Abstract
RhoB is a short-lived protein whose expression is increased by a variety of extra-cellular stimuli including UV irradiation, epidermal growth factor (EGF) and transforming growth factor β (TGF-β). Whereas most Rho proteins are modified by the covalent attachment of a geranylgeranyl group, RhoB is unique in that it can exist in either a geranylgeranylated (RhoB-GG) or a farnesylated (RhoB-F) form. Although each form is proposed to have different cellular functions, the signaling events that underlie these differences are poorly understood. Here we show that RhoB can activate NF-κB signaling in multiple cell types. Whereas RhoB-F is a potent activator of NF-κB, much weaker activation is observed for RhoB-GG, RhoA, and RhoC. NF-κB activation by RhoB is not associated with increased nuclear translocation of RelA/p65, but rather, by modification of the RelA/p65 transactivation domain. Activation of NF-κB by RhoB is dependent upon ROCK I but not PRK I. Thus, ROCK I cooperates with RhoB to activate NF-κB, and suppression of ROCK I activity by genetic or pharmacological inhibitors blocks NF-κB activation. Suppression of RhoB activity by dominant-inhibitory mutants, or siRNA, blocks NF-κB activation by Bcr, and TSG101, but not by TNFα or oncogenic Ras. Collectively, these observations suggest the existence of an endosome-associated pathway for NF-κB activation that is preferentially regulated by the farnesylated form of RhoB.
Keywords: Rho proteins, Nuclear factor κB, ROCK I, Endosome, Farnesylation
1. Introduction
RhoB is a member of a closely related (85% identity) subgroup of the Rho family that also includes RhoA and RhoC. RhoB displays several properties which suggest that it is functionally distinct from the other isoforms. First, it is an early response gene whose expression is regulated by growth factors, the non-receptor tyrosine kinase Src, and by ultraviolet radiation [1, 2]. It also has a unique pattern of cellular distribution. It has been localized to specialized membrane compartments including the Golgi, endosomal vesicles, and the multivesicular body (MVB) [3, 4]. This localization is consistent with several studies which suggest an important role for RhoB in endosome-mediated trafficking of growth factor receptors [4, 5]. Finally, RhoB displays a unique pattern of posttranslational modification.
Like most low molecular weight GTPases, Rho proteins are posttranslationally modified by the covalent addition of an isoprenoid lipid to the highly conserved cysteine residue within their COOH-terminal CAAX motif (C=cysteine, A=aliphatic residue, X=any residue) [6]. If the terminal residue is either a serine or a methionine it will serve as a substrate for a farnesyl transferase, and a farnesyl group will be added. If the residue is a leucine it will be recognized by a geranylgeranyltransferase, and a geranylgeranyl group will be added. Thus, Ras proteins are typically farnesylated while Rho proteins such as RhoA, RhoC and Rac are geranylgeranylated [6]. Although RhoB is predicted to be geranylgeranylated (CKVL), it is unique among the prenylated proteins in that it can be either farnesylated or geranylgeranylated [6]. The existence of both species of RhoB has been demonstrated in vitro, and recent studies suggest that both modifications can occur in vivo [7, 8].
Compared to other Rho family members, RhoB also appears to have a unique role in cell transformation. RhoA and RhoC are overexpressed in a variety of tumor types and are generally considered to have oncogenic properties. Although several studies suggest that RhoB may also promote cell growth, and possess the properties of an oncogene [9, 10], most studies suggest that it may in fact be a tumor suppressor [11, 12]. RhoB expression is low in many tumor types [13–15], and knockout mice display increased susceptibility to carcinogenic agents [12]. Recent studies suggest that a change in prenylation status of RhoB may account for the pro-apoptotic and antineoplastic effects associated with farnesyl transferase inhibitors (FTIs) [16, 17]. Thus, when farnesylation is blocked by FTIs, a shift from RhoB-F to RhoB-GG occurs [16, 17]. Since deletion of RhoB blocks the pro-apoptotic response of FTIs, RhoB-GG is implicated in this response [17]. These observations underscore the need to define changes in signaling activity that occur in response to a change in prenylation status of RhoB.
Another biological activity that distinguishes RhoB from other closely related family members is its failure to activate nuclear factor κB (NF-κB). NF-κB is a transcription factor that has an important role in the regulation of inflammation, proliferation and apoptosis [18]. In its latent state NF-κB resides in the cytoplasm in complex with the inhibitor of NF-κB (IκB) regulatory protein. In response to a variety of signaling events, IκB is phosphorylated and targeted for proteasome-mediated degradation. The newly liberated NF-κB can then migrate into the nucleus where it regulates the transcription of a wide array of target genes. Whereas Rho family members such as RhoA, Rac1 and Cdc42 have been shown to be activators of NF-κB [19], RhoB blocks both the basal and genotoxic agent-stimulated activity of NF-κB when expressed in murine fibroblasts [20]. Here we report the surprising observation that RhoB is a potent activator of NF-κB in multiple cell types, and that activation is preferentially associated with RhoB-F.
2. Materials and Methods
2.1. Molecular constructs
cDNAs that encode human wild-type RhoA, RhoB and RhoC fused to an NH2-terminal hemagluttinin (HA)-epitope tag were generated and cloned into pAX142 [21]. An identical mutant panel was then generated for each isoform: Rho-19N (dominant inhibitory), Rho-30L (fast-cycling), and Rho-63L (constitutively active). pAX142-rhoA/B(HV) encodes a chimeric protein in which the COOH-terminal hypervariable region of RhoA (residues 181–193) is replaced by that of RhoB (residues 181–196). pAX142-rhoB-F, pAX142-rhoB-GG, and pAX142-rhoB-ΔCAAX contain cDNAs that encode mutations of the CAAX motif of RhoB. RhoB-F protein has a CAAX motif amino acid sequence that reads CAIM and can only be modified by a farnesyl lipid moiety, whereas the RhoB-GG protein has a CAAX motif amino acid sequence that reads CLLL and can only be geranylgeranylated [8]. RhoB-ΔCAAX lacks the CAAX motif and cannot be prenylated. pAX142-rhoB-189/192A has alanine substitutions at residues 189 and 192 and cannot be palmitoylated. Construction of pAX142-IκB(SS), pAX142-H-ras-61L, pAX142-bcr(491–876) and pAX142-Tsg101 have been described previously [22, 23]. pCAG-rock(KD) which encodes the kinase-defective version of ROCK I and pCAG-rock(WT) constructs were provided by S. Narumiya [24]. The reporter constructs utilized in the luciferase-coupled transcriptional assays, have been described previously [22, 25].
2.2. Cell culture and reagents
HeLa, T47D, 293T and Cos-7 cells were maintained in Dulbecco’s modified Eagle medium (DMEM; high glucose) supplemented with 10% fetal bovine serum (BenchMark). NIH 3T3 cells were maintained in DMEM supplemented with 10% bovine calf serum (JRH). TNFα cytokine (Sigma) was used to treat cells 18 hours prior to lysate collection at a working concentration of 25 pg/ml. The ROCK inhibitor (Y27632-Calbiochem) was used to treat cells 18 hours prior to lysate collection at a working concentration of 50 μM. siRNA targeting RhoB was purchased (Santa Cruz Biotechnology) and used at a final concentration of 100 nM.
2.3. Transient expression reporter gene assays
For transient expression reporter assays, Lipofectamine 2000 (Invitrogen) was used to transfect HeLa, 293T, T47D and NIH 3T3 cells according to the manufacturer’s instructions, and DEAE-dextran was used for Cos-7 cell transfections as described previously [21]. For reporter assays performed in 293T cells, siRNAs were co-transfected with the plasmid DNA where indicated. In Cos-7 cells, siRNAs were transfected 24 hours after the plasmids using siLentFect reagent according to the manufacturer’s instructions (Bio-Rad). Analysis of luciferase and β-galactosidase expression was performed as described previously [22]. β-galactosidase activity was determined using Lumi-Gal substrate (Lumigen) according to the manufacturer’s instructions. All assays were performed in triplicate.
2.4. Protein expression
Protein expression was determined by Western blot analysis as described previously [21]. Protein was visualized with enhanced chemiluminescence reagents (Santa Cruz Biotechnology).
2.5. Electrophoretic mobility shift assays
Nuclear extracts from 293T cells were prepared as described previously [26]. γ32P labeling of NF-κB consensus oligonucleotides and subsequent binding reactions were done using the Gel Shift Assay System (Promega) using the protocols provided by the manufacturer. Two micrograms of each of the following antibodies was added to the binding reaction step of the EMSA for supershift analysis: p65 (F-6, Santa Cruz Biotechnology) and p105/p50 (Cell Signaling Technology). Reactions were then resolved on 5% polyacrylamide gels.
2.6. Immunofluorescence
Immunostaining of 293T cells was performed as described previously [27]. Cells were viewed with a ZEISS Axiovert 200M microscope fitted with an ApoTome Imaging system. Images shown are from a representative axial plane.
3. Results
3.1. RhoB activates NF-κB
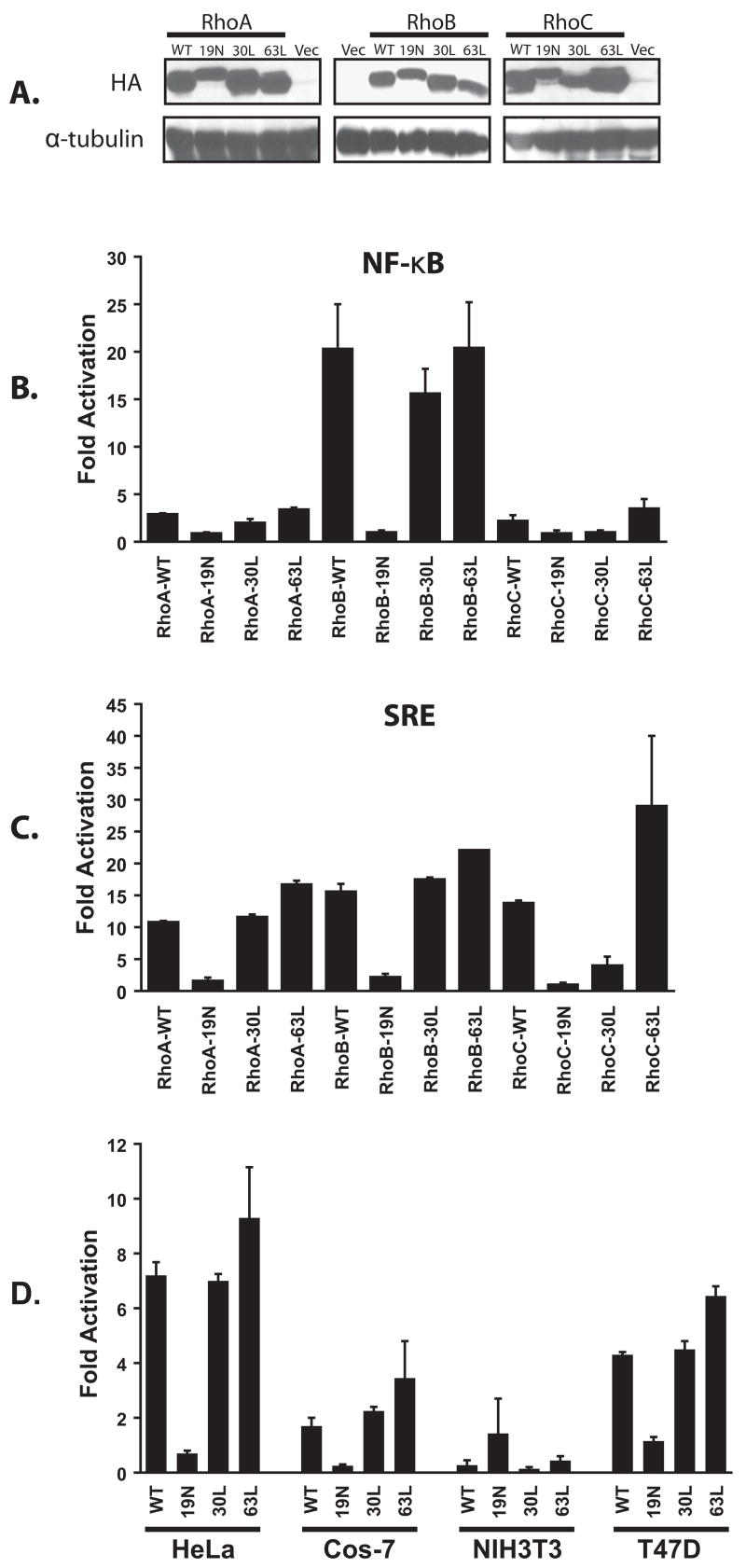
In order to compare the signaling activities of the closely related RhoA, RhoB and RhoC GTPases, a panel of mammalian expression constructs was generated that includes wild-type, dominant inhibitory (19N) and constitutively activated versions. Two different activating mutants were constructed for each GTPase; the GTPase deficient mutants (63L) [28] and the fast-cycling mutants (30L) [29]. All constructs were derived from human cDNAs and were fused at their NH2-terminus to an HA-epitope tag. Stable expression of all panel members was confirmed by transient expression in 293T cells followed by Western blots using an anti-HA antibody (Fig. 1A).
Fig. 1.
Activation of NF-κB signaling by RhoB. (A) Initially, all panel members were transiently expressed in 293T cells and detected by Western blot using a mouse anti-HA monoclonal antibody (vec = cognate vector). Panel members were then transiently transfected into 293T cells along with a luciferase reporter plasmid to determine stimulation of NF-κB-mediated (NF-κB-luc) (B) or serum response element-mediated ((SREm)2-luc) (C) transcriptional activity. pCMVnlac was included in all transfections as an internal control for transfection efficiency and/or growth inhibition. Luciferase and κ-galactosidase activity was measured and expressed as fold activation relative to the level of activation seen with the vector control. Luciferase activity was then standardized relative to β-galactosidase activity. Data shown are representative of at least three independent assays performed on duplicate plates. The error bars indicate standard deviations. (D) RhoB activates NF-κB signaling in multiple cell types. HeLa, Cos-7, NIH 3T3, and T47D cells were transiently transfected with the luciferase reporter plasmid along with plasmids encoding the indicated RhoB derivatives. Reporter activity was determined and represented as described above.
To compare signaling activity, all panel members were then tested in a luciferase-coupled transcriptional assay for activation of NF-κB response elements (Fig. 1B). Initially all assays were done in the human 293T cell line. Whereas RhoA and RhoC exhibited a modest activation of the reporter (2–3 fold), a potent activation (> 20 fold) was associated with the expression of either wild-type RhoB or the two constitutively active mutants. To confirm that RhoA, RhoB and RhoC all have signaling capacity in this cell type, we also compared our panel for activation of a luciferase reporter that is fused to the serum response element (Fig. 1C). All three GTPases exhibited a roughly equivalent ability to activate the reporter (10–20 fold) suggesting that all constructs are functionally competent.
3.2. RhoB activates NF-κB in multiple cell types
Since it has been shown in a previous report that RhoB inhibits NF-κB-mediated transcriptional activity in NIH 3T3 cells [20], we wondered whether the activation of NF-κB by RhoB is limited to 293T cells. Thus, we also examined RhoB for its ability to activate NF-κB in HeLa, T47D, Cos-7 and NIH 3T3 cells (Fig. 1D). RhoB activated the reporter to varying degrees in HeLa, T47D and Cos-7 cells, but not in NIH 3T3 cells. We conclude that the inhibitory effect of RhoB towards NF-κB may be limited to murine fibroblasts.
3.3. RhoB activates NF-κB through the non-classical pathway
IκB(SS) is a derivative of IκBκ that is mutated on serines 32 and 36 [30]. Since this mutant cannot be phosphorylated by the IKK complex, it binds irreversibly to NF-κB and blocks nuclear translocation. To determine whether activation of the NF-κB response element by RhoB requires nuclear NF-κB, we co-transfected wild-type and activated RhoB along with an expression plasmid that encodes IκB(SS) (Fig. 2A). In the presence of the inhibitor, activation of the reporter by wild-type RhoB was reduced below basal levels suggesting that reporter activation is dependent upon the presence of nuclear NF-κB.
Fig. 2.
RhoB activates NF-κB through a non-classical pathway. (A) Activation of NF-κB by RhoB is suppressed by the superrepressor IκB(SS). 293T cells were co-transfected with wild-type RhoB along with pAX142-Ikb(SS) or cognate vector. Data are calculated and presented as in Fig. 1. (B) RhoB does not cause the nuclear accumulation of NF-κB. 293T cells were transiently transfected with pAX142-rhoB(WT) and then examined by indirect immunofluorescence for the subcellular distribution of RhoB and NF-κB. RhoB and NF-κB were visualized using Alexa Fluor 568 (Red) or 488 (Green) conjugated secondary antibodies (Molecular Probes), respectively. Images shown are from a representative axial plane and are representative of over 50 cells examined. (C) EMSA performed with nuclear extracts collected from 293T cells that were either treated with TNFα, or transiently transfected with vector or RhoB-WT. The identity of the p65/p50 complexes (arrow) was confirmed by gel shift assays as indicated. (D) RhoB activates the transactivation domain of RelA/p65. 293T cells were transfected with plasmids encoding the indicated RhoB derivatives, along with vectors for the Gal4 DNA binding domain fused to transactivation domain 1 of p65 (Gal-p65) and a Gal4 luciferase reporter (Gal4-luc). Data are calculated and presented as in Figure 1.
Next we wished to directly examine whether RhoB activates NF-κB by increasing its nuclear accumulation. For this analysis we transiently transfected 293T cells with pAX142-rhoB(WT) or cognate vector, and then examined the cellular distribution of NF-κB using indirect immunofluorescence. As shown in Fig 2B, cells that express RhoB exhibit two discrete regions of immunoreactivity; at the plasma membrane and within a dense region of foci adjacent to the nucleus (lower plate). However, there is no discernable accumulation of NF-κB in the nucleus in RhoB transfected cells when compared to adjacent vector-transfected cells (shown in upper plate). Similar results were obtained for HeLa cells (not shown).
Since nuclear accumulation of NF-κB can also be detected using more sensitive electrophoretic mobility shift assays (EMSA), nuclear lysates were collected from 293T cells that transiently express RhoB and incubated with a 32P-labeled oligonucleotide containing an NF-κB consensus sequence. Lysates were also tested from cells transfected with vector, or treated with TNFα, as negative and positive controls respectively. To confirm the identity of complexes, supershift assays were also performed using antibodies specific for the p50 and p65 subunits of NF-κB. In response to TNFα treatment, a retarded band was visualized by EMSA indicating activation of NF-κB (lane 2). Antibodies to both p50 (lane 3) and p65 (lane 4) shifted the band to a higher molecular mass suggesting that the complex contains both subunits. In contrast, we were unable to detect an increased concentration of active p65/p50 heterodimers in the RhoB expressing cells when compared to vector or mock treated controls.
Besides phosphorylation of inhibitory binding partners, the optimal activation of NF-κB also requires phosphorylation of specific functional domains [18]. Phosphorylation can occur in the cytoplasm and precede nuclear translocation, or can occur directly in the nucleus. Thus, we co-transfected 293T cells with RhoB(WT) and with a reporter system that was designed to detect phosphorylation of the transactivation domain of RelA/p65 [31]. Overexpression of RhoB(WT) resulted in a 7–9-fold stimulation of the reporter relative to vector, while the dominant-inhibitory RhoB(19N) had no effect (Fig. 2D). Thus, activation of NF-κB by RhoB is associated with direct modification of the RelA/p65 transactivation domain.
3.4. Farnesylated RhoB preferentially activates NF-κB
Since RhoB is a potent activator of NF-κB in 293T cells, while RhoA and RhoC activate more weakly, we wondered whether this difference could be attributed to a difference in posttranslational modification. For this analysis we constructed a panel of RhoB mutants that are selectively restricted in their lipid modifications. RhoB-ΔCAAX lacks the CAAX motif and cannot be prenylated or palmitoylated. RhoB-189A/192A has alanine substitutions at residues 189 and 192 and cannot be palmitoylated. RhoB-F is a mutant that is exclusively farnesylated, while RhoB-GG is exclusively geranylgeranylated. Initially, the equal expression of all mutants was verified by Western blot in 293T cells (Fig. 3A). When we block both palmitoylation and prenylation of RhoB by deleting the CAAX motif we are unable to detect activation of the NF-κB reporter suggesting that one or more lipid modifications is required for activation (Fig. 3B). Consistent with this, the double mutant that blocks palmitoylation at residues 189 and 192 also cannot activate the reporter. When we substituted the CAAX motif with a motif that exclusively specifies farnesylation (RhoB-F) or geranylgeranylation (RhoB-GG), we observed that RhoB-F is a potent activator of the reporter (18-fold) while RhoB-GG is consistently weaker (3-fold). A similar difference in signaling capacity was also observed in T47D cells (data not shown). We conclude that activation of the NF-κB reporter by RhoB is enhanced by the presence of a farnesyl group on residue 193, plus one or more palmitoyl groups on residues 189 or 192.
Fig. 3.
Farnesylated RhoB selectively activates NF-κB. A panel of HA-epitope tagged Rho mutants was assembled that included wild-type (WT), dominant-inhibitory (19N), ΔCAAX, RhoB-F (F), RhoB-GG (GG) and RhoB-189/192A (189/192A). (A) Initially, all panel members along with cognate vector were transiently expressed in 293T cells and detected by Western blot using a mouse anti-HA monoclonal antibody. Panel members were then transiently transfected into 293T cells along with NF-κB-luc (B) or (SREm)2-luc (C). Data are calculated and presented as in Figure 1.
For comparison, we also examined our panel of lipid-binding mutants in an SRE reporter assay (Fig. 3C). Although removal of the CAAX box or mutation of the palmitoylation sites impairs activation of the reporter by greater than 70%, both RhoB-F and RhoB-GG are able to activate the reporter with an efficiency equivalent to wild-type. This confirms that both mutants are functionally competent in this cell type, and suggests that SRE activation is more dependent upon the presence of a palmitoyl group, than on the identity of the prenyl group.
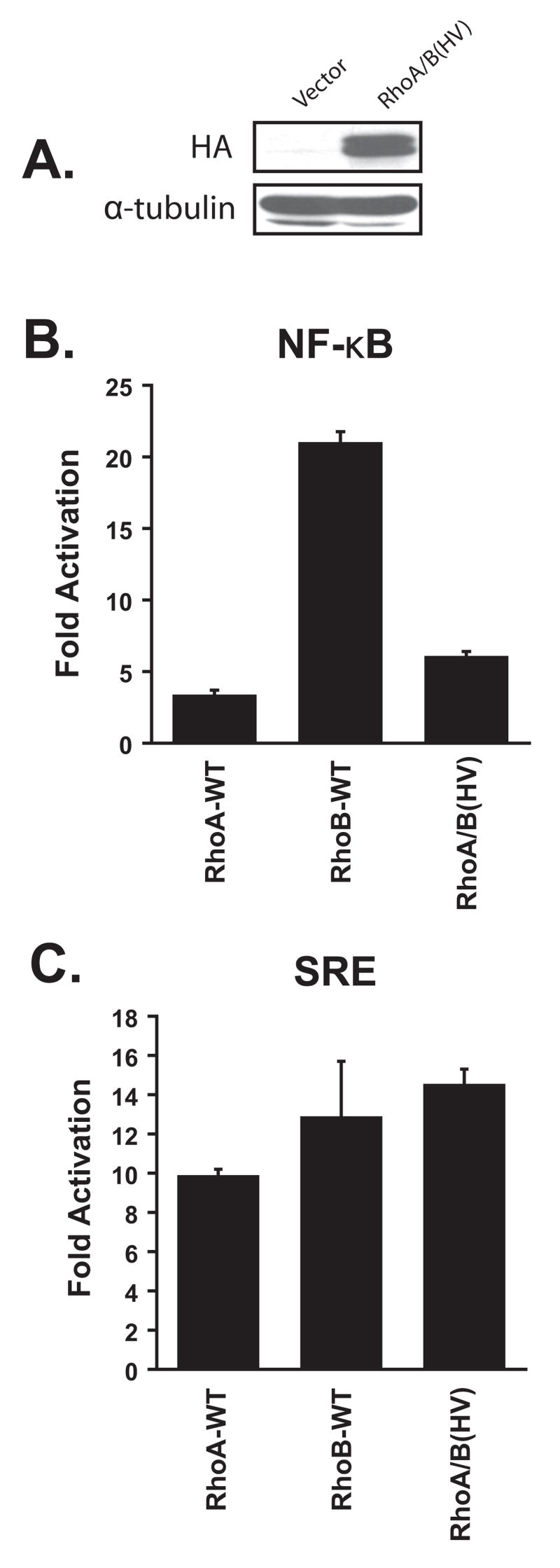
3.5. Activation of NF-κB requires sequences outside of the hypervariable domain
Since the ability of RhoB to activate NF-κB is largely dependent upon a lipid modification that is not associated with RhoA (farnesylation), we wondered whether this difference is sufficient to account for the difference in signaling activity. Thus, we constructed a chimeric molecule (RhoA/B(HV)) in which the hypervariable domain of RhoA was substituted with the equivalent domain from RhoB. When expressed in 293T cells the chimeric molecule consistently migrated as a doublet suggesting that incomplete posttranslational modification may be occurring (Fig. 4A). When we co-expressed the mutant with the NF-κB reporter, we observed only a marginal increase in NF-κB activity (Fig. 4B). However, the failure to activate the reporter could not be attributed to an overall defect in signaling potential since the chimeric molecule was able to activate the SRE reporter to the same extent as wild-type RhoA and RhoB (Fig. 4C). We conclude that sequences that lie outside of the hypervariable domain also contribute to the different signaling capacities of RhoA and RhoB.
Fig. 4.
Activation of NF-κB by RhoB requires sequences that lie outside the hypervariable domain. (A) 293T cells were transiently transfected with expression plasmids that encode vector or RhoA/B(HV). Expression was verified by Western blot using a mouse anti-HA monoclonal antibody. Panel members were then transiently transfected into 293T cells along with NF-κB-luc (B) or (SREm)2-luc (C). Data are calculated and presented as in Figure 1.
3.6. RhoB-dependent activation of NF-κB by Bcr
It has been previously shown that oncogenes such as Bcr, and Ras also activate NF-κB through phosphorylation of p65 [26, 31, 32]. Thus, we wondered whether either oncogene activates NF-κB in a RhoB-dependent manner. To address this possibility, we co-expressed mammalian expression vectors that encode Bcr or activated Ras, along with dominant-inhibitory or wild-type RhoB (Fig. 5). For comparison we also stimulated cells with TNFα. The Bcr studies were performed in Cos-7 cells since Bcr is a more potent activator of NF-κB in this cell type than in 293T cells (S.S. unpublished observations). Consistent with previous reports we observed strong activation of NF-κB by TNFα (20-fold; Fig. 5A) and Bcr (25-fold; Fig 5C). In contrast Ras exhibited a relatively modest 4-fold activation of the reporter (Fig. 5B). Dominant-inhibitory RhoB had no effect on the activation of NF-κB by either TNFα or Ras, and when we co-expressed wild-type RhoB in the presence of TNFα or Ras we observed increased reporter activity that was consistent with an additive effect. In contrast, dominant-inhibitory RhoB substantially blocked activation of NF–κB by Bcr (Fig. 5C). A consistent but statistically insignificant reduction in NF-κB activity was also observed when we co-expressed wild-type RhoB with Bcr. However, we also observed an extremely high level of growth inhibition in this condition which may account for the low level of NF-κB activity (not shown). This growth inhibition was not observed when we expressed wild-type RhoB in the presence of Ras suggesting that the combination of RhoB and Bcr may be toxic to Cos-7 cells. To confirm that Bcr activates NF-κB in a RhoB-dependent manner we then repeated the reporter assays in the presence of RhoB specific siRNAs (Fig. 5D). In the presence of siRNAs that suppress RhoB, but not the closely related RhoA, we observed a 60–70% reduction in NF-κB activation by Bcr. Collectively these results suggest that Bcr activates NF-κB in a RhoB-dependent manner, while Ras and TNFα activate NF-κB in a RhoB-independent manner.
Fig. 5.
RhoB mediates NF-κB activation by Bcr and TSG101, but not by Ras or TNFα. 293T cells (A, B, E, F) or Cos-7 cells (C, D) were transiently transfected with the indicated plasmids along with NF-κB-luc and pCMVnlac. All data are calculated and presented as in Figure 1. (A) Cells were mock-treated (mock), or treated with 25 pg/ml TNFα, prior to lysate collection. (D & F) Cells were co-transfected with siRNAs that target RhoB. Lower panels indicate Western blots on lysates demonstrating specific suppression of RhoB expression.
3.7. RhoB-dependent activation of NF-κB by TSG101
Since both RhoB and Bcr have been implicated in endosomal sorting we wondered whether activation of NF-κB by RhoB and Bcr may be attributable to this shared cellular function. RhoB has been localized to the limiting membrane of the mammalian MVB [4], while Bcr interacts with subunits of the ESCRT sorting complexes that are found on the same membrane [25, 33]. TSG101 is a subunit of the ESCRT complex that has been shown to directly interact with Bcr [25]. Despite the fact that TSG101 has no described catalytic activity, we wondered whether other components of the ESCRT complex could also regulate transcriptional signaling. When expressed alone, TSG101 stimulated NF-κB activity by 8-fold, as compared to 16-fold activation by RhoB(WT) (Fig. 5E). As was the case for Bcr, dominant-inhibitory RhoB blocked activation of NF-κB by TSG101 (Fig 5E), as did siRNAs that specifically suppress RhoB expression (Fig. 5F). We conclude that RhoB, Bcr and TSG101 may be activating NF-κB through a common endosome-mediated pathway.
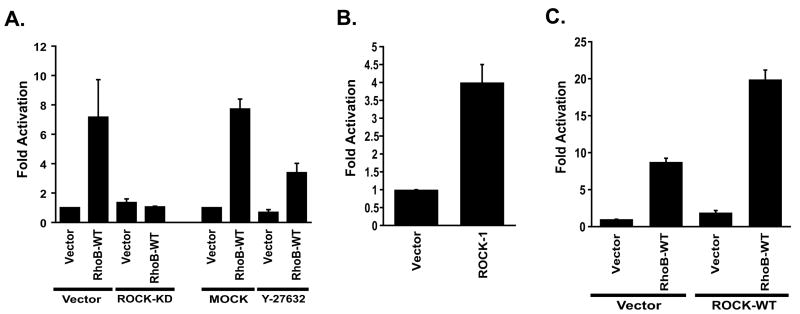
3.8. RhoB activates NF-κB in a ROCK-dependent manner
Next we wished to determine which effector protein is used by RhoB to activate NF-κB. In a recent study Milia et al. showed that RhoB-F (but not RhoB-GG) is required to confer resistance to radiation-induced mitotic cell death in mouse embryonic fibroblasts and that this function is dependent on ROCK I [34]. In addition, ROCK I has been reported to mediate thrombin-induced NF-κB activation in endothelial cells, which in part can be attributed to increased phosphorylation of the transactivation domain of RelA/p65 [35]. Since we observed that RhoB also activates NF–κB activity through modification of the RelA/p65 transactivation domain, we wondered whether this occurs in a ROCK I dependent manner. Initially we determined whether inhibitors of ROCK I could block NF-κB activation by RhoB. In the presence of a dominant-inhibitory version of ROCK I (ROCK I(KD)), or a pharmacological inhibitor (Y-27632), activation of NF-κB by RhoB is substantially reduced (Fig. 6A). In contrast, activation by RhoB is not blocked by a dominant-inhibitory version of PRK I which is a known effector for RhoB (not shown). Collectively, these results suggest that ROCK I mediated signaling is necessary to support NF-κB activation by RhoB.
Fig. 6.
RhoB activates NF-κB in a ROCK-dependent manner. 293T cells were transiently transfected with the indicated plasmids along with NF-κB-luc and pCMVnlac. All data are calculated and presented as in Figure 1. (A) Inhibitors of ROCK I block NF-κB activation by RhoB. Cells were transfected with RhoB along with dominant-inhibitory ROCK I (ROCK-KD), or in the presence of 50 μM Y-27632. (B) ROCK I activates NF-κB. 293T cells were transfected with 3 μg of wild-type ROCK I or cognate vector as indicated. (C) ROCK I cooperates with RhoB to activate NF-κB. Cells were transfected with 250 ng of ROCK I in order to observe the synergistic interaction with RhoB.
Next we wondered whether RhoB-mediated ROCK I activation is sufficient for NF-κB activation. When we expressed wild-type ROCK I alone in 293T cells we observed a 4-fold increase in NF-κB activity suggesting that endogenous ROCK I may in fact be a viable target for RhoB (Fig. 6B). We then reduced the amount of ROCK I in our transfection to a level at which it can no longer activate NF-κB, and then co-expressed it with RhoB or cognate vector (Fig. 6C). When co-expressed with ROCK I, RhoB-mediated activation of NF-κB was substantially higher than with vector (20-fold vs. 8-fold). These results are consistent with a synergistic interaction between RhoB and ROCK I, and suggest that RhoB may be recruiting and activating endogenous ROCK I.
4. Discussion
Unlike RhoA and RhoC which are constitutively expressed, RhoB is induced by both physical and chemical agents, which suggests that it may be a component of the cellular stress response [1, 2]. Consistent with this, fibroblasts derived from RhoB null mice are resistant to both physical and chemical induced apoptosis [12], and RhoB induces apoptosis if overexpressed in transformed cells [9]. RhoB expression is often suppressed during tumor progression which may impair normal apoptotic signaling, and thus, support tumor growth [13–15]. It has been previously reported that RhoB suppresses NF-κB signaling in murine fibroblasts which may account for this propapoptotic activity [20]. However, in the current study we have observed that RhoB activates NF-κB in most cell types examined including a tumor-derived breast epithelial cell line. Consistent with our observations it has been recently reported that RhoB can also activate an NF-κB-responsive reporter in a human ovarian tumor cell line [36]. This suggests that the high levels of NF-κB activity that is observed in many tumor types can not be attributed to a loss of RhoB expression, and is unlikely to be related to RhoB tumor suppressor activity.
Previous studies indicate that both RhoB-GG and RhoB-F may exist in vivo [8], and that they may have distinct cellular activities. Thus, RhoB-GG suppresses Ras transformation in NIH 3T3 cells while RhoB-F potentiates transformation [9]. In contrast, RhoB-F can provide resistance to radiation-induced cell death in NIH 3T3 cells while RhoB-GG does not provide such protection [34]. The precise signaling activities that mediate these differences in signaling potential have not been delineated. In this current study we have determined that RhoB is a potent activator of NF-κB-mediated transcription, and that this activation is primarily associated with the farnesylated variant. Geranylgeranylated RhoB only weakly activates NF-κB transcription at a level equivalent to what we observe for RhoA and RhoC. To our knowledge, this constitutes the first example of a signaling pathway that is preferentially activated by RhoB-F, and provides additional support for its in vivo relevance.
Although the prototypical mechanism of NF-κB activation involves the proteasome-mediated degradation of IκBκ, followed by the rapid translocation of NF-κB into the nucleus, we see no evidence of increased levels of nuclear NF-κB or increased binding activity in RhoB transfected cells. This is similar to what we have previously observed for Bcr [22]. However, an alternate mechanism for activating NF-κB involves phosphorylation of the transactivation domain [37–39]. Several studies have shown that the transcriptional activity of NF-κB is increased by phosphorylation at ser276, ser311, ser529 or ser536 [40–43]. The precise site of phosphorylation and the kinase that is responsible, can vary depending upon the mechanism of activation, and the cell-type that is examined [44–48]. Our observation that Bcr can activate an NF-κB reporter system which is designed to specifically examine changes in the transcriptional function of the transactivation domain suggests that RhoB may be activating NF-κB through this alternate mechanism.
Our observation that RhoB activation of NF-κB occurs in a ROCK I dependent manner is consistent with a recent study which suggests that thrombin-mediated activation of NF-κB in endothelial cells is also mediated by ROCK I [35]. Thus, thrombin induces NF-κB dependent upregulation of ICAM-I expression through activation of IκB kinase, and by phosphorylation of RelA/p65 on serine 536. However, we do not observe increased phosphorylation of RelA/p65 on serine 536 in RhoB transfected cells (data not shown), suggesting that activation of NF-κB by RhoB and thrombin occur through discrete downstream signaling events. Although ROCK I is considered to be a major effector for RhoA, there is limited information regarding the relationship between ROCK I and RhoB. It is clear that RhoB can bind to ROCK I in vitro, albeit weaker than RhoA and RhoC, and the sequences that are essential for ROCK I binding are identical between RhoA, RhoB and RhoC [49]. Additionally, the assembly of aderens junctions in Sertoli germ cell cultures is regulated by a RhoB-ROCK-LIMK pathway indicating that RhoB can engage ROCK I in cell-based studies [50]. Since in our hands RhoA is a much weaker activator of NF-κB than RhoB when expressed in equivalent cell types, it is possible that overexpressed RhoB can recruit ROCK I to signaling foci that are discrete from those that contain RhoA.
Our observation that both Bcr and TSG101 activate NF-κB in a RhoB-dependant manner suggests the existence of a novel signaling pathway that links trafficking events at the MVB with transcriptional activation. The formation of the MVB, and the sorting of cargo, is controlled by a large collection of proteins that was originally identified in yeast as the Class E vacuolar sorting proteins (Vps; reviewed in [51]). The mammalian homologs of the Vps proteins include TSG101, which serves to recognize ubiquitylated proteins for MVB internalization. In a recent study we have determined that Bcr interacts with TSG101, and is also a subunit of the mammalian sorting complex [25, 26]. Although the precise role that RhoB plays in this sorting pathway is unclear, it has also been localized to the MVB, and interference with RhoB, Bcr or TSG101 have an equivalent phenotype with regard to receptor turnover [5, 25, 52]. Although RhoB has not been shown to directly interact with TSG101 or Bcr, Bcr contains a Rho-specific guanine nucleotide exchange factor activity, and thus, may utilize endogenous RhoB as a substrate for NF-κB activation. Similarly, although TSG101 does not possess any known catalytic activities, it may signal through RhoB by activating endogenous Bcr. Alternatively, overexpression of either Bcr or TSG101 may disturb the stoichiometry of the ESCRT complexes and trigger an intracellular stress response. Although such a pathway has not been described for the endosome, it would be analogous to the intracellular stress response pathway that links the overloading of the endoplasmic reticulum to the activation of the NF-κB transcriptional pathway [53].
5. Conclusions
The role of RhoB in human tumorigenesis remains unclear. In Ras-transformed NIH 3T3 fibroblasts it has been proposed that RhoB-F has a pro-growth activity while RhoB-GG suppresses tumor growth by inducing apoptosis [9]. Thus, treatment with farnesyl transferase inhibitors (FTI) may shift RhoB from the farnesylated to the geranylgeranylated form and uncover a tumor suppressor function. Consistent with this hypothesis it has been shown recently that FTIs can block the transforming activity of RhoC transformed breast epithelial cells by targeting RhoB [10]. In addition, RhoB-GG suppresses RhoC-mediated transformation in breast epithelial cells [10]. However, aspects of this model have been challenged by studies that demonstrate that both RhoB-F and RhoB-GG can inhibit malignant transformation when expressed in pancreatic sarcoma, osteosarcoma or cervical carcinoma cells lines [11], and that RhoB null mice exhibit increased susceptibility to tumorigenesis [12]. When viewed in their entirety these studies suggest that the contributions of RhoB to transformation may be sensitive to both the prevalence of a particular prenylated variant, and the type of tumor that these variants are expressed in. Our observation that RhoB-F can preferentially activate NF-κB would provide a molecular explanation for the ability of RhoB to function as an oncogene in some cellular contexts.
Acknowledgments
This work was supported by Public Health Service grants CA097066 (IPW) and F31 CA117049 (O. Olabisi) from the National Cancer Institute. PL Rodriguez is a recipient of a predoctoral fellowship from the New Jersey Commission for Cancer Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fritz G, Kaina B. J Biol Chem. 1997;272(49):30637–30644. doi: 10.1074/jbc.272.49.30637. [DOI] [PubMed] [Google Scholar]
- 2.Jahner D, Hunter T. Mol Cell Biol. 1991;11(7):3682–3690. doi: 10.1128/mcb.11.7.3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adamson P, Paterson HF, Hall A. J Cell Biol. 1992;119(3):617–627. doi: 10.1083/jcb.119.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wherlock M, Gampel A, Futter C, Mellor H. J Cell Sci. 2004;117(Pt 15):3221–3231. doi: 10.1242/jcs.01193. [DOI] [PubMed] [Google Scholar]
- 5.Gampel A, Parker PJ, Mellor H. Curr Biol. 1999;9(17):955–958. doi: 10.1016/s0960-9822(99)80422-9. [DOI] [PubMed] [Google Scholar]
- 6.Zhang FL, Casey PJ. Annu Rev Biochem. 1996;65:241–269. doi: 10.1146/annurev.bi.65.070196.001325. [DOI] [PubMed] [Google Scholar]
- 7.Armstrong SA, Hannah VC, Goldstein JL, Brown MS. J Biol Chem. 1995;270(14):7864–7868. doi: 10.1074/jbc.270.14.7864. [DOI] [PubMed] [Google Scholar]
- 8.Baron R, Fourcade E, Lajoie-Mazenc I, Allal C, Couderc B, Barbaras R, Favre G, Faye JC, Pradines A. Proc Natl Acad Sci U S A. 2000;97(21):11626–11631. doi: 10.1073/pnas.97.21.11626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazieres J, Tillement V, Allal C, Clanet C, Bobin L, Chen Z, Sebti SM, Favre G, Pradines A. Exp Cell Res. 2005;304(2):354–364. doi: 10.1016/j.yexcr.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 10.van Golen KL, Bao L, DiVito MM, Wu Z, Prendergast GC, Merajver SD. Mol Cancer Ther. 2002;1(8):575–583. [PubMed] [Google Scholar]
- 11.Chen Z, Sun J, Pradines A, Favre G, Adnane J, Sebti SM. J Biol Chem. 2000;275(24):17974–17978. doi: 10.1074/jbc.C000145200. [DOI] [PubMed] [Google Scholar]
- 12.Liu AX, Rane N, Liu JP, Prendergast GC. Mol Cell Biol. 2001;21(20):6906–6912. doi: 10.1128/MCB.21.20.6906-6912.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adnane J, Muro-Cacho C, Mathews L, Sebti SM, Munoz-Antonia T. Clin Cancer Res. 2002;8(7):2225–2232. [PubMed] [Google Scholar]
- 14.Forget MA, Desrosiers RR, Del M, Moumdjian R, Shedid D, Berthelet F, Beliveau R. Clin Exp Metastasis. 2002;19(1):9–15. doi: 10.1023/a:1013884426692. [DOI] [PubMed] [Google Scholar]
- 15.Mazieres J, Antonia T, Daste G, Muro-Cacho C, Berchery D, Tillement V, Pradines A, Sebti S, Favre G. Clin Cancer Res. 2004;10(8):2742–2750. doi: 10.1158/1078-0432.ccr-03-0149. [DOI] [PubMed] [Google Scholar]
- 16.Du W, Lebowitz PF, Prendergast GC. Mol Cell Biol. 1999;19(3):1831–1840. doi: 10.1128/mcb.19.3.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu A, Du W, Liu JP, Jessell TM, Prendergast GC. Mol Cell Biol. 2000;20(16):6105–6113. doi: 10.1128/mcb.20.16.6105-6113.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Viatour P, Merville MP, Bours V, Chariot A. Trends Biochem Sci. 2005;30(1):43–52. doi: 10.1016/j.tibs.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 19.Perona R, Montaner S, Saniger L, Sanchez-Perez I, Bravo R, Lacal JC. Genes Dev. 1997;11(4):463–475. doi: 10.1101/gad.11.4.463. [DOI] [PubMed] [Google Scholar]
- 20.Fritz G, Kaina B. J Biol Chem. 2001;276(5):3115–3122. doi: 10.1074/jbc.M005058200. [DOI] [PubMed] [Google Scholar]
- 21.Whitehead I, Kirk H, Tognon C, Trigo-Gonzalez G, Kay R. J Biol Chem. 1995;270(31):18388–18395. doi: 10.1074/jbc.270.31.18388. [DOI] [PubMed] [Google Scholar]
- 22.Korus M, Mahon GM, Cheng L, Whitehead IP. Oncogene. 2002;21(30):4601–4612. doi: 10.1038/sj.onc.1205678. [DOI] [PubMed] [Google Scholar]
- 23.Olabisi OO, Mahon GM, Kostenko EV, Liu Z, Ozer HL, Whitehead IP. Cancer Res. 2006;66(12):6250–6257. doi: 10.1158/0008-5472.CAN-06-0536. [DOI] [PubMed] [Google Scholar]
- 24.Liu Z, Kostenko EV, Mahon GM, Olabisi OO, Whitehead IP. J Biol Chem. 2006 doi: 10.1074/jbc.M601823200. [DOI] [PubMed] [Google Scholar]
- 25.Olabisi NMG, Kostenko E, Liu Z, Ozer HL, Whitehead IP. 2006 [Google Scholar]
- 26.Korus M, Mahon GM, Cheng L, Whitehead IP. 2001 doi: 10.1038/sj.onc.1205678. in press. [DOI] [PubMed] [Google Scholar]
- 27.Rossman KL, Cheng L, Mahon GM, Rojas RJ, Snyder JT, Whitehead IP, Sondek J. J Biol Chem. 2003;278(20):18393–18400. doi: 10.1074/jbc.M300127200. [DOI] [PubMed] [Google Scholar]
- 28.Khosravi-Far R, Solski PA, Clark GJ, Kinch MS, Der CJ. Mol Cell Biol. 1995;15(11):6443–6453. doi: 10.1128/mcb.15.11.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin R, Cerione RA, Manor D. J Biol Chem. 1999;274(33):23633–23641. doi: 10.1074/jbc.274.33.23633. [DOI] [PubMed] [Google Scholar]
- 30.Whitehead IP, Lambert QT, Glaven JA, Abe K, Rossman KL, Mahon GM, Trzaskos JM, Kay R, Campbell SL, Der CJ. Mol Cell Biol. 1999;19(11):7759–7770. doi: 10.1128/mcb.19.11.7759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reuther JY, Reuther GW, Cortez D, Pendergast AM, Baldwin AS., Jr Genes Dev. 1998;12(7):968–981. doi: 10.1101/gad.12.7.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galang CK, Der CJ, Hauser CA. Oncogene. 1994;9(10):2913–2921. [PubMed] [Google Scholar]
- 33.Babst M. Traffic. 2005;6(1):2–9. doi: 10.1111/j.1600-0854.2004.00246.x. [DOI] [PubMed] [Google Scholar]
- 34.Milia J, Teyssier F, Dalenc F, Ader I, Delmas C, Pradines A, Lajoie-Mazenc I, Baron R, Bonnet J, Cohen-Jonathan E, Favre G, Toulas C. Cell Death Differ. 2005;12(5):492–501. doi: 10.1038/sj.cdd.4401586. [DOI] [PubMed] [Google Scholar]
- 35.Anwar KN, Fazal F, Malik AB, Rahman A. J Immunol. 2004;173(11):6965–6972. doi: 10.4049/jimmunol.173.11.6965. [DOI] [PubMed] [Google Scholar]
- 36.Chen YX, Li ZB, Diao F, Cao DM, Fu CC, Lu J. J Steroid Biochem Mol Biol. 2006;101(4–5):179–187. doi: 10.1016/j.jsbmb.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 37.Chen LF, Mu Y, Greene WC. Embo J. 2002;21(23):6539–6548. doi: 10.1093/emboj/cdf660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quivy V, Van Lint C. Biochem Pharmacol. 2004;68(6):1221–1229. doi: 10.1016/j.bcp.2004.05.039. [DOI] [PubMed] [Google Scholar]
- 39.Schmitz ML, Bacher S, Kracht M. Trends Biochem Sci. 2001;26(3):186–190. doi: 10.1016/s0968-0004(00)01753-9. [DOI] [PubMed] [Google Scholar]
- 40.Buss H, Dorrie A, Schmitz ML, Frank R, Livingstone M, Resch K, Kracht M. J Biol Chem. 2004;279(48):49571–49574. doi: 10.1074/jbc.C400442200. [DOI] [PubMed] [Google Scholar]
- 41.Rocha S, Garrett MD, Campbell KJ, Schumm K, Perkins ND. Embo J. 2005;24(6):1157–1169. doi: 10.1038/sj.emboj.7600608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Teusch N, Lombardo E, Eddleston J, Knaus UG. J Immunol. 2004;173(1):507–514. doi: 10.4049/jimmunol.173.1.507. [DOI] [PubMed] [Google Scholar]
- 43.Yeh PY, Yeh KH, Chuang SE, Song YC, Cheng AL. J Biol Chem. 2004;279(25):26143–26148. doi: 10.1074/jbc.M402362200. [DOI] [PubMed] [Google Scholar]
- 44.Bohuslav J, Chen LF, Kwon H, Mu Y, Greene WC. J Biol Chem. 2004;279(25):26115–26125. doi: 10.1074/jbc.M313509200. [DOI] [PubMed] [Google Scholar]
- 45.Madrid LV, Mayo MW, Reuther JY, Baldwin AS., Jr J Biol Chem. 2001;276(22):18934–18940. doi: 10.1074/jbc.M101103200. [DOI] [PubMed] [Google Scholar]
- 46.Sakurai H, Chiba H, Miyoshi H, Sugita T, Toriumi W. J Biol Chem. 1999;274(43):30353–30356. doi: 10.1074/jbc.274.43.30353. [DOI] [PubMed] [Google Scholar]
- 47.Vermeulen L, De Wilde G, Van Damme P, Vanden Berghe W, Haegeman G. Embo J. 2003;22(6):1313–1324. doi: 10.1093/emboj/cdg139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang F, Tang E, Guan K, Wang CY. J Immunol. 2003;170(11):5630–5635. doi: 10.4049/jimmunol.170.11.5630. [DOI] [PubMed] [Google Scholar]
- 49.Wheeler AP, Ridley AJ. Exp Cell Res. 2004;301(1):43–49. doi: 10.1016/j.yexcr.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 50.Lui WY, Lee WM, Cheng CY. Biol Reprod. 2003;68(6):2189–2206. doi: 10.1095/biolreprod.102.011379. [DOI] [PubMed] [Google Scholar]
- 51.Katzmann DJ, Odorizzi G, Emr SD. Nat Rev Mol Cell Biol. 2002;3(12):893–905. doi: 10.1038/nrm973. [DOI] [PubMed] [Google Scholar]
- 52.Babst M, Odorizzi G, Estepa EJ, Emr SD. Traffic. 2000;1(3):248–258. doi: 10.1034/j.1600-0854.2000.010307.x. [DOI] [PubMed] [Google Scholar]
- 53.Pahl HL, Baeuerle PA. Embo J. 1995;14(11):2580–2588. doi: 10.1002/j.1460-2075.1995.tb07256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]