rebec GV, Langley PE, Pierce RC, Wang Z, Heidenreich BA. A simple micromanipulator for multiple uses in freely moving rats: electrophysiology, voltammetry, and simultaneous intracerebral infusions. J Neurosci Methods. 1993;47:53–59. doi: 10.1016/0165-0270(93)90021-i. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors of dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- Servan-Shreiber D, Perlstein WM, Cohen JD, Mintun M. Selective pharmacological activation of limbic structures in human volunteers: A positron emission tomorgaphy study. J Neuropsychiatry. 1998;10:148–159. doi: 10.1176/jnp.10.2.148. [DOI] [PubMed] [Google Scholar]
- Sherer MA, Kumor KM, Jaffe JH. Effects of intravenous cocaine are partially attenuated by haloperidol. Psychiatry Res. 1989;27:117–125. doi: 10.1016/0165-1781(89)90127-3. [DOI] [PubMed] [Google Scholar]
- Shriver DA, Long IP. A pharmacological comparison of some quaternary derivatives of cocaine. Arch Int Pharmacodyn. 1971;189:198–208. [PubMed] [Google Scholar]
- Steinfels GF, Heym J, Stricker RE, Jacobs BL. Behavioral correlates of dopaminergic unit activity in freely moving cats. Brain Res. 1983;258:217–228. doi: 10.1016/0006-8993(83)91145-9. [DOI] [PubMed] [Google Scholar]
- Waterhouse BD, Stowe ZN, Jimenez-Rivera CA, Sessler FM, Woodward DJ. Cocaine actions in a central noradrenergic circuit: enhancement of cerebellar Purkinje neuron responses to iontophoretically applied GABA. Brain Res. 1991;546:297–309. doi: 10.1016/0006-8993(91)91494-l. [DOI] [PubMed] [Google Scholar]
- West MO. Anesthetics eliminate somatosensory-evoked discharges on neurons in the somatotopically organized sensorimotor striatum of the rat. J Neurosci. 1998;18:9055–9068. doi: 10.1523/JNEUROSCI.18-21-09055.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ. The generation of natural firing patterns in neostriatal neurons. Prog Brain Res. 1993;99:277–297. doi: 10.1016/s0079-6123(08)61352-7. [DOI] [PubMed] [Google Scholar]
- Windels F. Neuronal activity: from in vitro preparation to behaving animals. Mol Neurobiol. 2006;34:1–26. doi: 10.1385/mn:34:1:1. [DOI] [PubMed] [Google Scholar]
- Wilkerson RD. Cardiovascular effects of cocaine in conscious dogs: importance of fully functional autonomic and central nervous system. J Pharmacol Exp Ther. 1988;246:466–471. [PubMed] [Google Scholar]
- Willatts DG, Reynolds F. Comparison of the vasoactivity of amide and ester local anesthetics. An intradermal study. Br J Anesth. 1985;57:1006–1011. doi: 10.1093/bja/57.10.1006. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- Woolverton WL, Balster RL. Reinforcing properties of some local anesthetics in rhesus monkey. Pharmacol Biochem Behav. 1979;11:669–672. doi: 10.1016/0091-3057(79)90260-0. [DOI] [PubMed] [Google Scholar]
- Wu SN, Chang HD, Sung BJ. Cocaine-induced inhibition of ATP-sensitive K+ channels in rat ventricular myocytes and in heart-derived H9c2 cells. Basic Clin Pharmacol Toxicol. 2006;98:510–517. doi: 10.1111/j.1742-7843.2006.pto_354.x. [DOI] [PubMed] [Google Scholar]
- Zhang S, Rajamani S, Chen Y, Gong Q, Rong Y, Zhou Z, Ruoho A, January CT. Cocaine blocks HERG, but not KvLQT1+mink, potassium channels. Mol Pharmacol. 2002;59:1069–1076. doi: 10.1124/mol.59.5.1069. [DOI] [PubMed] [Google Scholar]
- Zernig G, Giacomuzzi S, Riemer Y, Wakonigg G, Sturm K, Saria A. Intravenous drug injection habits: Drug users’ self-reports versus researchers’ perception. Pharmacology. 2003;68:49–56. doi: 10.1159/000068731. [DOI] [PubMed] [Google Scholar]