Abstract
A novel approach to H-phosphonates from hypophosphorous acid using a transfer hydrogenation process was developed. This method is atom-economical, environmentally friendly, catalytic, and efficient, leading easily to H-phosphonate monoesters or ammonium salt in moderate to good yields.
H-Phosphonate monoesters (1) are important synthetic intermediates in general phosphorus chemistry1 but mostly in the synthesis of phosphates. Phosphate groups are present in numerous biological molecules such as nucleic acids, proteins, carbohydrates, lipids, coenzymes, and steroids. Therefore, in order to study biological pathways or for therapeutic applications, analogs of these compounds have been synthesized and phosphorylation reactions were developed. Among phosphorylating agents, phosphoric acid monoesters ((RO)P(O)(OH)2), phosphoric acid diesters ((HO)P(O)(OR)2), phosphorus pentoxide (P2O5) and phosphorus trichloride (PCl3) are commonly used.2 In the more recent literature, H-phosphonate monoesters have emerged as an interesting option to obtain phosphates in an atom-economical way. Several examples of phospholipid analogs3, carbohydrate analogs4, peptide analogs5, and nucleotide analogs6 syntheses involved H-phosphonate monoesters intermediates.7
Current methods to access to H-phosphonate monoesters (1) can be organized into two groups. The first uses P(III) phosphorus compounds such as PCl38 or salicylchlorophosphite9 in the presence of an alcohol and a base such as tetrazole or imidazole. The second group uses P(V) phosphorus compounds such as H3PO310 and diphenyl-H-phosphonate11 in the presence of an alcohol, or the hydrolysis of H-phosphonate diesters12.
Our laboratory has been involved in the development of phosphorus-carbon bond formation using inexpensive and easily handled hypophosphorous acid (H3PO2) and its derivatives.13 Based on the transfer hydrogenation mechanism,14 we developed the hydrophosphinylation of alkenes and alkynes. We postulated that H3PO2 and its derivatives in the presence of a metal catalyst undergo an equilibrium (Scheme 1) between the P(V) form (2) and phosphinidene oxide complex (3) and we showed this equilibrium can be controlled by using different phosphine ligands. Based on this mechanism, we found a new atom-economical, efficient, and catalytic way to prepare H-phosphonate monoesters (1) by trapping phosphinidene oxide (3) with an alcohol.
Scheme 1.
Hydrophosphinylation and transfer hydrogenation mechanisms
Menthol was selected as test substrate to investigate the different parameters of the reaction : catalyst, solvent, temperature, and stoichiometry (Table 1).15H-Menthyl phosphonate was isolated by a basic-acidic extractive work-up to afford the acid form. First, and as expected, no product is formed in the absence of catalyst (entry 1). Although the desired reaction takes place in all other cases, significant differences are observed. Since we were looking for an inexpensive catalytic P-O bond formation, Pd/C and Ni/Al2O3-SiO2 were selected as catalysts. These catalysts proceed differently depending on solvents (entries 2a-c versus 3a-d). Although the best conditions are Pd/C in a toluene/CH3CN mixture (1:1, v/v) (entry 3a), it should be noted that Ni/Al2O3-SiO2 in toluene (entry 2b) leads to the product in comparable yield. Entries 3a and 4 show the influence of H3PO2 stoichiometry. In this process, residual water competes with the alcohol for 3 thereby transforming H3PO2 into H3PO3. Therefore, decreasing the amount of H3PO2 leads to lower yields (entries 4a-b versus entries 3a and 4c) and the optimum appears to be 1.5 equivalents (entries 2b, 3a). As expected, catalyst loading controls the rate of the reaction. With Pd at 1 mol % loading in Pd/C, the reaction still proceeds in 63% yield after 24h (entry 5c). On the other hand, reaction temperature is a critical parameter, and influences dramatically the speed of the reaction. Reactions performed at room temperature and at 40°C, led to only 4% and 6% yields, respectively.
Table 1.
Optimization of the catalytic oxidative phosphorylation of menthol a
| Entry | H3PO2 (equiv)b | Catalyst | Solventc | Yield (%)d | |
|---|---|---|---|---|---|
| Type | Amount (mol %) | ||||
| 1 | 3.0 | - | - | Toluene | 0e |
|
| |||||
| 2a | 1.5 | Ni on Al2O3/SiO2 | 5 | Toluene/CH3CN | 13 |
| 1/1 | |||||
| 2b | 1.5 | 5 | Toluene | 85 | |
| 2c | 1.3 | 5 | Toluene | 61 | |
|
| |||||
| 3a | 1.5 | Pd/C | 5 | Toluene/CH3CN | 88 |
| 1/1 | |||||
| 3b | 1.5 | 5 | Toluene | 70 | |
| 3c | 1.5 | 5 | CH3CN | 74 | |
| 3d | 1.5 | 5 | DMF | 49 | |
|
| |||||
| 4a | 1.2 | Pd/C | 5 | 77 | |
| 4b | 1.0 | 5 | Toluene/CH3CN | 62 | |
| 4c | 3.0 | 5 | 1/1 | 80 | |
|
| |||||
| 5a | 1.5 | Pd/C | 3 | 80 | |
| 5b | 1.5 | 2 | Toluene/CH3CN | 76 | |
| 5c | 1.5 | 1 | 1/1 | 63 | |
Unless otherwise noted, reactions were conducted with 1.0 equiv of menthol (0.32 M in solvent), 5 mol % catalyst, under N2 at 85°C for 24h.
Commercial 50 wt % aqueous H3PO2 was concentrated in vacuo (0.5 mmHg) for 30 min at rt.
Toluene and CH3CN were freshly distilled over CaH2, and DMF was stored over 4Å sieves before use.
H-Menthyl phosphonate was isolated by extraction of the acid form.
Unreacted H3PO2 only.
Reactivities of different H3PO2 salts (NH3, PhNH2, Et3N, Na) were also tested but unsuccessfully (the sodium salt gave a 5% yield in CH3CN or DMF). Dorfman and Aleshkova have studied the reaction of NaH2PO2 with methanol or butanol catalyzed by PdCl2.16 The alcohols were employed as solvents and no yields were reported.
After the establishment of the best conditions for menthol, we investigated the scope of the reaction (Table 2).15 In most cases, the H-phosphonate monoester can be isolated in its acid form, in moderate to good yield by a basic-acidic extractive work-up (entries 1-9). In the case of more water-soluble products (entries 10-12), a simple extractive work-up followed by precipitation of the ammonium salt was necessary. Primary and secondary alcohols react well in this reaction (entries 7-12 and 1-6, respectively). But in spite of various efforts, the products derived from tertiary alcohols could not be isolated. As Cherbuliez and al. reported, it appears that these products are unstable in acidic conditions and elimination takes place.17
Table 2.
Scope of the catalytic oxidative phosphorylation
| Entrya | Alcohol | H-phosphonate | Isolated Yield %b |
|---|---|---|---|
| 1 | (−) Menthol |

|
88 |
| 2 | Borneol |

|
70 |
| 3 | (+) Fenchyl Alcohol |
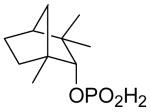
|
97 |
| 4 | 3,3-Dimethyl-butan-2-ol |

|
69 |
| 5 | 2,4-Dimethyl-pentan-3-ol |

|
78 |
| 6 | Pregnenolone |

|
67 |
| 7 | 3-Phenyl-propan-1-ol |
|
67 |
| 8 | Dibenzyl glycerol |

|
68 |
| 9 | Dec-5-yn-1-ol |

|
80 |
| 10 | 3-Chloro-1-propanol |
|
49c |
| 11 | 3-Bromo-1-propanol |
|
37c |
| 12 | Benzyl Alcohol |

|
56c |
See Supplementary Material for detailed experimental procedures. Unless otherwise noted, reactions were conducted with 1.0 equiv of alcohol (0.32 M), 1.5 equiv of concentrated H3PO2 (prepared by evaporation of a commercial aqueous solution in vacuo (0.5 mmHg), for 30 min at rt) and 5 mol % Pd/C, in distilled toluene/CH3CN (1:1, v/v), under nitrogen, at 85°C for 24h.
Unless otherwise noted, the H-phosphonate is isolated by extraction as the acid form.
H-phosphonate was isolated by extraction and precipitation of the ammonium salt.
Hindered substrates give as good a yield as the unhindered alcohols (entries 3-5) thus supporting a highly reactive phosphinidene oxide intermediate. It should be noted that various functionalities, such as ketone (entry 6), alkene (entry 6), alkyne (entry 9), and halides (entries 10-11) are tolerated.
In conclusion, a simple and catalytic phosphorus-oxygen bond forming-reaction was developed based on the postulated transfer hydrogenation mechanism. Various primary and secondary alcohols, including hindered compounds, reacted satisfactorily. Hypophosphorous acid was shown to be a useful and inexpensive reagent to prepare various H-phosphonate monoesters via an oxidative phosphorylation which likely involves a highly reactive phosphinidene oxide intermediate. The method is atom-economical as it does not rely on protecting group strategies or on phosphorus (III) chlorides.
Supplementary Material
Supplementary Material
Representative experimental procedures and spectroscopic data. This material can be found in the online version, at doi :.
Acknowledgments
We gratefully acknowledge the National Institute of General Medical Sciences/NIH (1R01 GM067610) for the financial support of this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.For examples of different reactions involving H-phosphonate monoesters as starting material, see Laskorin BN, Yakshin VV, Bulgakova VB. J Gen Chem USSR. 1976;46:2372–2376.Iyer VV, Griesgraber GW, Radmer MR, McIntee EJ, Wagner CR. J Med Chem. 2000;43:2266–2274. doi: 10.1021/jm000110g.Chang SL, Griesgraber G, Wagner CR. Nucleosides Nucleotides. 2001;20:1571–1582. doi: 10.1081/NCN-100105248.Michalski J, Modro T, Zweirzak A. J Chem Soc. 1961:4904–4906.
- 2.Slotin LA. Synthesis. 1977:737–752. [Google Scholar]
- 3.For examples of phospholipids analogs synthesis via H-phosphonates : Lindh I, Stawinski J. J Org Chem. 1989;54:1338–1342.García ML, Pascual J, Borràs L, Andreu JA, Fos E, Mauleón D, Carganico G, Arcamone F. Tetrahedron. 1991;47:10023–10034.
- 4.For examples of carbohydrate analogs synthesis via H-phosphonates : Garegg PJ, Hansson J, Helland AC, Oscarson S. Tetrahedron Lett. 1999;40:3049–3052.Yashunsky DV, Nikolaev AV. J Chem Soc Perkin Trans 1. 2000;8:1195–1198.
- 5.For examples of peptide analogs synthesis via H-phosphonates : Larsson E, Lüning B. Tetrahedron Lett. 1994;35:2737–2738.Hu YJ, Rajagopalan PT, Pei D. Bioorg Med Chem Lett. 1998;8:2479–2482. doi: 10.1016/s0960-894x(98)00443-0.
- 6.For examples of nucleotide analogs synthesis via H-phosphonates : Stawinski J, Strömberg R. Trends Org Chem. 1993;4:31–67.Whalen LJ, McEvoy KA, Halcomb RL. Bioorg Med Chem Lett. 2003;13:301–304. doi: 10.1016/s0960-894x(02)00735-7.Ludwig PS, Schwendener RA, Schott H. Eur J Med Chem. 2005;40:494–504. doi: 10.1016/j.ejmech.2004.12.006.Yan Z, Kern ER, Gullen E, Cheng YC, Drach JC, Zemlicka J. J Med Chem. 2005;48:91–99. doi: 10.1021/jm040149b.Puech F, Gosselin G, Balzarini J, De Clercq E, Imbach JL. J Med Chem. 1988;31:1897–1907. doi: 10.1021/jm00118a006.
- 7.(a) Stawinski J. In: Handbook of Organophosphorus Chemistry. Engel R, editor. Marcel Dekker; New York: 1992. pp. 377–434. [Google Scholar]; (b) Stawinski J, Kraszewski A. Acc Chem Res. 2002;35:952–960. doi: 10.1021/ar010049p. [DOI] [PubMed] [Google Scholar]
- 8.(a) Boyd DR. J Chem Soc. 1901;79:1221–1227. [Google Scholar]; (b) Garegg PJ, Regberg T, Stawinski J, Strömberg R. Chem Scr. 1986;26:59–62. [Google Scholar]; (c) Froehler BC, Ng PG, Matteucci MD. Nucleic Acids Res. 1986;14:5399–5407. doi: 10.1093/nar/14.13.5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Marugg JE, Tromp M, Kuyl-Yeheskiesly E, van der Marel GA, van Boom JH. Tetrahedron Lett. 1986;27:2661–2664. [Google Scholar]; (b) Knerr L, Pannecoucke X, Schmitt G, Luu B. Tetrahedron Lett. 1996;37:5123–5126. [Google Scholar]; (c) Lartia R, Asseline U. Tetrahedron Lett. 2004;45:5949–5952. [Google Scholar]
- 10.(a) Cherbuliez E, Hunkeler F, Weber G, Rabinowitz J. Helv Chim Acta. 1964;47:1647–1653. [Google Scholar]; (b) Nifant'ev EE, Kil'disheva VR, Nasonovskii IS. J Appl Chem USSR. 1969;42:2443–2446. [Google Scholar]
- 11.(a) Jankowska J, Sobkowski M, Stawinski J, Kraszewski A. Tetrahedron Lett. 1994;35:3355–3358. [Google Scholar]; (b) Kers A, Kers I, Stawinski J, Sobkowski M, Kraszewski A. Synthesis. 1995;4:427–430. [Google Scholar]
- 12.Hammond P. J Chem Soc. 1962:2521–2522. [Google Scholar]
- 13.(a) Montchamp JL, Dumond YR. J Am Chem Soc. 2001;123:510–511. [Google Scholar]; (b) Dumond YR, Montchamp JL. Organomet Chem. 2002;653:252–260. [Google Scholar]; (c) Deprèle S, Montchamp JL. J Am Chem Soc. 2002;124:9386–9387. doi: 10.1021/ja0261978. [DOI] [PubMed] [Google Scholar]; (d) Deprèle S, Montchamp JL. Org Lett. 2004;6:3805–3808. doi: 10.1021/ol0484198. [DOI] [PubMed] [Google Scholar]; (e) Ribière P, Bravo-Altamirano K, Antczak MI, Hawkins JD, Montchamp JL. J Org Chem. 2005;70:4064–4072. doi: 10.1021/jo050096l. [DOI] [PubMed] [Google Scholar]; For a review see: Montchamp JL. J Organomet Chem. 2005;690:2388–2406.
- 14.Reviews: Brieger G, Nestrick TJ. Chem Rev. 1974;74:567–580.Johnstone RAW, Wilby AH, Entwistle AI. Chem Rev. 1985;85:129–170. Examples: Boyer SK, Bach J, McKenna J, Jagdmann E., Jr J Org Chem. 1985;50:3408–3411.Khai BT, Arcelli A. J Org Chem. 1989;54:949–953.Brigas AF, Johnstone RAW. Tetrahedron. 1992;48:7735–7746.
- 15.General procedure : Aqueous hypophosphorous acid (50 wt %) was concentrated in vacuo (0.5 mmHg) for 30 min at rt. H3PO2 (1.5 equiv, 4.8 mmol), alcohol (1.0 equiv, 3.2 mmol), Pd/C (10 wt %, 5 mol % Pd, 0.16 mmol) in a mixture of distilled toluene/acetonitrile (10 mL, 1:1 v/v) were heated at 85°C under a nitrogen atmosphere for 24h. After cooling, the reaction was filtered through Celite® and concentrated in vacuo. The resulting oil was dissolved in ethyl acetate (25 mL) and washed with brine (10 mL, 3x). The organic layer was extracted with NaHCO3 (0.5 M, 10 mL, 1x), then water (10 mL, 2x). The combined aqueous layers were acidified with 10% aqueous HCl until pH 1, and extracted with ethyl acetate (25 mL, 3x). The combined organic layers were washed with brine (5 mL, 1x), dried over MgSO4, and concentration in vacuo to afford the acid form of H-phosphonate monoesters.
- 16.Dorfman YA, Aleshkova MM. Kinet Catal. 1998;39:852–858. [Google Scholar]
- 17.Cherbuliez E, Prince R, Rabinowitz J. Helv Chim Acta. 1964;47:1653–1659. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Representative experimental procedures and spectroscopic data. This material can be found in the online version, at doi :.



