Introduction
Many exciting new variations of MRI have been tested in MR research, but only a few find their way to clinical use. Sodium (23Na) MRI is one of those techniques that at first glance appeared to be very promising. 23Na MRI has the potential to extend MR imaging beyond anatomical imaging by providing information on physiology and cellular metabolism. Signal strength is related to disease specific changes in the tissue. This type of ‘disease specific’ contrast can compensate for the much lower signal to noise ratio of these techniques. The contrast between ischaemic (1) and healthy cardiac tissue and between benign tissue and malignant tumors (2) is based on significant changes in Tissue Sodium Concentration (TSC). For stroke in humans an increase in TSC was recorded of 50%(3).
The feasibility of 23Na MRI on human subjects has been demonstrated as far back as 1989 (4) and the image contrast obtainable in stroke(3), cancer and edema (2) has also been demonstrated some time ago. In spite of this, 23Na MRI has yet to find a wider acceptance as a possible clinical tool. The technical difficulty of obtaining 23Na scans can only be part of the reason. If it had been deemed worth it commercially, the MR scanner manufacturers would have found a way to simplify the procedure to almost a push button solution. Apparently, the progression of 23Na MRI from research to clinical use was halted by a failure to create a demand for the great potential of 23Na MRI.
Currently 23Na MRI appears to have been rediscovered. Even though the technical advances since the early 90's have been incremental, a threshold has been reached by reducing scan times to the point where the addition of a 23Na MRI to an existing 1H-MRI protocol becomes an option for studies on patients rather than just very patient volunteers.
An overview of recent advances in the use of 23Na-MRI for clinical MR research will illustrate the very significant consequences of the reduction of the length of a 23Na scan from more than 45 minutes in earlier attempts to less than 15 minutes at 1.5T. Now 23Na-MRI is no longer a stand-alone modality with 1H-MRI used only as a scout image. Instead, 23Na-MRI is used as an add-on to improve specificity of established 1H-MRI protocols. To understand what kind of information 23Na-MR can and cannot add to an MRI exam we have to explore the properties of this nucleus and what imaging techniques we can use to observe it.
23Na-MRI techniques
The signal to noise ratio (SNR) of 23Na-MRI is much lower than that of 1H-MRI, mainly because of the difference in abundance between water protons and sodium ions in the body and also because of the lower gyro-magnetic ratio. The short longitudinal relaxation time, T1, in the order of 25-30 milliseconds (ms) allows fairly rapid repetition rates and this partially compensates for the low SNR, even when repetition rate, TR, is chosen relatively long to allow quantification of TSC without T1 relaxation corrections. The transverse relaxation time, T2, of sodium is very fast and is bi-exponential in most biological tissues and in gels. Part of the sodium experiences anisotropic interactions with proteins. In very ordered environments such as cartilage or gels this can lead to a short T2 in the order of 1-2 ms for 60% of the signal and a longer T2 in the order of 20-30ms for 40% of the signal. In most biological tissues the fraction of the fast relaxing component is less than 60%, but still large signal losses can occur as a result of T2 relaxation. For quantification of TSC short TE in the order of 0.2-0.4 ms is required to reduce those T2 losses to less than 5-10% of the total signal. This requirement for a short TE together combined with the need for small receiver bandwidths for better SNR, makes it difficult to use gradient recalled echo sequences with little T2 losses and good SNR.
One method to overcome this problem is to use 3D projection imaging (PI) methods. In 3D-PI sequences there is no need for a selective pulse and no need for slice select gradient refocusing or phase encode gradient pulse. Thus, the signal acquisition can start very shortly after the excitation pulse with an effective TE that is only limited by the pulse duration and the switching time between transmit and receive.
To reduce the large number of excitations needed for a complete 3D image with PI using constant projection gradients during readout, the projections can be twisted by using time varying gradients. This method has been successfully applied to quantitation of TSC in humans(2,3).
Neither the increase in intracellular sodium concentration, nor an increase in extracellular space is entirely unique for cancer. In perfused ex-vivo organs and in some animal models intraand extra cellular sodium can be separated with shift reagents that remain exclusively extracellular, but so far all suitable shift reagents are toxic and thus of no use for human studies. The Triple Quantum Filtered (TQF) 23Na-MRI technique has been suggested as an alternative way to separate intra-and extra-cellular sodium. TQF certainly provides some suppression of extracellular signals, thus accentuating the tumor specific changes in the intracellular sodium, but the price in Signal to Noise ratio (SNR) of such filters is very high, leading to much longer scan times and reduced resolution and T2 dependencies of the TQF signals further complicate quantification.
Without a reliable means to exclusively quantify intra-cellular sodium we can still use 23NaMR imaging of the total tissue sodium to our advantage, but only in combination with an optimized comprehensive 1H-MRI protocol (see Figure 1). To appreciate what type of information is added by 23Na MRI adds to an MRI exam we need to understand the origin of the disease specific contrast delivered by measurement of TSC with 23Na-MRI.
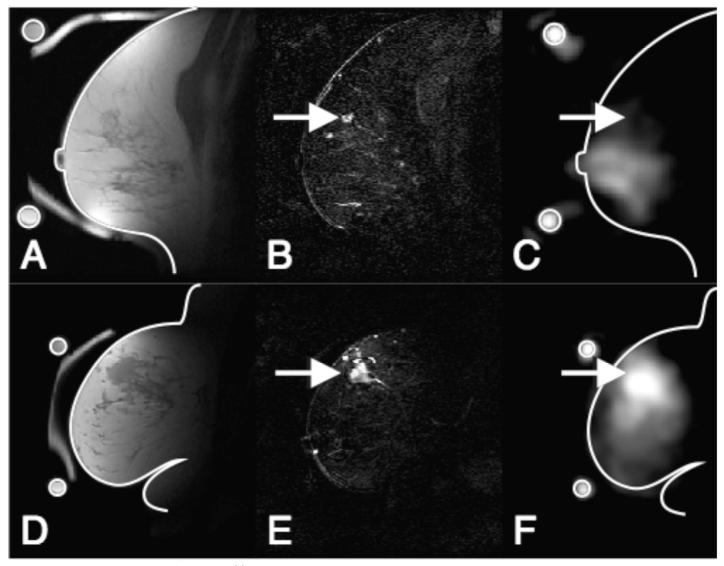
Figure 1.
Registered 1H and 23Na-MRI breast images of a benign (ABC) and a malignant breast lesion (DEF). Images ABC are from a 55 year-old woman with proliferative fibrocystic changes and sclerosing adenosis (benign). Images DEF are from a 54 year-old woman with an infiltrating poorly differentiated ductal carcinoma (T3, malignant). A,D) T1 weighted 1H-MRI showing anatomical details B,E) Difference images of pre and post Gd injection CE 1H-MRI showing enhancement in the lesion indicated by arrows C,F) 23Na images with arrows to the position of enhancing lesions. Outlines of the breast and a ring shaped phantom, containing a 150 mMol/l saline solution, taken from images A and D are superimposed on the 23Na images for position reference. The 23Na images show hyperintensity in the Gd positive region of the malignant case but not in the benign case.
23Na-MRI and heart disease
In a dog model of acute ischemia the TSC increase right after reperfusion was in excess of 200% of the baseline myocardial TSC. The increase in sodium in older infarcts is less pronounced, typically about 50%. The origin of this increase is unknown. Ex-vivo hearts from rats with four-week old myocardial infarcts (MI) showed normal intracellular sodium content and an increase in the extracellular volume fraction of the scar tissue. Whether increase in the extarcellular space is the dominant mechanism leading to the increased sodium signals observed in human hearts with non-acute myocardial infarcts is not known. The most interesting feature from a clinical point of view would be the intracellular sodium concentration in at-risk areas around the MI, rather that the TSC in non-viable tissue. Elevated intracellular sodium in myocytes could well be one factor leading to arrhythmias, particularly under stress.
23Na-MRI to monitor therapy
Lack of substrate and oxygen can cause very significant changes in tissue sodium content. As soon as the energy dependend Na+/K+-ATPase stops pumping sodium out of the cell, passive sodium influx from the extracellular environment will rapidly raise the intracellular levels of sodium several fold. This effect is exacerbated if a stress on the cells increases the permeability of the cell wall for sodium ions.
The acute effect of a therapy that causes cell death on an appreciable scale should therefore be easy to monitor with 23Na-MRI. The effect of the Highly Focused Ultrasound (HIFUS) ablation of uterine fibroids could be seen in 23Na-MRI images taken within 24 hours of therapy. Long-term effects of such therapies may be more difficult to predict. In a limited number of patients with breast cancer pre-operative systemic chemotherapy the effect of the therapy in responders showed as a decline in the tumor TSC along with a decline in lesion size. In this application of 23Na MRI it is important to have a reference or a quantification method to make comparison of the results of baseline images and multiple post-chemo therapy images possible.
23Na-MRI and cancer
Proliferating cells have an abnormally high sodium content, because the normally very low intracellular sodium concentration of about 10-15 mmol/l is elevated as a result of altered Na+/H+ transport kinetics (5) and pH. Outside the cells continuous perfusion of living tissue will ensure a constant sodium concentration of approximately 140 mmol/l. Thus, an increase in the extracellular partial tissue volume through the increased vascularization (angiogenesis) and the increased interstitial space in tumors will also lead to in crease in tissue sodium concentration (TSC) in tumors.
Using a quantitative 23Na-MRI technique, a 50% increase of in TSC relative to non-involved contra-lateral tissues was found in malignant brain tumors (2). Likewise, in malignant breast tumors an increase of 50% in TSC was found relative to non-involved glandular tissue and benign lesions. This information could augment the specificity of Contrast Enhanced (CE)–MRI. The unique sensitivity of 23Na-MRI to both extra-cellular volume and intracellular changes related to cell proliferation can provide information that is supplemental to a full coverage high resolution CE-MRI scan (see Figure 1).
Conclusion
If we want to retain the best possible resolution in 23Na MRI, we must look other relatively high-resolution 1H-MR techniques such as Fluid Attenuated Inversion Recovery (FLAIR)-MRI, CE–MRI or Diffusion Weighted (DW)-MRI to distinguish non-cancer-high -TSC tissues from malignant tumors. This goes for any clinical application of 23Na-MRI. The protocol will be different for each particular application and in each application we have to examine whether 23Na-MRI has enough added value to justify the extra scan time and effort. But in doing so we must not forget that practically all MRI exams consist of a series of scans and only the whole set will yield the desired diagnostic sensitivity and specificity. Thus we cannot expect that 23Na-MRI can compete with this in an exam consisting of only a scout image and a 23Na-MRI.
This is where the reduced scan time for 23Na MRI has made an impact. The total scan time for a protocol yielding for a good diagnostic quality set of 1H-MR images and a 23Na image can be limited to less than an hour. This is a really important factor in continued patient recruitment even for repeat MRI scans on the same patients. Quantitative imaging of TSC used in longitudinal studies in stroke (3) or to monitor treatment in cancer may prove to be one of the more interesting uses of 23Na-MRI. If we consistently use 23Na-MRI in synergy with 1H-MRI we may learn a lot about progressive changes in TSC related to edema, ischemia or cancer.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Ronald Ouwerkerk, Johns Hopkins University, School of Medicine, Division of MR Research.
Russell H. Morgan, Department of Radiology and Radiological Science.
References
- 1.Constantinides CD, Kraitchman DL, O'Brien KO, Boada FE, Gillen J, Bottomley PA. Noninvasive quantification of total sodium concentrations in acute reperfused myocardial infarction using 23Na MRI. Magn Reson Med. 2001;46(6):1144–1151. doi: 10.1002/mrm.1311. [DOI] [PubMed] [Google Scholar]
- 2.Ouwerkerk R, Bleich KB, Gillen JS, Pomper MG, Bottomley PA. Tissue Sodium Concentration in Human Brain Tumors as Measured with 23Na MR Imaging. Radiology. 2003;227:529–537. doi: 10.1148/radiol.2272020483. [DOI] [PubMed] [Google Scholar]
- 3.Thulborn KR, Davis D, Snyder J, Yonas H, Kassam A. Sodium MR imaging of acute and subacute stroke for assessment of tissue viability. Neuroimaging Clin N Am. 2005;15(3):639–653. doi: 10.1016/j.nic.2005.08.003. xi-xii. [DOI] [PubMed] [Google Scholar]
- 4.Ra JB, Hilal SK, Oh CH. An algorithm for MR imaging of the short T2 fraction of sodium using the FID signal. J Comput Assist Tomogr. 1989;13(2):302–309. doi: 10.1097/00004728-198903000-00022. [DOI] [PubMed] [Google Scholar]
- 5.Reshkin SJ, Bellizzi A, Caldeira S, Albarani V, Malanchi I, Poignee M, Alunni-Fabbroni M, Casavola V, Tommasino M. Na+/H+ exchanger-dependent intracellular alkalinization is an early event in malignant transformation and plays an essential role in the development of subsequent transformation-associated phenotypes. Faseb J. 2000;14(14):2185–2197. doi: 10.1096/fj.00-0029com. [DOI] [PubMed] [Google Scholar]