Abstract
Increasing evidence suggests mutations in human breast cancer cells that induce inappropriate expression of the 18kDa cytokine pleiotrophin (PTN, Ptn) initiate progression of breast cancers to a more malignant phenotype. Pleiotrophin signals through inactivating its receptor, the Receptor Protein Tyrosine Phosphatase (RPTP)β/ζ, leading to increased tyrosine phosphorylation of different substrate proteins of RPTPβ/ζ, including β-catenin, β-adducin, Fyn, GIT1/Cat-1, and P190RhoGAP. PTN signaling thus has wide impact on different important cellular systems. Recently, PTN was found to activate Anaplastic Lymphoma Kinase (ALK) through the PTN/RPTPβ/ζ signaling pathway; this discovery potentially is very important, since constitutive ALK activity of nucleophosmin (NPM)-ALK fusion protein is causative of anaplastic large cell lymphomas, and, activated ALK is found in other malignant cancers. Recently ALK was identified in each of 63 human breast cancers from 22 subjects. We now demonstrate that RPTPβ/ζ is expressed in each of these same 63 human breast cancers that previously were found to express ALK and in 10 additional samples of human breast cancer. RPTPβ/ζ furthermore was localized not only in its normal association with the cell membrane but also scattered in cytoplasm and in nuclei in different breast cancer cells and, in the case of infiltrating ductal carcinomas, the distribution of RPTPβ/ζ changes as the breast cancer become more malignant. The data suggest that the PTN/RPTPβ/ζ signaling pathway may be constitutively activated and potentially function to constitutively activate ALK in human breast cancer.
Introduction
Pleiotrophin (PTN, Ptn) is a cytokine that has important roles in development and differentiation of different cells [1, 2]. Pleiotrophin is expressed in different human cancers [3–11], and recently, PTN was identified in different subtypes of human breast cancers [12–14]. Targeting of constitutive PTN signaling in human breast cancers that inappropriately express Ptn by a dominant negative PTN reverses their malignant phenotype both in vitro and in vivo [15]. These studies suggest constitutive PTN signaling in human breast cancers may have an important place in the pathogenesis of human breast cancers.
To address the mechanism through which inappropriate expression of PTN may stimulate progression of breast cancer, mouse mammary tumor virus (MMTV) promoter-driven Ptn expressed in MMTV-Polyoma Virus Middle T antigen (PyMT)-Ptn transgenic mice were analyzed and shown to induce rapid growth of morphologically identified foci of “scirrhous” carcinoma, to extensively remodel the microenvironment, and to induce tumor angiogenesis in the breast cancers of MMTV-PyMT-Ptn mice, supporting directly the conclusion that inappropriate expression of Ptn promotes breast cancer progression in mice; the data linked the consequences of constitutive PTN signaling directly with progression of mouse breast cancers to a phenotype closely resembling a most malignant form of human cancer (Chang et al. In Press)1; this study also linked the critical importance of secretion of PTN to the critical features of tumor angiogenesis and remodeling of the tumor microenvironment needed to support aggressive breast cancers.
Pleiotrophin signals by inactivating its receptor, the Receptor Protein Tyrosine Phosphatase (RPTP)β/ζ [16, 17]; the loss of the tyrosine phosphatase activity of RPTPβ/ζ in PTN-stimulated cells leaves tyrosine kinases that target the same sites normally dephosphorylated by RPTPβ/ζ free to phosphorylate these same sites, thereby increasing levels of tyrosine phosphorylation of the different substrates of RPTPβ/ζ. The downstream targets of the PTN/RPTPβ/ζ signaling pathway and thus the substrates of RPTPβ/ζ include β-catenin [16], β-adducin [18, 19], Fyn [20], GIT1/Cat-1 [21], and P190RhoGAP [22], proteins critical to functions of different cellular systems. PTN through the PTN/RPTPβ/ζ signaling pathway thus appears to have a wide impact on the function of PTN-stimulated cells. It is suggested that PTN through the PTN/RPTPβ/ζ signaling pathway regulates diverse systems to coordinately influence cell function, a suggestion supported by the recent demonstration that PTN stimulates an epithelial to mesenchymal transition (EMT) in PTN-stimulated cells.
Recently, we demonstrated that Anaplastic Lymphoma Kinase (ALK) is activated through the PTN/RPTPβ/ζ signaling pathway (Perez-Pinera et al. Submitted)2 and not through the direct interaction of PTN with ALK, as recently suggested [23–25]. Thus, ALK is activated through an Alternative Mechanism of Receptor Tyrosine Kinase (RTK) Activation that is dependent on enforced dimerization and inactivation of RPTPβ/ζ.
ALK is a receptor protein tyrosine kinase of the insulin receptor superfamily [26] known to have an essential role in normal development; however, ALK was first discovered as the constitutive active nucleophosmin (NPM)-ALK fusion protein that results from of the (2;5)(p23;q35) chromosomal translocation; NPM-ALK is the oncoprotein that initiates anaplastic large cell lymphomas. We also recently demonstrated that ALK is highly expressed in each of 63 samples of human breast cancer of different subtypes from 22 subjects and furthermore, that the subcellular location and patterns of ALK expression in these different breast cancers differs significantly from its pattern of expression in normal breast tissues [27]. These studies appear to be important, since they suggest that ALK may be activated through the constitutively activated PTN/RPTPβ/ζ signaling pathway in breast cancers that inappropriately express Ptn.
To pursue the significance of these findings and the possibility that RPTPβ/ζ is expressed in these same breast cancers that express ALK, we have now analyzed the expression of RPTPβ/ζ and its distribution in human breast cancers by immunohistochemistry.
Methods
Immunohistochemistry
Breast cancer tissue arrays (Catalog number CC08-01-005) were obtained from Cybrdi (Frederick, Maryland). Tissue samples were obtained from the Tissue Bank of the Principado de Asturias.
Tissue slides were deparaffinized (2×10min) in xylene, and hydrated (2×10min) with 100% 95% (2×10min), (1×10min) 90%, (1×10min) 70% ethanol and distilled water (10 min). The slides were then incubated in antigen retrieval solution (Trypsin 0.05%, CaCl2 0.1% pH 7.8) for 20 minutes at 37°C and then for 10 minutes at room temperature in a humidified chamber as previously described [28]. Endogenous peroxidase was quenched by incubating the sections with 3% hydrogen peroxide for 5 minutes and the tissues were permeabilized by incubating the samples in Tris-buffered saline (TBS, 10 mM Tris pH 7.6, 150 mM NaCl) with 1% Triton X-100 for 30 minutes. Non-specific binding of the antibodies was reduced by incubating the sections for 30 minutes in a blocking solution containing 2% bovine calf serum, 2% goat serum, 1%BSA, 0.1% gelatin, 0.1% Triton X-100, 0.05% Tween 20 in 10 mM PBS, pH 7.2. The sections were incubated overnight with anti-RPTPβ/ζ antibodies (BD Biosciences, La Jolla, CA) diluted 1:100 in PBS pH 7.2, 1% BSA, 0.1% gelatin overnight. The slides were then washed with permeabilization solution (2×10min), incubated with SuperPicTure polymer from Zymed for 30 minutes or the Envision secondary antibody-conjugated polymer from Dako, washed in PBS (2×3min), and developed with DAB provided with the in the SuperPicTure kit Zymed. The slides were rinsed in distilled water 10 min and dehydrated with 70% (1×10min), 90% (1×10min), 95% (2×10min), 100% ethanol (2×10min), and cleared in xylene (2×10min), mounted, observed with a Nikon TE2000U microscope coupled with a Confocal Cell Imaging CARV system, and photographed.
Slides with breast cancers from mouse mammary tumor virus (MMTV)-Polyoma Middle T antigen (PyMT) mice were used as positive control for RPTPβ/ζ expression. Samples from MMTV-PyMT mice in which primary antibodies were omitted were used as negative control.
The tumor staging we used as follows:
| Tumor staging | |
| Grade I: 3–5 points | |
| Grade II: 6–7 points | |
| Grade III: 8–9 points | |
| Tubule formation: | |
| More than 75% of the tumor | 1 |
| From 10–75% of the tumor | 2 |
| Less than 10% of the tumor | 3 |
| Nuclear pleomorphism: | |
| Small and regular nuclei | 1 |
| Moderate increase in nuclear size | 2 |
| Marked pleomorphism or nucleolus | 3 |
| Mitotic index: | |
| 0–8 mitosis per 10 fields | 1 |
| 9–16 mitosis per 10 fields | 2 |
| More than 17 mitosis per field | 3 |
Results
Expression of RPTPβ/ζ was tested in 63 samples of human breast cancer from 22 subjects using immunohistochemistry. The histological phenotypes of the breast cancers studies included infiltrating duct carcinomas, infiltrating lobular carcinomas, medullary carcinomas, mucinous adenocarcinomas, intraductal carcinomas, comedocarcinoma, and Paget’s disease. RPTPβ/ζ was expressed in all breast cancer samples analyzed (Figure 1) and in normal tissue (Figure 1, B). RPTPβ/ζ was expressed in the breast cancer cells themselves, but importantly, RPTPβ/ζ also was expressed in the carcinoma associated fibroblast within the breast cancers.
Figure 1. Expression of RPTPβ/ζ in different human breast cancers.

A. Infiltrating ductal carcinoma. B. Normal breast tissue. C. Infiltrating lobular carcinoma. D. Infiltrating ductal carcinoma (papillary pattern). E. Medullary carcinoma. F. Mucinous adenocarcinoma. G. Intraductal carcinoma. H. Paget’s disease.
To assess the relative levels of expression of RPTPβ/ζ protein, the levels of its expression were scored according to the intensity of immunoreactivity observed using light microscopy and a scale of 1 (low) to 3 (high). The results demonstrated that 75% of the infiltrating ductal carcinomas and 75% of the infiltrating lobular carcinomas expressed low but readly detectable levels of RPTPβ/ζ whereas 100% of the medullary carcinomas, 100% of the mucinous carcinomas, and 100% of the intraductal carcinomas express moderate levels of RPTPβ/ζ. Interestingly, 80% of the cases of Paget’s disease were found to express high levels of RPTPβ/ζ (Figure 2). The results also demonstrated that the subcellular localization of RPTPβ/ζ is not homogeneous in any of the breast cancers studied, in contrast to the expression of RPTPβ/ζ in normal breast tissues. As example, in Paget’s disease of the breast, RPTPβ/ζ expression was also found in the nuclei (Figure 1, H, arrows).
Figure 2. Levels of expression of RPTPβ/ζ in different human breast cancers.

The levels of expression of ALK were quantified using light microscopy and scored in a scale from 1 to 3. The results demonstrated that 75% of the infiltrating ductal carcinomas and 75% of the infiltrating lobular carcinomas expressed low levels of RPTPβ/ζ whereas 100% of the medullary carcinomas, 100% of the mucinous carcinomas, and 100% of the intraductal carcinomas express moderate levels of RPTPβ/ζ. Interestingly, 80% of the cases of Paget’s disease were found to express high levels of ALK and the single sample of comedocarcinoma only weakly expressed RPTPβ/ζ.
To develop a better understanding of the subcellular location of RPTPβ/ζ, we used ten biopsies from human breast cancers stained using immunohistochemistry with anti-RPTPβ/ζ antibodies (Figure 3). The results confirmed that RPTPβ/ζ is expressed in ductal carcinomas, in lobular carcinomas, in mucinous carcinomas, and in medullary carcinomas. In the ductal carcinomas, nuclear staining was found in each of the grades of increasing malignancy of infiltrating ductal carcinomas from grades I to III. However, cytoplasmic expression that is prominent in I and II is lost in the most aggressive grade III infiltrating ductal carcinoma. These results suggest that possibly different isoforms of RPTPβ/ζ may be expressed in different types of tumors and that changes in the expression pattern of RPTPβ/ζ are associated with tumor progression to higher grades of malignancy. The data also suggest the possibility that RPTPβ/ζ that has been inactivated through the PTN-enforced dimerization may be processed and appear in different stages of degradation.
Figure 3. Levels and pattern of expression of RPTPβ/ζ in infiltrating ductal carcinomas.

Immunohistochemistry for RPTPβ/ζ in grade I (top panels), grade II (middle panels), and grade III (bottom panels). Nuclear staining was observed in all grades whereas the cytoplasmic expression was lost in the highest grade. Magnification: ×20 on the left panels, ×40 on the right panels.
In addition, other patterns of expression of RPTPβ/ζ were seen in subtypes of breast cancers. Apocrine carcinomas express RPTPβ/ζ in an unequal cytoplasmic and nuclear staining (Figure 4 A) whereas mucinous carcinomas express RPTPβ/ζ in a mixed nuclear and cytoplasmic pattern (Figure 4 B).
Figure 4. Patterns of expression of RPTPβ/ζ in different carcinomas.
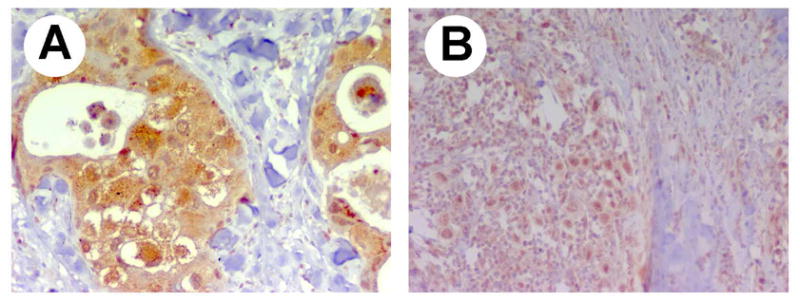
A, unequal cytoplasmic and nuclear staining in an uncommon variant of apocrine carcinoma associated to androgen receptors. B, nuclear and cytoplasmic staining in a mucinous carcinoma. Magnification: ×40.
Discussion
We recently demonstrated that ALK is a substrate of RPTPβ/ζ and that the PTN-dependent inactivation of RPTPβ/ζ is a mechanism through which ALK is activated (Perez-Pinera et al. Submitted)2. This mechanism of activation of ALK is unique; it is independent of the classically described direct interaction of a growth factor with its cognate receptor tyrosine kinase (RTK). We also demonstrated that ALK is expressed in each of 63 human breast cancers taken from 22 subjects, suggesting the possibility that constitutive activation of ALK by the PTN/RPTPβ/ζ signaling pathway may stimulate progression of human breast cancers. In studies in progress, we also have identified PTN and midkine (MK) also known to signal through RPTPβ/ζ [29] in human breast cancers, in further support of the possibility that ALK may be activated through the PTN(MK)/RPTPβ/ζ signaling pathway.
In these studies it is demonstrated that RPTPβ/ζ is expressed in each of the breast cancer samples studied [30]. It is furthermore demonstrated that the distribution of RPTPβ/ζ in each of the subtypes of human breast cancer examined differs from that found in normal breast epithelium and furthermore, the distribution of RPTPβ/ζ in infiltrating ductal carcinomas changes as the breast cancers of this subtype become increasingly aggressive.
The demonstration that PTN, RPTPβ/ζ, and ALK are expressed in human breast cancers is consistent with the hypothesis that ALK may be activated through the PTN/RPTPβ/ζ signaling pathway and activated ALK may function as a potent oncogenic protein in the pathogenesis of human breast cancer.
Acknowledgments
This is manuscript number 18956 from the Scripps Research Institute. This work was supported by grant CA84400 from The National Institutes of Health. The Instituto Universitario de Oncologia del Principado de Asturias IUOPA is supported by Cajastur and RTICC funds. The MEM core laboratory is supported by Sam and Rose Stein Endowment Fund. Pablo Perez-Pinera was supported by grant 2 T32 DK007022-26 from the National Institutes of Health. Yunchao Chang was supported by Skaggs training grant. We thank Maria Jose Rodriguez-Arguello for her assistance in the preparation of the samples.
Footnotes
Y. Chang, M. Zuka, P. Perez-Pinera, A. Astudillo, J. Mortimer, J.R. Berenson, Z. Wang, and T.F. Deuel. Inappropriate Expression of Pleiotrophin (PTN) Stimulates Breast Cancer Progression Through Secretion of PTN and PTN-dependent Remodeling of the Tumor Microenvironment. In Press.
P. Perez-Pinera, W. Zhang, Y. Chang, J.A. Vega and T.F. Deuel. Anaplastic Lymphoma Kinase (ALK) is Activated Through the Pleiotrophin (PTN)/Receptor Protein Tyrosine Phosphatase (RPTP)β/ζ Signaling Pathway: An “Alternative Mechanism of Receptor Tyrosine Kinase (RTK) Activation”. Submitted.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Li YS, Milner PG, Chauhan AK, Watson MA, Hoffman RM, Kodner CM, Milbrandt J, Deuel TF. Cloning and expression of a developmentally regulated protein that induces mitogenic and neurite outgrowth activity. Science. 1990;250:1690–1694. doi: 10.1126/science.2270483. [DOI] [PubMed] [Google Scholar]
- 2.Deuel TF, Zhang N, Yeh HJ, Silos-Santiago I, Wang ZY. Pleiotrophin: a cytokine with diverse functions and a novel signaling pathway. Arch Biochem Biophys. 2002;397:162–171. doi: 10.1006/abbi.2001.2705. [DOI] [PubMed] [Google Scholar]
- 3.Vacherot F, Caruelle D, Chopin D, Gil-Diez S, Barritault D, Caruelle JP, Courty J. Involvement of heparin affin regulatory peptide in human prostate cancer. Prostate. 1999;38:126–136. doi: 10.1002/(sici)1097-0045(19990201)38:2<126::aid-pros6>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 4.Soulie P, Heroult M, Bernard I, Kerros ME, Milhiet PE, Delbe J, Barritault D, Caruelle D, Courty J. Immunoassay for measuring the heparin-binding growth factors HARP and MK in biological fluids. J Immunoassay Immunochem. 2002;23:33–48. doi: 10.1081/IAS-120002273. [DOI] [PubMed] [Google Scholar]
- 5.Zhang L, Mabuchi T, Satoh E, Maeda S, Nukui H, Naganuma H. Overexpression of heparin-binding growth-associated molecule in malignant glioma cells. Neurol Med Chir (Tokyo) 2004;44:637–643. doi: 10.2176/nmc.44.637. discussion 644–635. [DOI] [PubMed] [Google Scholar]
- 6.Wu H, Barusevicius A, Babb J, Klein-Szanto A, Godwin A, Elenitsas R, Gelfand JM, Lessin S, Seykora JT. Pleiotrophin expression correlates with melanocytic tumor progression and metastatic potential. J Cutan Pathol. 2005;32:125–130. doi: 10.1111/j.0303-6987.2005.00282.x. [DOI] [PubMed] [Google Scholar]
- 7.Souttou B, Juhl H, Hackenbruck J, Rockseisen M, Klomp HJ, Raulais D, Vigny M, Wellstein A. Relationship between serum concentrations of the growth factor pleiotrophin and pleiotrophin-positive tumors. J Natl Cancer Inst. 1998;90:1468–1473. doi: 10.1093/jnci/90.19.1468. [DOI] [PubMed] [Google Scholar]
- 8.Klomp HJ, Zernial O, Flachmann S, Wellstein A, Juhl H. Significance of the expression of the growth factor pleiotrophin in pancreatic cancer patients. Clin Cancer Res. 2002;8:823–827. [PubMed] [Google Scholar]
- 9.Jager R, List B, Knabbe C, Souttou B, Raulais D, Zeiler T, Wellstein A, Aigner A, Neubauer A, Zugmaier G. Serum levels of the angiogenic factor pleiotrophin in relation to disease stage in lung cancer patients. Br J Cancer. 2002;86:858–863. doi: 10.1038/sj.bjc.6600202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jager R, Noll K, Havemann K, Pfluger KH, Knabbe C, Rauvala H, Zugmaier G. Differential expression and biological activity of the heparin-binding growth-associated molecule (HB-GAM) in lung cancer cell lines. Int J Cancer. 1997;73:537–543. doi: 10.1002/(sici)1097-0215(19971114)73:4<537::aid-ijc14>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 11.Kadomatsu K, Muramatsu T. Midkine and pleiotrophin in neural development and cancer. Cancer Lett. 2004;204:127–143. doi: 10.1016/S0304-3835(03)00450-6. [DOI] [PubMed] [Google Scholar]
- 12.Garver RI, Jr, Radford DM, Donis-Keller H, Wick MR, Milner PG. Midkine and pleiotrophin expression in normal and malignant breast tissue. Cancer. 1994;74:1584–1590. doi: 10.1002/1097-0142(19940901)74:5<1584::aid-cncr2820740514>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 13.Riegel AT, Wellstein A. The potential role of the heparin-binding growth factor pleiotrophin in breast cancer. Breast Cancer Res Treat. 1994;31:309–314. doi: 10.1007/BF00666163. [DOI] [PubMed] [Google Scholar]
- 14.Wellstein A, Fang WJ, Khatri A, Lu Y, Swain SS, Dickson RB, Sasse J, Riegel AT, Lippman ME. A heparin-binding growth factor secreted from breast cancer cells homologous to a developmentally regulated cytokine. J Biol Chem. 1992;267:2582–2587. [PubMed] [Google Scholar]
- 15.Zhang N, Zhong R, Wang ZY, Deuel TF. Human breast cancer growth inhibited in vivo by a dominant negative pleiotrophin mutant. J Biol Chem. 1997;272:16733–16736. doi: 10.1074/jbc.272.27.16733. [DOI] [PubMed] [Google Scholar]
- 16.Meng K, Rodriguez-Pena A, Dimitrov T, Chen W, Yamin M, Noda M, Deuel TF. Pleiotrophin signals increased tyrosine phosphorylation of beta beta-catenin through inactivation of the intrinsic catalytic activity of the receptor-type protein tyrosine phosphatase beta/zeta. Proc Natl Acad Sci U S A. 2000;97:2603–2608. doi: 10.1073/pnas.020487997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukada M, Fujikawa A, Chow JP, Ikematsu S, Sakuma S, Noda M. Protein tyrosine phosphatase receptor type Z is inactivated by ligand-induced oligomerization. FEBS Lett. 2006 doi: 10.1016/j.febslet.2006.06.041. [DOI] [PubMed] [Google Scholar]
- 18.Pariser H, Herradon G, Ezquerra L, Perez-Pinera P, Deuel TF. Pleiotrophin regulates serine phosphorylation and the cellular distribution of {beta}-adducin through activation of protein kinase C. Proc Natl Acad Sci U S A. 2005;102:12407–12412. doi: 10.1073/pnas.0505901102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pariser H, Perez-Pinera P, Ezquerra L, Herradon G, Deuel TF. Pleiotrophin stimulates tyrosine phosphorylation of beta-adducin through inactivation of the transmembrane receptor protein tyrosine phosphatase beta/zeta. Biochem Biophys Res Commun. 2005;335:232–239. doi: 10.1016/j.bbrc.2005.07.060. [DOI] [PubMed] [Google Scholar]
- 20.Pariser H, Ezquerra L, Herradon G, Perez-Pinera P, Deuel TF. Fyn is a downstream target of the pleiotrophin/receptor protein tyrosine phosphatase beta/zeta-signaling pathway: Regulation of tyrosine phosphorylation of Fyn by pleiotrophin. Biochem Biophys Res Commun. 2005;332:664–669. doi: 10.1016/j.bbrc.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Kawachi H, Fujikawa A, Maeda N, Noda M. Identification of GIT1/Cat-1 as a substrate molecule of protein tyrosine phosphatase zeta/beta by the yeast substrate-trapping system. Proc Natl Acad Sci U S A. 2001;98:6593–6598. doi: 10.1073/pnas.041608698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tamura H, Fukada M, Fujikawa A, Noda M. Protein tyrosine phosphatase receptor type Z is involved in hippocampus-dependent memory formation through dephosphorylation at Y1105 on p190 RhoGAP. Neurosci Lett. 2006 doi: 10.1016/j.neulet.2006.01.045. [DOI] [PubMed] [Google Scholar]
- 23.Powers C, Aigner A, Stoica GE, McDonnell K, Wellstein A. Pleiotrophin signaling through anaplastic lymphoma kinase is rate-limiting for glioblastoma growth. J Biol Chem. 2002;277:14153–14158. doi: 10.1074/jbc.M112354200. [DOI] [PubMed] [Google Scholar]
- 24.Bowden ET, Stoica GE, Wellstein A. Anti-apoptotic signaling of pleiotrophin through its receptor, anaplastic lymphoma kinase. J Biol Chem. 2002;277:35862–35868. doi: 10.1074/jbc.M203963200. [DOI] [PubMed] [Google Scholar]
- 25.Stoica GE, Kuo A, Aigner A, Sunitha I, Souttou B, Malerczyk C, Caughey DJ, Wen D, Karavanov A, Riegel AT, Wellstein A. Identification of anaplastic lymphoma kinase as a receptor for the growth factor pleiotrophin. J Biol Chem. 2001;276:16772–16779. doi: 10.1074/jbc.M010660200. [DOI] [PubMed] [Google Scholar]
- 26.Pulford K, Morris SW, Turturro F. Anaplastic lymphoma kinase proteins in growth control and cancer. J Cell Physiol. 2004;199:330–358. doi: 10.1002/jcp.10472. [DOI] [PubMed] [Google Scholar]
- 27.Perez-Pinera P, Chang Y, Astudillo A, Mortimer J, Deuel TF. Anaplastic lymphoma kinase is expressed in different subtypes of human breast cancer. Biochem Biophys Res Commun. 2007 doi: 10.1016/j.bbrc.2007.04.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perez-Pinera P, Garcia-Suarez O, Prieto JG, Germana A, Ciriaco E, del Valle ME, Vega JA. Thymocyte depletion affects neurotrophin receptor expression in thymic stromal cells. J Anat. 2006;208:231–238. doi: 10.1111/j.1469-7580.2006.00514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maeda N, Ichihara-Tanaka K, Kimura T, Kadomatsu K, Muramatsu T, Noda M. A receptor-like protein-tyrosine phosphatase PTPzeta/RPTPbeta binds a heparin-binding growth factor midkine. Involvement of arginine 78 of midkine in the high affinity binding to PTPzeta. J Biol Chem. 1999;274:12474–12479. doi: 10.1074/jbc.274.18.12474. [DOI] [PubMed] [Google Scholar]
- 30.Fang W, Hartmann N, Chow DT, Riegel AT, Wellstein A. Pleiotrophin stimulates fibroblasts and endothelial and epithelial cells and is expressed in human cancer. J Biol Chem. 1992;267:25889–25897. [PubMed] [Google Scholar]


