Abstract
Parkinson's disease (PD) is a neurodegenerative disease whose hallmark pathological features include a selective loss of dopaminergic neurons in the midbrain. Recent studies have described the activation of a stress-induced signal cascade, c-Jun N-terminal kinase (JNK)-mediated activation of c-Jun, and an increase in the expression of a downstream effector, cyclooxygenase 2 (COX-2), in postmortem PD brains. The neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), which induces selective neuronal loss in the midbrain similar to that seen in PD, also induces JNK-mediated activation of c-Jun and generates a COX-2 response in C57BL/6J mice. However, mice exhibit a strain-dependent susceptibility to MPTP. Identifying the point(s) of molecular divergence in the MPTP-induced response may provide insight into the cause of PD or a means to identify susceptibility to PD in humans. Here we examined JNK signaling and COX-2 induction in two strains of mice, the MPTP-sensitive C57BL/6J and the MPTP-resistant Swiss Webster (SW). We show that C57BL/6J and SW strains differ in JNK and c-Jun activation in response to MPTP. In addition, the MPTP-induced COX-2 response occurs exclusively in C57BL/6J mice. Furthermore, strain–specific responses to MPTP are not due to differences in MPP+ levels and are not secondary to cell death. These results provide evidence toward a mechanism of strain-dependent sensitivity to MPTP.
Keywords: Parkinson's disease, oxidative stress, dopamine transporter, VMAT2, signal transduction, neuroinflammation, COX-2
1. Introduction
Parkinson's disease (PD) is a common movement disorder characterized by bradykynesia, muscle rigidity, resting tremor, and postural instability (Przedborski and Vila, 2001). The primary neuropathological feature of PD is the selective loss of dopaminergic cells in the substantia nigra pars compacta (SNpc). The etiology of Parkinson's disease is complex, and although the mutation of several genes results in rare forms of PD (Bathory et al., 1987; Nussbaum and Polymeropoulos, 1997; Burke, 2004), the vast majority of PD (more than 90% of cases) is the sporadic form (Olanow and Tatton, 1999). Current hypotheses regarding the etiology of PD suggest an interaction of genetic predisposition coupled with exposure to an environmental agent (Tanner and Goldman, 1996). Recent reports suggest that neuroinflammatory processes also contribute to the pathogenesis of PD (Hunot and Hirsch, 2003). This hypothesis is supported by the report of oxidative stress–induced signaling in postmortem PD brains (Hunot et al., 2004). Together, stress-induced signaling and subsequent activation of downstream neuroinflammatory responses may contribute to the demise of the dopaminergic neurons in the SNpc in PD.
There are several animal models of PD that have been developed, including a mouse model in which a parkinsonian pathology develops in response to administration of the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). MPTP causes many of the hallmark features of PD in humans and nonhuman primates, and it induces SNpc-specific dopaminergic cell loss in other mammalian species, including mice (Dauer and Przedborski, 2003). We, and others, have shown that the effects of MPTP on neuronal cell loss in mice are strain-dependent (Heikkila, 1985; Sundstrom et al., 1987; Sonsalla and Heikkila, 1988; Hoskins and Davis, 1989; Hamre et al., 1999).
MPTP toxicity leads to an increase in oxidative stress and a reduction of ATP (Mizuno et al., 1987; Nicklas et al., 1987). The generation of free radicals and a decrease in cellular energetics are thought to promote cellular stress signaling and downstream activation of inflammation. One mediator of inflammation in the CNS is cyclooxygenase 2 (COX-2). COX-2 has been suggested to contribute to neurodegeneration via the formation of reactive oxygen species (Hoozemans et al., 2002; Teismann et al., 2003b). In fact, COX-2 has been shown to be one of the earliest markers of oxidative stress, and may also contribute to a generalized increase in oxidative stress within its environment (Madrigal et al., 2003). Recently, Hunot et al. (Hunot et al., 2004) have reported that MPTP induces JNK-mediated activation of c-Jun leads to an increase in the expression of COX-2 in MPTP-sensitive C57BL/6J mice. In addition, the authors showed that pharmacologic inhibition or genetic ablation of JNK resulted in protection against MPTP-induced cell loss in the SNpc as well as attenuated induction of COX-2. In light of these findings, we compared the MPTP-induced JNK signaling and COX-2 increase in the MPTP-sensitive C57BL/6J strain to that of the MPTP-resistant SWR strain at times before the physical induction of cell death. We hypothesize that the identification of points of divergence in the MPTP toxicity cascade (Smeyne and Jackson-Lewis, 2005) between strains will identify key molecular events and thus, potential targets for interfering with the cell death that occurs following environmental exposure to toxins that have been shown to induce parkinsonism. Here we report evidence of an intrinsic difference in the MPTP-mediated JNK activation of c-Jun between the two mouse strains. In addition, we confirm that MPTP-mediated induction of COX-2 occurs exclusively in C57BL/6J.
2. Results
Time course of MPTP-induced cell death in C57BL/6J mice
To assess MPTP-induced cellular changes between C57BL/6J and SW mice, we first analyzed MPTP-induced degeneration in C57BL/6J mice using stereological assessment of SNpc cell number. Mice were killed and cell loss in each brain was analyzed at 3, 4, 5, 6, and 7 days after MPTP administration. Cells were stained by using TH immunohistochemistry and counterstained with a Nissl stain to ensure that all dopaminergic neurons were assessed (Jackson-Lewis et al., 1995). A significant loss of neurons was first observed 5 days after MPTP administration; the greatest loss was observed at 7 days (Figure 1A, 1D). Although no significant neuronal loss was seen 4 days after MPTP challenge, TH expression in the these mice (Figure 1C) was less than that in the controls (Figure 1B).
Figure 1. Timeline of cell death following acute MPTP administration in C57BL/6J mice.

A, Number of TH-positive and Nissl stain–positive neurons in the SNpc. Significant cell loss occurred 5 days after administration of acute administration of MPTP (4 × 20 mg/kg, each injection spaced 2 hours apart), and the maximum loss was observed at 7 days after acute MPTP administration. Mean ± S.E., n = 3 at each timepoint. *, p < 0.05 in comparison with the control. (B-D) Representative section of TH-positive, Nissl-counterstained SN of control mice (B), C57BL/6J mice 4 days after acute MPTP administration (C), and C57BL/6J mice 7 days after acute MPTP administration (D).
To discriminate any intrinsic differences in MPTP-induced cellular responses between C57BL/6J and SW strains, all further analysis was done before day 4 in the period that followed MPTP administration. This timing ensured that any reported observations were not secondary to cell loss.
Expression of DAT, VMAT2 and MPP+ in C57BL/6J and SW mice
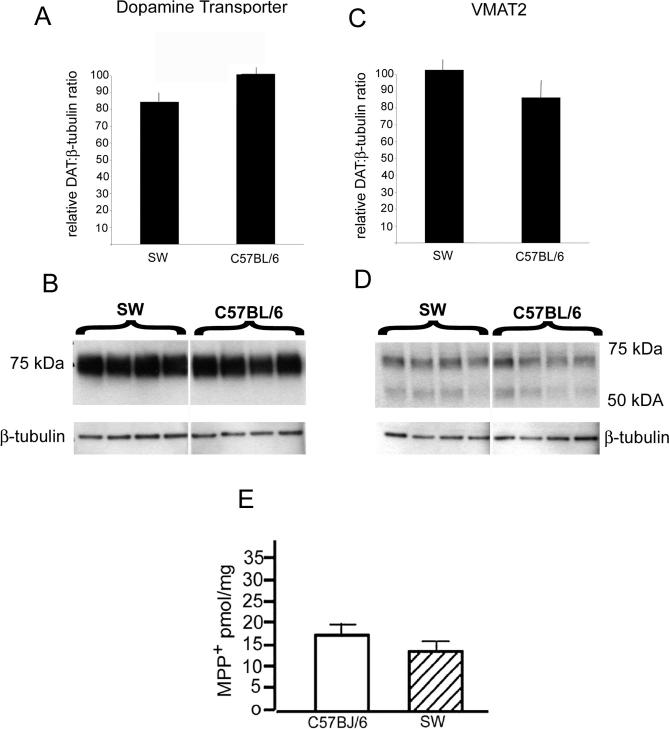
Regulation of MPTP entry, storage and metabolism have been reported to determine MPTP sensitivity (Donnan et al., 1986; Staal and Sonsalla, 2000). To test whether intrinsic differences in MPTP sensitivity were a result of differences in toxin entry, sequestration or toxin levels, we quantified the protein levels of DAT and VMAT2, and monitored the amount of striatal MPP+. No significant differences were observed in the levels of DAT (Figure 2A,B) or VMAT2 (Fig 2C,D) in striatum of MPTP-resistant SW mice compared to the MPTP-sensitive C57BL/6J mice. Likewise, no significant differences in MPP+ levels were observed between mouse strains 15 minutes following the fourth 20 mg/kg injection of MPTP (Figure 2E). These results suggest that differences MPTP sensitivity between C57BL/6J and SW mice are mediated possibly by downstream signaling independent of MPTP absorption, sequestration or metabolism.
Figure 2. Dopamine transporter, vesicular monoamine transporter and MPP+ levels in C57BL/6J and SW brain.
(A) Quantitative analysis of DAT levels in striatum of C57BL/6J and SW mice. Bars represent the ratio of DAT and β-tubulin measured using NIH image. (B) Representative western blot of DAT and β-tubulin levels in striatum of C57BL/6J and SW brain used to generate bar graphs in (A). Each lane represents 1 animal. (C) Quantitative analysis of VMAT2 levels in SN of C57BL/6J and SW mice. Bars represent the ratio of VMAT2 and β-tubulin measured using NIH image. (D) Representative western blot of VMAT2 and β-tubulin levels in SN of C57BL/6J and SW brain. Each lane represents 1 animal. (E) HPLC measurement of MPP+ levels in the striatum of C57BL/6J and SW (n = 4), 15 minutes after the 4th injection of 20 mg/kg MPTP.
MPTP-induced activation of JNK and c-Jun
Both chronic and acute administration of MPTP induces JNK-mediated activation of c-Jun in C57BL/6J mice (Hunot et al., 2004; Wang et al., 2004). The studies by Hunot et al. and Wang et al. also showed that pharmacologic inhibition and genetic ablation of JNK signaling protects against MPTP-induced cell loss. Here, we examined the MPTP-induced JNK-mediated activation of c-Jun by using phospho-specific antibodies in the ventral midbrain of MPTP-sensitive C57BL/6J mice and MPTP-resistant SW mice to determine whether molecular divergence in the response to MPTP exists. Western blot analysis revealed that MPTP-induced activation of phospho-JNK and phospho–c-Jun exhibit strain dependence in C57BL/6J and SW mice. Signaling profiles were examined at 2 × 20mg/kg MPTP (4 hours after initial administration of MPTP), 4×20mg/kg MPTP + 12h (20 hours after initial administration of MPTP) and 4×20mg/kg + 1d (28 hours after initial administration of MPTP). C57BL/6J mice exhibit a higher basal level of phospho-JNK:JNK than SW mice (Figure 3A,B), a trend that continues in all of the experimental timepoints examined (Figure 3B). Following administration of 2×20mg/kg MPTP, there is no significant increase in phospho-JNK levels in either strain (Figure 3A,B). In contrast, by 4×20mg/kg + 12h, there is a significant increase in phospho-JNK in the C57BL/6J mice compared to control (p ≤ 0.05). This increase in expression of JNK returns to baseline levels 24 hours after MPTP administration (4 × 20 mg/kg) has been completed (Figure 3B,C).
Figure 3. MPTP-mediated activation of JNK signaling and downstream transcription factor, c-Jun, in C57BL/6J and SW substantia nigra.
(A) C57BL/6J and SW mice were injected with 20mg/kg MPTP at 2 hour intervals with a maximum dose of 80 mg/kg MPTP. A representative immunoblot with control, 2×20mg/kg MPTP, 4×20mg/kg MPTP + 12h, and 4×20mg/kg MPTP + 1d is shown. (B) Ratio of phospho-JNK:total JNK in striatum. Levels of phospho-JNK are represented as percent total respective protein. (C) Ratio of phospho-Jun: total c-Jun in striatum. Levels of phospho-JNK are represented as percent total respective protein. For each experiment, Mean ± S.E.; n = 7 per strain, per time point; *, p < 0.05 level of significance compared to control within strains across conditions; #, p < 0.05 level of significance between strains at each condition.
The expression profile of the JNK downstream effector, c-Jun, reveals a more profound strain-dependent response to MPTP compared to phospho-JNK. There is no significant difference between C57BL/6J mice and SW mice basal phospho-c-Jun levels (Figure 3A,C). Although, not significantly different than the control (p = 0.068), C57BL/6J mice, following administration of 2×20mg/kg MPTP, there is increased c-Jun phosphorylation. Both strains exhibit an MPTP-induced elevation of phospho-c-Jun 12 hours following 4×20mg/kg MPTP, with a significantly greater phospho-c-Jun response in C57BL/6J mice (p < 0.05). 24 hours after 4 × 20 mg/kg MPTP, C57BL/6J mice continue to express an elevation in c-Jun phosphorylation, whereas levels of phospho-c-Jun return to levels comparable to baseline in SW mice. These findings suggest that the effects of MPTP on the JNK effector c-Jun, are greater in C57BL/6J compared to SW (Figure 3A,C).
Phospho–c-Jun expression distribution in response to MPTP is strain-dependent
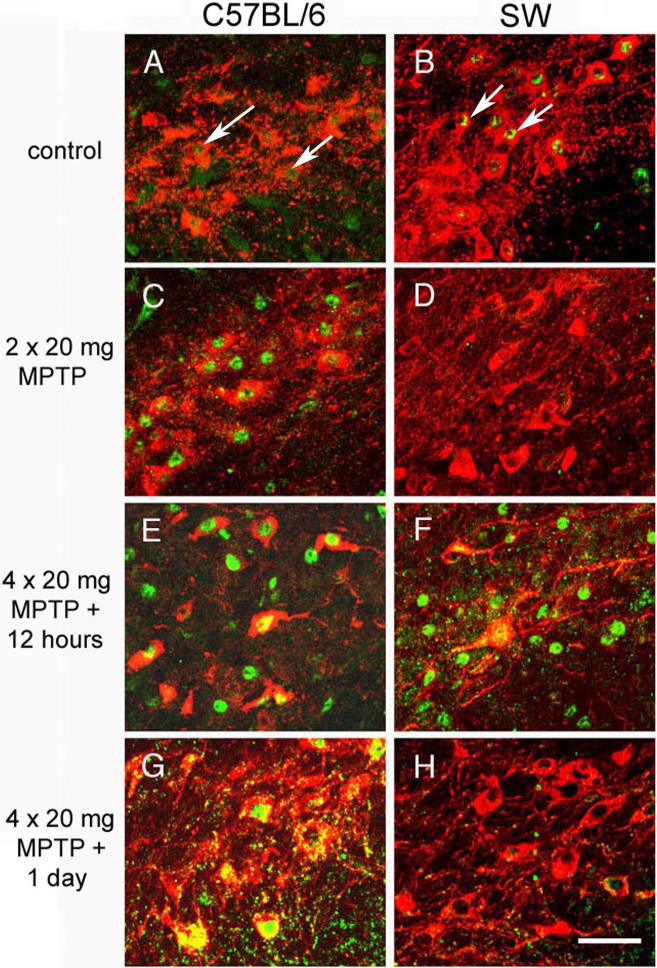
To determine whether the MPTP-induced JNK-mediated activation of c-Jun occurs in the SNpc dopaminergic neurons, we performed immunofluorescent double labeling in which TH was the marker of dopaminergic cells and phospho–c-Jun was the indicator of JNK-mediated signaling. Close examination of the SNpc revealed a striking difference in the MPTP-mediated signal response between strains (Figure 4). There were low levels of phospho–c-Jun in the SNpc dopaminergic neurons of C57BL/6J and SW control mice (Figure 4A,B). Phospho–c-Jun was abundant in SNpc TH-positive neurons in dopaminergic neurons of C57BL/6J mice given only two doses of MPTP, of C57BL/6J mice 12 hours after the fourth dose of MPTP, and of C57BL/6J mice 24 hours after the fourth dose (Figure 4C, E, G); in contrast, an increase in phospho–c-Jun labeling in SNpc dopaminergic neurons was seen in SW mice only 12 hours after the fourth dose of MPTP was given (Figure 4D, F, H). Examination of the phospho–c-Jun distribution after MPTP administration indicated that the stress response to MPTP in C57BL/6J SNpc dopaminergic cells occurred early and was prolonged, as previously described (Hunot et al., 2004). The MPTP-mediated signal response in the SNpc dopaminergic cells of SW mice appeared later. In addition to being delayed, this response to MPTP was briefer in SW SNpc dopaminergic cells than in C57BL/6J SNpc dopaminergic cells; this result is consistent with those from our Western blot analysis (Figure 3C). The strain-specific signaling response to MPTP in the dopaminergic cells may contribute to further divergence of downstream effectors, mediating the differences in MPTP-mediated cell loss between the strains.
Figure 4. Immunohistochemical analysis of MPTP-induced c-Jun activation in C57BL/6J and SW mice.
Basal expression level of phospho–c-Jun in (A) C57BL/6J and (B) SW mice. MPTP-induced phospho–c-Jun (green) co-localized (yellow, green nuclear staining, white arrows) with TH (red) in C57BL/6J mice given (C) two doses of MPTP (20 mg/kg per dose), (E) 12 hours after the fourth dose of MPTP (20 mg/kg per dose) and (G) in mice 24 hours after the fourth dose of MPTP (20 mg/kg per dose) was given. MPTP-induced up-regulation of phospho–c-Jun is observed in SW mice 12 hours after the fourth dose of MPTP (20 mg/kg per dose) (F). No change in expression of phospho–c-Jun was observed in SW mice given only two doses of MPTP (20 mg/kg per dose) (D) and in SW mice 24 hours after the fourth dose of MPTP (20 mg/kg per dose) was given (H). . Representative data from one of four mice per strain per time point are shown. Original magnification, ×40; scale bar = 25 μm.
COX-2 expression distribution in response to MPTP is strain-dependent
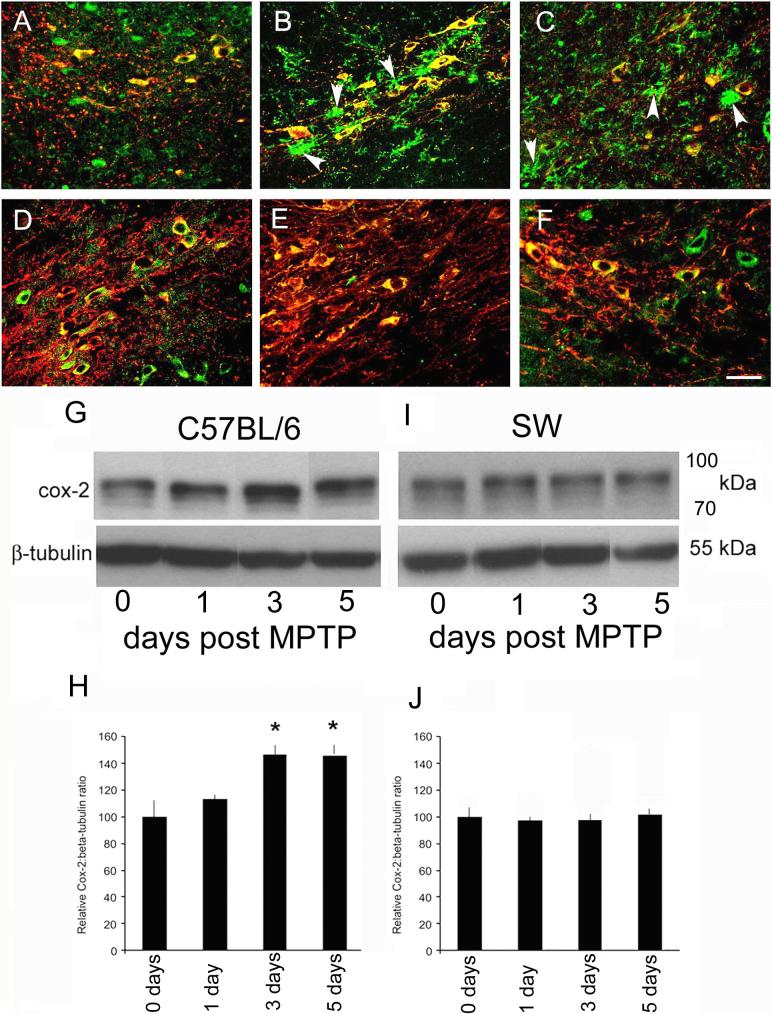
To assess a downstream effector of MPTP-induced c-Jun activation between strains, we examined the immunohistochemical distribution of COX-2 expression in control and MPTP-treated mice at 2 days and 3 days after drug administration. COX-2 is reportedly increased at these time points after MPTP administration (Teismann et al., 2003a; Hunot et al., 2004), and the increase in COX-2 precedes MPTP-induced neuronal loss (Figure 1A). Basal distribution of COX-2 between C57BL/6J and SW mice appeared to be similar: expression of COX-2 occurred in TH-positive neurons, and faint staining of neuropil was observed (Figure 5A, D). In C57BL/6J mice 2 and 3 days after 4 × 20 mg/kg MPTP, COX-2 immunostaining was increased within both TH-positive neurons, including neuronal processes and the surrounding neuropil (Figure 5B,C). The increase in COX-2 immunolabeling was confirmed by Western blot analysis of COX-2 expression (Figure 5G-J). In C57Bl/6J SN, COX-2 expression is increased as early as 1 day following MPTP, and remains elevated 7 days following MPTP administration (Figure 5G,I). In contrast to the MPTP-induced changes in COX-2 distribution observed in the C57BL/6J mice, no MPTP-induced alteration in COX-2 expression was observed in SW mice (Figure 5H,J). These results demonstrate that the MPTP-induced change in COX-2 distribution occurs only in C57BL/6J mice, which are MPTP-sensitive.
Figure 5. MPTP-induced COX-2 expression is strain-dependent.
COX-2 expression (green) in SNpc of C57BL/6J control mice (A), in C57BL/6J SNpc 2 days after the fourth dose of MPTP was given (B), and in C57BL/6J mice 3 days after the fourth dose (20 mg/kg per dose) of MPTP (C). Arrowheads depict non-neuronal expression of COX-2. Baseline expression of COX-2 in SNpc of SW control mice is similar to that seen in C57BL/6J SNpc (D). Two days (E) and three days (F) after the fourth dose of MPTP (20 mg/kg per dose) was given, there was no apparent increase in COX-2 expression or change in its localization. Representative data from one of four mice per strain per time point are shown. (G) Representative western blot of COX-2 expression 0, 1, 3, and 5 days post-MPTP in striatum of C57BL/6J mice. Loading control for the western blot was β-tubulin. (H) Quantitative analysis of COX-2: β-tubulin ratios 0, 1, 3 and 5 days following MPTP measured using NIH Image. At 1 day there is a slight increase in COX-2 expression. Statistically significant changes were detected at 3 days post MPTP. (I) Representative western blot of COX-2 expression 0, 1, 3, and 5 days post-MPTP in striatum of SW mice. Loading control for the western blot was β-tubulin. (J) Quantitative analysis of COX-2: β-tubulin ratios 0, 1, 3 and 5 days following MPTP measured using NIH image. No change in COX-2 expression was detected in SW striatum. Scale bar (A-F) = 25 μm.
3. Discussion
There is considerable evidence that inbred strains of mice, including C57BL/6J and SW, differ in their sensitivity to MPTP, as indicated by their differences in toxin-induced dopaminergic cell loss in the SNpc (Sonsalla and Heikkila, 1988; Muthane et al., 1994; Freyaldenhoven et al., 1995; Hamre et al., 1999; Sedelis et al., 2000). This study examined several factors emerging as mediators of MPTP sensitivity. In order to determine if there were baseline strain differences that could account for strain-dependent sensitivity to MPTP, we examined the levels of MPP+, an indicator of MAO-B expression (Chiba et al., 1984; Ransom et al., 1987), expression of the dopamine transporter, which modulates the entry of MPP+ into the DA neuron (Javitch et al., 1985; Gainetdinov et al., 1997), and the expression of the monoamine vesicular transporter, which regulates the sequestration of MPP+ inside the cell (Gainetdinov et al., 1998; Staal et al., 2000; Staal and Sonsalla, 2000). Alterations in each of these proteins have been shown to affect the toxicity of MPTP (Gainetdinov et al., 1998; Bezard et al., 1999; Kupsch et al., 2001). We found no baseline strain differences in the levels of MPP+ and in the levels of DAT and VMAT2 proteins. Thus, it is unlikely that these factors function as primary determinants of MPTP sensitivity between mouse strains.
In contrast to DAT, VMAT2 and MPP+ levels, we observed evidence of an intrinsic difference in the MPTP-mediated phosphorylation of JNK and c-Jun between the C57BL/6J and SW mouse strains. C57BL/6J mice exhibit a transient elevation in phospho-JNK:JNK following administration of MPTP. JNK-mediated activation of c-Jun quickly elevates with MPTP administration and is prolonged, displaying a robust expression in dopaminergic neurons of the SNpc for several days. In contrast, SW mice exhibit a relatively low and stable level of JNK phosphorylation. Downstream activation of c-Jun is delayed following MPTP and returns to baseline levels by 24 hours. In addition, COX-2 expression, which has been shown to respond to MPTP-induced c-Jun activation (Hunot et al., 2004), changes exclusively in C57BL/6J SN.
Postmortem analyses of PD brains have found evidence of JNK-mediated activation of c-Jun, a stress-induced transcription factor (Hunot et al., 2004). Several studies of neurotoxin models of PD have shown that inhibiting JNK-mediated activation of c-Jun mitigates MPTP-induced cell loss (Hunot et al., 2004; Peng et al., 2004; Wang et al., 2004). These studies include the chronic and acute administration of MPTP and the widely used herbicide, paraquat, which is chemically similar to the toxic metabolite of MPTP (Nishi, 1997; Hunot et al., 2004; Wang et al., 2004). Reports of these studies suggested that JNK signaling can be generalized as an important component of toxin-induced cell death of SNpc dopaminergic neurons. By finding that C57BL/6J and SW strains differ transcriptionally in the signal response to MPTP, we sought to identify a point(s) of divergence that is functionally relevant to the strain-dependent cell loss. Similar findings of strain-dependent cellular responses to toxic challenge have been reported. Specifically, strain-dependent activation of JNK signaling was reportedly observed in kainic acid sensitive mice that experienced seizure-induced neuronal death but not in kainic acid resistant strains (Schauwecker, 2000).
The cause of the strain-dependent difference in MPTP-induced signaling remains unknown. Although we have found that MPP+ levels in both strains after acute administration of MPTP are comparable, it is possible that C57BL/6J mice sequester MPP+ longer than SW mice. In addition, it is possible that there are variations in the expression of endogenous JNK signaling inhibitors. JNK-interacting protein-1b/islet-brain (JIP-1b/IB1) is one such JNK inhibitor and has been shown to protect dopaminergic cells against MPTP-mediated toxicity (Xia et al., 2001). Interestingly, JIP-1b/IB1 has been associated with alpha-synuclein, a protein commonly associated with inclusion bodies found in PD brains. One study suggested that alpha-synuclein expression promotes JNK inhibition via JIP-1b/IB1 redistribution (Hashimoto et al., 2002). Examination of JIPs between strains may identify a means by which JNK signaling differs between C57BL/6J and SW strains. Another inhibitor of JNK signaling that may have important implications to strain-dependent toxicity is the detoxifying enzyme, glutathione S-transferase class pi (GSTpi). GSTpi is an abundant antioxidant that facilitates the conjugation of xenobiotics to reduced glutathione. Not only does this enzyme protect against xenobiotics and oxidative stress, but also it functions as a JNK inhibitor. GSTpi protein inhibits JNK phosphorylation independently of its enzymatic activity (Monaco et al., 1999) and inhibits JNK signaling by a direct protein-protein interaction (Wang et al., 2001). In addition, inducible expression of GSTpi protects cells against peroxide-induced death by JNK inhibition (Yin et al., 2000). A GSTpi polymorphism is associated with PD in persons exposed to pesticides (Menegon et al., 1998) and it has been shown that strain-dependent expression of GSTpi mediates a defense against MPTP-induced oxidative stress between C57BL/6 and SW(Smeyne et al., 2007). It is possible that GSTpi expression contributes to MPTP sensitivity by providing a defense against MPTP-induced oxidative stress and protection against JNK-mediated cell death signaling.
In conclusion, we have identified divergence in the MPTP-mediated stress response between MPTP-sensitive C57BL/6J mice and MPTP-resistant SW mice, and this divergence precedes dopaminergic cell death in the SNpc. Our findings are consistent with those given in a previous report (Hunot et al., 2004): early and prolonged activation of c-Jun in the SNpc dopaminergic cells of C57BL/6J mice mediates COX-2 induction and subsequent cell loss. The relatively delayed and brief activation of c-Jun in SW mice fails to induce COX-2 expression. Consistent with results from earlier studies in which JNK signaling and the COX-2 response were inhibited and/or genetically ablated (Teismann et al., 2003b; Hunot et al., 2004), the reduced JNK-mediated activation of c-Jun in the SW strain appears to contribute to resistance to MPTP. Our findings support a molecular basis of MPTP-sensitivity. Discovering mediators of strain-dependent sensitivity to MPTP provides a potential means to identify those persons susceptible to PD and promising targets for better management of the disease.
4. Experimental Procedure
Animals and treatment
Three- to four-month-old male and female C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME, USA) and Swiss Webster mice (SW; Harlan Laboratories, Indianapolis, IN, USA) were given intraperitoneal injections of 20 mg/kg MPTP (Sigma Chemical Co., St. Louis, MO) at 2-hour intervals; MPTP was dissolved in sterile saline, and the maximum dose of MPTP was 80 mg/kg. Control mice for all experiments were untreated because we have empirically determined that there is no difference in gene activation in animals that receive or do not receive saline injections (data not shown). Animals were treated in accordance with the National Institutes of Health guidelines, and the research protocol was approved by the Animal Care and Use Committee at St. Jude Children's Research Hospital. All efforts were made to minimize the number of animals used in this study.
Histologic processing of mouse brains
For cell number examination and immunohistochemical studies, mice were transcardially perfused with 0.1 M phosphate-buffered saline and 4% paraformaldehyde; subsequently each brain was carefully removed and underwent overnight postfixation. After postfixation, brains were split into two groups and either submerged in 30% sucrose in phosphate-buffered saline for cryopreservation or processed for paraffin embedding. Ten series of sections (14 μm) of fixed-frozen brains were produced, mounted on Super Frost Plus slides (Fisher Scientific, Pittsburgh, PA) for fluorescent immunohistochemistry. For quantitative analysis of SNpc number, serial sections (10 μm) of paraffin-embedded brains were mounted to Super Frost Plus slides for 3,3'-diaminobenzidine (DAB) immunohistochemistry per the manufacturer's specifications (Vector Laboratories, Burlingame, CA, USA).
Stereologic analysis of SNpc cell number
Dopaminergic neurons in the SNpc were visualized by immunolabeling with rabbit polyclonal antibody to tyrosine hydroxylase (TH; dilution ratio, 1:250; Cat# P40101−0, Pel-Freez, Rogers, AR, USA) and subsequent incubation in biotinylated horse anti–rabbit IgG antibody (dilution ratio, 1:200; Vector Laboratories). Because previous studies have suggested that MPTP can downregulate the activity of this phenotypic marker (Jackson-Lewis et al., 1995), we counterstained every immunostained section with a Nissl stain, neutral red. The number of SNpc dopaminergic neurons (TH + Nissl, n=5) were estimated by using stereological techniques (Smeyne et al., 2005).
Immunohistochemistry
Two adjacent series of frozen sectioned tissue from 3 individual mouse brains were used to determine the cellular localization of phospho–c-Jun and COX-2. One series was labeled with rabbit polyclonal antibody to phospho–c-Jun (Cat# 9164, Cell Signaling, Beverly, MA, USA) and mouse monoclonal anti-TH antibody (Cat# 1299, Sigma); subsequently the tissue was incubated in TRITC-conjugated goat anti–mouse IgG antibody (dilution ratio, 1:100) and FITC–conjugated goat anti–rabbit IgG1 antibody (dilution ratio, 1:100) (Molecular Probes, Eugene, OR, USA). The second series of sections was labeled with mouse monoclonal anti–COX-2 antibody (1:100; Cat# 610203, BD Biosciences, San Jose, CA, USA) and rabbit polyclonal anti-TH antibody (Pel-Freez); the secondary antibodies were TRITC-conjugated goat anti-rabbit antibody and FITC–conjugated secondary antibody (Molecular Probes). Sections were mounted by using Vectashield mounting medium for fluorescence (Vector Laboratories) and visualized by using computer-assisted confocal laser microscopy (Leica Microsystems, Inc., Bannockburn, IL).
Western blot analysis
After the mice were killed by cervical dislocation, each brain was rapidly removed and snap-frozen for use in Western blot analysis (7 mice per strain per condition were analyzed). Substantia nigra from control and experimental brains were dissected as previously described 15 to 30 minutes after different doses of MPTP were administered (Faherty et al., 2005). The dosages of MPTP and the time of brain dissection were the following: 20 mg/kg MPTP was administered twice, and brain dissection occurred within 15 to 30 minutes after the last dose of MPTP was given (2 × 20 mg/kg); 20 mg/kg MPTP was administered four times, and brain dissection occurred 12 hours after the last dose of MPTP (4 × 20 mg + 12 hours); and 20mg/kg MPTP was administered four times, and brain dissection occurred 24 hours after the last dose (4 × 20 mg/kg + 24 hours). Once the SN was dissected from the whole brain, the tissue was placed into and homogenized in RIPA lysis buffer (1% NP-40, 0.5% deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 150 mM NaCl and 50 mM Tris at pH 7.4) with a protease-inhibitor cocktail (Roche, Indianapolis, IN, USA??). Protein concentration was determined by using a commercially available protein assay in accordance with the manufacturer's specifications (Bio-Rad, Hercules, CA). Briefly, aliquots from the protein lysis solutions were added to a dilute dye reagent, and spectrophotometric analysis at 595 nm was conducted. Protein concentrations were calculated by using a standard curve generated by spectrophotometric analysis of known concentrations of bovine serum albumin. Thirty micrograms of total protein underwent electrophoresis in a 4%-12% SDS gradient polyacrylamide gel and electrotransferred to a polyvinylidene fluoride (PVDF) membrane (Millipore, Bedford, MA). Phospho-JNK protein levels were determined by using a rabbit polyclonal antibody to phospho-JNK (dilution ratio, 1:500; Cat# 44−682G, Biosource, Camarillo, CA, USA). The levels of phospho–c-Jun (Ser 73) protein were determined by using a rabbit antibody (1:500; Cat# 9164, Cell Signaling). All signals were visualized by using Supersignal West Dura chemiluminescence (Pierce, Rockford, IL). Membranes were stripped and re-probed by using a rabbit polyclonal anti-JNK antibody (1:1000; Cat# 9252, Cell Signaling), a rabbit monoclonal anti–c-Jun antibody (1:1000; Cat# 9165, Cell Signaling), and a mouse monoclonal anti—beta-actin antibody (1:10,000; Cat# A-5441, Sigma). Protein bands were visualized by using chemiluminescence.
Protein quantification
Kodak Biomax MR film was used to detect chemiluminescence at exposure times of 5 seconds, 10 seconds, 30 seconds, 1 minute, 2 minutes, and 5 minutes to ensure that the analysis of protein bands was performed within the film's linear phase of exposure. Western blots were digitally scanned and analyzed with NIH Image (National Institutes of Health, Bethesda, MD, USA) for quantification in accordance with the manufacturer's directions (rsb.info.nih.gov/nih-image/). Briefly, digital images were transferred to TIFF files and imported into Image. After calibration, average gray scores were acquired by using a fixed box whose area approximated that of a loading control band to highlight areas of the protein blots for analysis. Background scores, which were determined by using the same fixed-box method, were subtracted from protein blot scores to obtain adjusted scores. Adjusted scores for phospho-JNK, phospho–c-Jun, JNK, and c-Jun were divided by the adjusted scores for beta-actin to obtain the normalized scores (as percent beta-actin).
Measurement of MPP+ levels in the SN
Three– to four–month-old C57BL/6J mice and SW mice (4 per group), received a single intraperitoneal injection of 20 mg/kg MPTP hydrochloride (Sigma). Fifteen minutes after the injection, mice were killed, and the SN of each mouse was dissected on ice and homogenized in 10 volumes of ice-cold 0.1 M perchloric acid (Fisher Scientific). The homogenate was centrifuged at 12,000× g for 15 minutes at 4°C. The supernatants were filtered through 0.2-μm filters for 2 minutes at 8000× g, and 100 μl of the supernatant were injected into a high-performance liquid chromatography (HPLC) system equipped with an Shimadzu autosampler, a programmable solvent module a Nucleosil 100−5 C18 (4 × 125 mm, 5 μm) column (Agilent Technology, Wilmington, DE), and a UV detector. The mobile phase consisted of 50 μM sulfuric acid (Sigma), 10% acetonitrile, 90% water, and 75 mM triethylamine (Sigma) adjusted to a final pH of 2.3; chromatography was done under isocratic conditions (flow rate1.0 ml/min). The UV detector was set to 295 nm for MPP+ detection, as previously described (Fornai et al., 2000). The concentrations of MPP+ were determined by running known amounts of MPP+ iodide (Sigma) dissolved in 0.1 M perchloric acid, using the data to create a standard curve, and extrapolating from the standard curve.
Statistical analysis
A two-way analysis of variance (ANOVA) followed by Bonferonni post hoc analysis was performed to determine the significance of the MPTP-induced loss of SNpc neurons over time. For determination of protein levels by densitometric analysis, ANOVA with Bonferroni post hoc analysis was used to determine significant differences in MPTP-induced changes in protein levels within and between strains. All statistical analyses were performed with Statview (SAS).
Acknowledgements
The authors thank Shivaprasad Bhuvanendran and Kenneth Barnes of Scientific Imaging at St. Jude Children's Research Hospital for access and assistance with the confocal microscope. This work was supported by NS 39006 (to R.J.S.), Cancer Center CORE Grant CA 21765 and the American Lebanese Syrian Associated Charities (ALSAC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bathory G, Szuts T, Magyar K. Studies on the melanin affinity of selegiline (deprenyl) and other amphetamine derivatives. Polish J Pharm Pharmacy. 1987;39:195–201. [PubMed] [Google Scholar]
- Bezard E, Gross CE, Fournier MC, Dovero S, Bloch B, Jaber M. Absence of MPTP-induced neuronal death in mice lacking the dopamine transporter. Exp Neurol. 1999;155:268–273. doi: 10.1006/exnr.1998.6995. [DOI] [PubMed] [Google Scholar]
- Burke RE. Recent advances in research on Parkinson disease: synuclein and parkin. Neurologist. 2004;10:75–81. doi: 10.1097/01.nrl.0000117822.90759.83. [DOI] [PubMed] [Google Scholar]
- Chiba K, Trevor A, Castagnoli N., Jr. Metabolism of the neurotoxic tertiary amine, MPTP, by brain monoamine oxidase. Biochem Biophys Res Commun. 1984;120:574–578. doi: 10.1016/0006-291x(84)91293-2. [DOI] [PubMed] [Google Scholar]
- Dauer W, Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- Donnan GA, Kaczmarczyk SJ, Solopotias T, Rowe P, Kalnins RM, Vajda FJ, Mendelsohn FA. The neurochemical and clinical effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in small animals. Clin Exp Neurol. 1986;22:155–164. [PubMed] [Google Scholar]
- Faherty CJ, Raviie Shepherd K, Herasimtschuk A, Smeyne RJ. Environmental enrichment in adulthood eliminates neuronal death in experimental Parkinsonism. Mol Brain Res. 2005;134:170–179. doi: 10.1016/j.molbrainres.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Fornai F, Battaglia G, Gesi M, Giorgi FS, Orzi F, Nicoletti F, Ruggieri S. Time course and dose-response study on the effects of chronic L-DOPA administration on striatal dopamine levels and dopamine transporter following MPTP toxicity. Brain Res. 2000;887:110–117. doi: 10.1016/s0006-8993(00)02999-1. [DOI] [PubMed] [Google Scholar]
- Freyaldenhoven TE, Ali SF, Hart RW. MPTP- and MPP(+)-induced effects on body temperature exhibit age- and strain-dependence in mice. Brain Res. 1995;688:161–170. doi: 10.1016/0006-8993(95)00529-y. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Fumagalli F, Jones SR, Caron MG. Dopamine transporter is required for in vivo MPTP neurotoxicity: evidence from mice lacking the transporter. J Neurochem. 1997;69:1322–1325. doi: 10.1046/j.1471-4159.1997.69031322.x. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Fumagalli F, Wang YM, Jones SR, Levey AI, Miller GW, Caron MG. Increased MPTP neurotoxicity in vesicular monoamine transporter 2 heterozygote knockout mice. J Neurochem. 1998;70:1973–1978. doi: 10.1046/j.1471-4159.1998.70051973.x. [DOI] [PubMed] [Google Scholar]
- Hamre K, Tharp R, Poon K, Xiong X, Smeyne RJ. Differential strain susceptibility following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) administration acts in an autosomal dominant fashion: quantitative analysis in seven strains of Mus musculus. Brain Res. 1999;828:91–103. doi: 10.1016/s0006-8993(99)01273-1. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Hsu LJ, Rockenstein E, Takenouchi T, Mallory M, Masliah E. alpha-Synuclein protects against oxidative stress via inactivation of the c-Jun N-terminal kinase stress-signaling pathway in neuronal cells. J Biol Chem. 2002;277:11465–11472. doi: 10.1074/jbc.M111428200. [DOI] [PubMed] [Google Scholar]
- Heikkila RE. Differential neurotoxicity of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in Swiss-Webster mice from different sources. Eur J Pharmacol. 1985;117:131–133. doi: 10.1016/0014-2999(85)90482-0. [DOI] [PubMed] [Google Scholar]
- Hoozemans JJ, Veerhuis R, Janssen I, van Elk EJ, Rozemuller AJ, Eikelenboom P. The role of cyclo-oxygenase 1 and 2 activity in prostaglandin E(2) secretion by cultured human adult microglia: implications for Alzheimer's disease. Brain Res. 2002;951:218–226. doi: 10.1016/s0006-8993(02)03164-5. [DOI] [PubMed] [Google Scholar]
- Hoskins JA, Davis LJ. The acute effect on levels of catecholamines and metabolites in brain, of a single dose of MPTP in 8 strains of mice. Neuropharmacology. 1989;28:1389–1397. doi: 10.1016/0028-3908(89)90015-4. [DOI] [PubMed] [Google Scholar]
- Hunot S, Hirsch EC. Neuroinflammatory processes in Parkinson's disease. Ann Neurol. 2003;53(Suppl 3):S49–58. doi: 10.1002/ana.10481. [DOI] [PubMed] [Google Scholar]
- Hunot S, Vila M, Teismann P, Davis RJ, Hirsch EC, Przedborski S, Rakic P, Flavell RA. JNK-mediated induction of cyclooxygenase 2 is required for neurodegeneration in a mouse model of Parkinson's disease. Proc Natl Acad Sci (USA) 2004;101:665–670. doi: 10.1073/pnas.0307453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson-Lewis V, Jakowec M, Burke RE, Przedborski S. Time course and morphology of dopaminergic neuronal death caused by the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neurodegeneration. 1995;4:257–269. doi: 10.1016/1055-8330(95)90015-2. [DOI] [PubMed] [Google Scholar]
- Javitch JA, D'Amato RJ, Strittmatter SM, Snyder SH. Parkinsonism-inducing neurotoxin, N-methyl-4-phenyl-1,2,3,6 -tetrahydropyridine: uptake of the metabolite N-methyl-4-phenylpyridine by dopamine neurons explains selective toxicity. Proc Nat Acad Sci (USA) 1985;82:2173–2177. doi: 10.1073/pnas.82.7.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupsch A, Sautter J, Gotz ME, Breithaupt W, Schwarz J, Youdim MB, Riederer P, Gerlach M, Oertel WH. Monoamine oxidase-inhibition and MPTP-induced neurotoxicity in the non-human primate: comparison of rasagiline (TVP 1012) with selegiline. J Neural Transm. 2001;108:985–1009. doi: 10.1007/s007020170018. [DOI] [PubMed] [Google Scholar]
- Madrigal JL, Garcia-Bueno B, Moro MA, Lizasoain I, Lorenzo P, Leza JC. Relationship between cyclooxygenase-2 and nitric oxide synthase-2 in rat cortex after stress. Eur J Neurosci. 2003;18:1701–1705. doi: 10.1046/j.1460-9568.2003.02888.x. [DOI] [PubMed] [Google Scholar]
- Menegon A, Board PG, Blackburn AC, Mellick GD, Le Couteur DG. Parkinson's disease, pesticides, and glutathione transferase polymorphisms. Lancet. 1998;352:1344–1346. doi: 10.1016/s0140-6736(98)03453-9. [DOI] [PubMed] [Google Scholar]
- Mizuno Y, Sone N, Saitoh T. Effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and 1-methyl-4-phenylpyridinium ion on activities of the enzymes in the electron transport system in mouse brain. J Neurochem. 1987;48:1787–1793. doi: 10.1111/j.1471-4159.1987.tb05737.x. [DOI] [PubMed] [Google Scholar]
- Monaco R, Friedman FK, Hyde MJ, Chen JM, Manolatus S, Adler V, Ronai Z, Koslosky W, Pincus MR. Identification of a glutathione-S-transferase effector domain for inhibition of jun kinase, by molecular dynamics. J Protein Chem. 1999;18:859–866. doi: 10.1023/a:1020679229110. [DOI] [PubMed] [Google Scholar]
- Muthane U, Ramsay KA, Jiang H, Jackson-Lewis V, Donaldson D, Fernando S, Ferreira M, Przedborski S. Differences in nigral neuron number and sensitivity to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in C57/bl and CD-1 mice. Exp Neurol. 1994;126:195–204. doi: 10.1006/exnr.1994.1058. [DOI] [PubMed] [Google Scholar]
- Nicklas WJ, Youngster SK, Kindt MV, Heikkila RE. MPTP, MPP+ and mitochondrial function. Life Sci. 1987;40:721–729. doi: 10.1016/0024-3205(87)90299-2. [DOI] [PubMed] [Google Scholar]
- Nishi K. Expression of c-Jun in dopaminergic neurons of the substantia nigra in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated mice. Brain Res. 1997;771:133–141. doi: 10.1016/s0006-8993(97)00862-7. [DOI] [PubMed] [Google Scholar]
- Nussbaum RL, Polymeropoulos MH. Genetics of Parkinson's disease. Hum Mol Gen. 1997;6:1687–1692. doi: 10.1093/hmg/6.10.1687. [DOI] [PubMed] [Google Scholar]
- Olanow CW, Tatton WG. Etiology and pathogenesis of Parkinson's disease. Ann Revi Neurosci. 1999;22:123–144. doi: 10.1146/annurev.neuro.22.1.123. [DOI] [PubMed] [Google Scholar]
- Peng J, Mao XO, Stevenson FF, Hsu M, Andersen JK. The herbicide paraquat induces dopaminergic nigral apoptosis through sustained activation of the JNK pathway. J Biol Chem. 2004;279:32626–32632. doi: 10.1074/jbc.M404596200. Epub 32004 May 32620. [DOI] [PubMed] [Google Scholar]
- Przedborski S, Vila M. The last decade in Parkinson's disease research. Basic sciences. Adv Neurol. 2001;86:177–186. [PubMed] [Google Scholar]
- Ransom BR, Kunis DM, Irwin I, Langston JW. Astrocytes convert the parkinsonism inducing neurotoxin, MPTP, to its active metabolite, MPP+. Neurosci Lett. 1987;75:323–328. doi: 10.1016/0304-3940(87)90543-x. [DOI] [PubMed] [Google Scholar]
- Schauwecker PE. Seizure-induced neuronal death is associated with induction of c-Jun N-terminal kinase and is dependent on genetic background. Brain Res. 2000;884:116–128. doi: 10.1016/s0006-8993(00)02888-2. [DOI] [PubMed] [Google Scholar]
- Sedelis M, Hofele K, Auburger GW, Morgan S, Huston JP, Schwarting RK. MPTP susceptibility in the mouse: behavioral, neurochemical, and histological analysis of gender and strain differences. Behav Genet. 2000;30:171–182. doi: 10.1023/a:1001958023096. [DOI] [PubMed] [Google Scholar]
- Smeyne M, Jiao Y, Shepherd KR, Smeyne RJ. Glia cell number modulates sensitivity to MPTP in mice. Glia. 2005;52:144–152. doi: 10.1002/glia.20233. [DOI] [PubMed] [Google Scholar]
- Smeyne M, Boyd J, Raviie Shepherd K, Jiao Y, Pond BB, Hatler M, Wolf R, Henderson C, Smeyne RJ. GSTpi expression mediates dopaminergic neuron sensitivity in experimental parkinsonism. Proc Natl Acad Sci (USA) 2007;104:1977–1982. doi: 10.1073/pnas.0610978104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeyne RJ, Jackson-Lewis V. The MPTP model of Parkinson's disease. Mol Brain Res. 2005;134:57–66. doi: 10.1016/j.molbrainres.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Sonsalla PK, Heikkila RE. Neurotoxic effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and methamphetamine in several strains of mice. Prog Neuropsychopharmacol Biol Psychiatry. 1988;12:345–354. doi: 10.1016/0278-5846(88)90054-1. [DOI] [PubMed] [Google Scholar]
- Staal RGW, Sonsalla PK. Inhibition of brain vesicular monoamine transporter (VMAT2) enhances 1-methyl-4-phenylpyridinium neurotoxicity in vivo in rat striata. JPET. 2000;293:336–342. [PubMed] [Google Scholar]
- Staal RGW, Hogan KA, Liang CL, German DC, Sonsalla PK. In vitro studies of striatal vesicles containing the vesicular monoamine transporter (VMAT2): Rat versus mouse differences in sequestration of 1-methyl-4-phenylpyridinium. JPET. 2000;293:329–335. [PubMed] [Google Scholar]
- Sundstrom E, Stromberg I, Tsutsumi T, Olson L, Jonsson G. Studies on the effect of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) on central catecholamine neurons in C57BL/6 mice. Comparison with three other strains of mice. Brain Res. 1987;405:26–38. doi: 10.1016/0006-8993(87)90986-3. [DOI] [PubMed] [Google Scholar]
- Tanner CM, Goldman SM. Epidemiology of Parkinson's disease. Neurol Clin. 1996;14:317–335. doi: 10.1016/S0733-8619(05)70259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teismann P, Vila M, Choi DK, Tieu K, Wu DC, Jackson-Lewis V, Przedborski S. COX-2 and neurodegeneration in Parkinson's disease. Ann N Y Acad Sci. 2003a;991:272–277. doi: 10.1111/j.1749-6632.2003.tb07482.x. [DOI] [PubMed] [Google Scholar]
- Teismann P, Tieu K, Choi DK, Wu DC, Naini A, Hunot S, Vila M, Jackson-Lewis V, Przedborski S. Cyclooxygenase-2 is instrumental in Parkinson's disease neurodegeneration. Proc Natl Acad Sci (USA) 2003b;100:5473–5478. doi: 10.1073/pnas.0837397100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Arifoglu P, Ronai Z, Tew KD. Glutathione S-transferase P1−1 (GSTP1−1) inhibits c-Jun N-terminal kinase (JNK1) signaling through interaction with the C terminus. J Biol Chem. 2001;276:20999–21003. doi: 10.1074/jbc.M101355200. [DOI] [PubMed] [Google Scholar]
- Wang W, Shi L, Xie Y, Ma C, Li W, Su X, Huang S, Chen R, Zhu Z, Mao Z, Han Y, Li M. SP600125, a new JNK inhibitor, protects dopaminergic neurons in the MPTP model of Parkinson's disease. Neurosci Res. 2004;48:195–202. doi: 10.1016/j.neures.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Xia XG, Harding T, Weller M, Bieneman A, Uney JB, Schulz JB. Gene transfer of the JNK interacting protein-1 protects dopaminergic neurons in the MPTP model of Parkinson's disease. Proc Natl Acad Sci (USA) 2001;98:10433–10438. doi: 10.1073/pnas.181182298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z, Ivanov VN, Habelhah H, Tew K, Ronai Z. Glutathione S-transferase p elicits protection against H2O2-induced cell death via coordinated regulation of stress kinases. Cancer Res. 2000;60:4053–4057. [PubMed] [Google Scholar]