Abstract
Mechanical stretch induces phosphorylation of the hydrophobic motif site Thr389 in p70S6k through a rapamycin-sensitive (RS) pathway that involves a unique PI3K-independent mechanism. Rapamycin is considered to be a highly specific inhibitor of the protein kinase mTOR; however, mTOR is also considered to be a PI3K-dependent signaling molecule. Thus, questions remain as to whether mTOR is the RS element that confers mechanically-induced signaling to p70S6k(389). In this study, rapamycin-resistant mutants of mTOR along with mechanical stretch were used to address this question. The results indicate that mTOR is the RS element and reveal that mTOR signaling can be activated through a PI3K-independent mechanism.
Keywords: Skeletal muscle, exercise, growth, mechanotransduction, hypertrophy, phospholipase D
1. Introduction
Over 30 years ago, it was proposed that mechanically-induced tension was the critical event for initiating compensatory growth of skeletal muscle [1]. Today, this hypothesis is still widely accepted, and current studies are now focused on identifying the molecular events that are involved in this process. These studies have revealed that mechanically-induced growth is inhibited by the macrolide antibiotic, rapamycin [2,3]. Rapamycin is a highly specific inhibitor of a protein kinase called the mammalian target of rapamycin (mTOR) and based on this specificity, many have concluded that mechanical stimuli induce skeletal muscle growth through the activation of mTOR signaling [4,5]
The activation of mTOR signaling is often evaluated by measuring changes in the phosphorylation of the Thr389 residue in the hydrophobic motif of the ribosomal S6 kinase 1 (p70S6k(389)). Consistent with a role for mTOR in mechanically-induced growth, several studies have shown that mechanical stimuli can induce p70S6k(389) phosphorylation, and the magnitude of this activation correlates with the extent of the concomitant growth response [6-8]. Furthermore, the mechanical activation of p70S6k(389) phosphorylation occurs through a rapamycin-sensitive mechanism [2,9]. Combined, these observations indicate that mechanical stimulation is sufficient for the activation of mTOR signaling. However, mechanical stimuli have been reported to activate p70S6k(389) phosphorylation through a phosphatidylinositol-3-kinase (PI3K)-independent mechanism [9,10]. Since signaling by mTOR is considered to be dependent on the PI3K, this point raises questions about whether mechanically-induced signaling to p70S6k(389) is indeed an mTOR-dependent signaling event [11-13].
To determine if mTOR is the rapamycin-sensitive element responsible for mechanically-induced signaling to p70S6k(389), we have combined the use of various rapamycin-resistant mutants of mTOR along with an in-vitro stretch model for mechanically stimulating skeletal muscle myoblasts. Consistent with other models of mechanical stimulation, it was determined that, in-vitro, the mechanical activation of p70S6k(389) phosphorylation occurs through a PI3K-independent mechanism. Furthermore, the rapamycin-resistant mutants of mTOR allowed us to demonstrate that mTOR is the rapamycin-sensitive element that confers mechanically-induced signaling to p70S6k(389), and that this event requires mTOR kinase activity. Taken together, this study demonstrates that p70S6k(389) phosphorylation is a valid marker of mechanically-induced mTOR signaling, and that mTOR signaling can be activated through a PI3K-independent mechanism.
2. Materials and Methods
2.1 Materials
Rapamycin, wortmannin and fibronectin were purchased from Sigma-Aldrich (St. Louis, MO). 1-Butanol was purchased from Fischer Scientific. [3H] Arachidonic acid (62.5Ci/mmol) was purchased from New England Nuclear (Chicago, IL). PVDF membranes were purchased from Millipore (Bedford, MA). All antibodies were purchased from Cell Signaling Technologies (Danvers, MA). Peroxidase-conjugated anti-rabbit goat secondary antibody was purchased from Vector Laboratories (Burlingame, CA). Enhanced Chemiluminescence detection reagents (ECL and ECL Plus) were purchased from Amersham Pharmacia Biotech (Pittsburgh, PA).
2.2 Cell Culture
Mouse C2C12 myoblasts were cultured in growth media consisting of high glucose DMEM supplemented with antibiotics (streptomycin 100μg/ml, penicillin 100U/ml and 0.25μg/ml Fungizone (Gibco)) and 10% fetal bovine serum (FBS). Stable C2C12 cell lines expressing RR-mTOR and RRKD-mTOR have been previously described [14] and were kindly provided by Dr. Jie Chen (Department of Cell and Developmental Biology, University of Illinois, Urbana IL). These cells were maintained in growth media containing 0.2mg/ml G418 (Calbiochem). G418 was not included in the media after the cells had been plated for mechanical stimulation experiments. All cell culture and stretch experiments were performed in a humidified 95% air, 5% CO2 incubator at 37°C.
2.3 Mechanical Stimulation
C2C12 myoblasts were subjected to 10-30min of cyclic 15% biaxial stretch at a frequency of 1 Hz using the system previously described by Sotoudeh et al. 1998 [15]. In this system, silicone membranes were coated with 2μg/cm2 fibronectin dissolved in phosphate buffered saline (PBS) for 1hr followed by treatment with UV light for 1hr. The membranes were rinsed with fresh PBS and then plated with C2C12 myoblasts at approximately 70% confluence. The myoblasts were allowed to grow on the membranes for 48hr at which point they were highly confluent. At 2hr prior to the initiation of mechanical stimulation, myoblasts were switched to serum-free high-glucose DMEM that did not contain any antibiotics. Myoblasts were subjected to 10-30min of stretch and then collected in lysis buffer as described below. For the control condition, culture plates were placed on the running stretch device, but were not subjected to the stretch condition.
2.4 Sample Preparation and Western Blot Analysis
Cells were collected in lysis buffer as previously described [10]. Cell lysates were centrifuged at 500g for 5min and the supernatant was saved for analysis. Protein concentration was determined with DC protein assay kit (Bio-Rad, Hercules, CA) and equivalent amounts of protein from each sample were subjected to western blot analysis as previously described [10].
2.5 Immunoprecipitation
Immunoprecipitations were carried out with EZview red ANTI-FLAG M2 agarose affinity gel beads (Sigma-Aldrich) according to the manufacturer's protocol. Briefly, 300μg of sample protein was dissolved in 1ml of lysis buffer plus 40μl of EZview beads, and allowed to incubate at 4°C for 2 hr with gentle rocking. The beads were pelleted and washed several times with lysis buffer. The pellets were dissolved in 2X Laemmli buffer and subjected to western blot analysis as described above.
2.6 Phospholipase D Activity
Phospholipase D (PLD) activity was measured as previously described with the exception that C2C12 myoblasts were labeled in growth media containing 1μCi/ml [3H] arachidonic acid for 24hr [16].
2.7 Statistical Analysis
All values are expressed as means ± SEM. Statistical significance was determined by using ANOVA, followed by Student Newman-Keuls post hoc analysis. Differences between groups were considered significant if p ≤ 0.05.
3. Results and Discussion
3.1 Time Course of Mechanically-Induced Signaling Events
To begin characterizing the in-vitro model of mechanical stimulation, we first evaluated the time dependent changes in the phosphorylation of p70S6k(389), protein kinase B (PKB) and Jun N-terminal kinase 2 (JNK2). The results indicated that mechanical stimulation induced a progressive increase in p70S6k(389) and PKB phosphorylation. The increase in p70S6k(389) and PKB phosphorylation reached a maximum within 20min and remained at this level for at least 30min. Mechanical stimulation also induced an increase in JNK2 phosphorylation which reached a maximum within 10min and was sustained at this level for at least 30min (Figure 1A).
Figure 1. Mechanically-induced p70S6k(389) phosphorylation requires PLD but not PI3K.
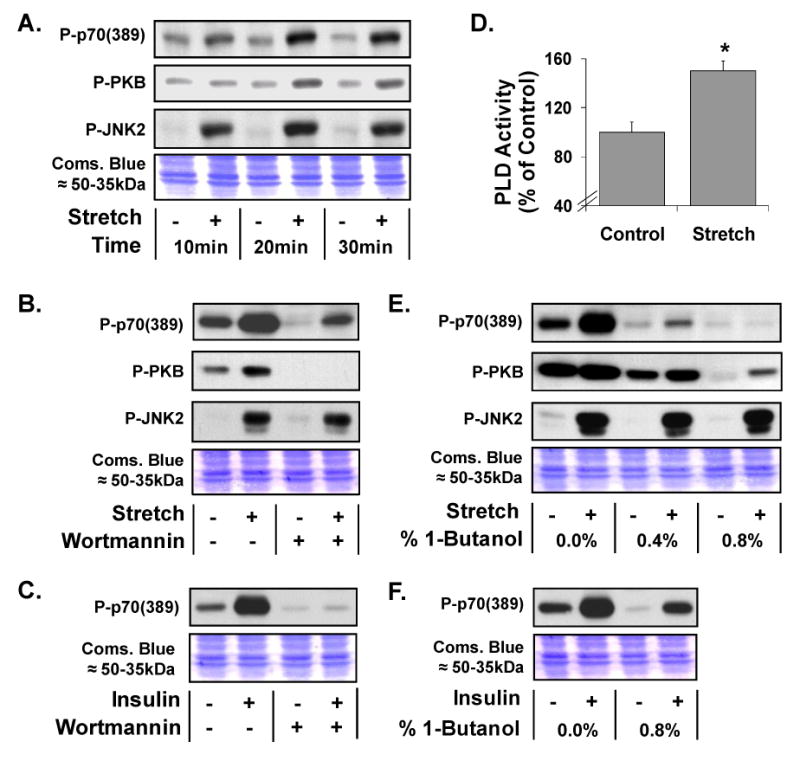
(A) Western blot analysis of phosphorylated p70S6k (Thr389) [P-p70(389)], PKB(Ser473) [P-PKB], JNK2 (Thr183/Tyr185) [P-JNK2] in C2C12 myoblasts following 10-30min of control or 15% cyclic (1Hz) biaxial stretch [Stretch +] conditions. (B and C) C2C12 myoblasts were pre-incubated with 500nM wortmannin or the solvent vehicle (0.2% DMSO) for 15min before being subjected to 20min of control or stretch conditions (B) or 10min of 100nM insulin stimulation [Insulin +] (C). (D) PLD activity during 0-20min of control or stretch conditions. (E and F) C2C12 myoblasts were pre-incubated with 0.0–0.8% 1-butanol for 5min before being subjected to 20min of control or stretch conditions (E) or 10min of 100nM Insulin stimulation (F). At the completion of western blotting, equal loading of protein in all lanes was verified with Coomassie Blue (Coms. Blue) staining of the transfer membranes (Images of the region containing the ≈50-35kDa proteins are shown). All western blots are representative of at least three experiments. * Significantly different from treatment control (p ≤ 0.05), (n = 3-6 / group).
3.2 The Role of PI3K in the Mechanical Activation of p70S6k(389) Phosphorylation
The PI3K inhibitor wortmannin was used to address the role of PI3K in the mechanical activation of p70S6k(389) phosphorylation. Incubating control cells with wortmannin promoted a reduction in the basal level of p70S6k(389) phosphorylation, however, wortmannin did not block the mechanically-induced increase in p70S6k(389) or JNK2 phosphorylation (Figure 1B). On the other hand, wortmannin completely eliminated P-PKB phosphorylation in both control and mechanically stimulated cells.
In order to confirm that wortmannin fully inhibited PI3K activity, myoblasts were stimulated with insulin in the presence or absence of wortmannin and the changes in p70S6k(389) phosphorylation were compared. The results indicated that stimulation with insulin promoted a robust increase in p70S6k(389) phosphorylation, and this effect was completely blocked by wortmannin, thus, confirming that wortmannin did inhibit PI3K activity (Figure 1C). Taken together, these experimental results indicate that mechanical stimulation of skeletal muscle myoblasts, in-vitro, induces p70S6k(389) phosphorylation through a PI3K-independent mechanism.
3.3 The Role of PLD in the Mechanical Activation of p70S6k(389) Phosphorylation
Using an ex-vivo model for mechanical stimulation of whole skeletal muscle explants, it was recently determined that a PLD-dependent synthesis of phosphatidic acid (PA) was required for the mechanical activation of p70S6k(389) phosphorylation [16]. Consistent with these ex-vivo observations, we have shown in the current in-vitro experiments that mechanical stimulation of skeletal muscle myoblasts promotes an increase in PLD activity (Figure 1D). Furthermore, inhibiting the PLD-dependent synthesis of PA with 1-butanol resulted in a dose-dependent inhibition in the mechanical activation of p70S6k(389) phosphorylation. At 0.8%, 1-butanol completely inhibited mechanically-induced signaling to p70S6k(389), but it did not have any significant effect on the magnitude of the mechanical activation of PKB or JNK2 phosphorylation (Figure 1E).
In an effort to confirm that the effects of 1-butanol were specific to the inhibition of PLD, myoblasts were stimulated with insulin in the presence or absence of 0.8% 1-butanol. The results indicated that 1-butanol did not inhibit the insulin-induced increase in p70S6k(389) phosphorylation. This observation demonstrates that signaling to p70S6k(389) can occur in the presence of inhibitory doses of 1-butanol, and also further reveals that mechanical stimuli and insulin utilize distinct mechanisms to activate p70S6k(389) phosphorylation.
3.4 The Roles of Rapamycin and mTOR in the Mechanical Activation of p70S6k(389) Phosphorylation
First, we used rapamycin to address the potential role of mTOR in the mechanical activation of p70S6k(389) phosphorylation. As shown in Figure 2A, rapamycin completely abolished p70S6k(389) phosphorylation in both control and mechanically-stimulated cells, while having no effect on JNK2 phosphorylation.
Figure 2. mTOR is the rapamycin-sensitive kinase that confers mechanically-induced phosphorylation of p70S6k(389).
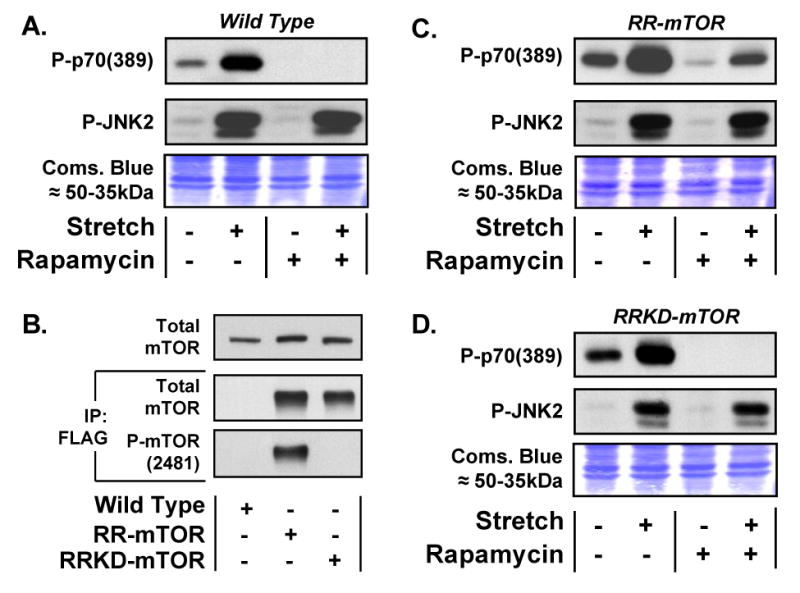
(A) C2C12 myoblasts were pre-incubated with 50nM rapamycin or the solvent vehicle (0.2% DMSO) for 30min before being subjected to 20min of control or 15% cyclic (1Hz) biaxial stretch [Stretch +] conditions. Samples were subjected to Western blot analysis of phosphorylated p70S6k (Thr389) [P-p70(389)] and phosphorylated JNK2 (Thr183/Tyr185) [P-JNK2]. (B) Lysates from C2C12 myoblasts [Wild Type] and C2C12 myoblasts stably expressing either a FLAG-tagged rapamycin-resistant mutant of mTOR [RR-mTOR] or a FLAG-tagged rapamycin-resistant kinase-dead mutant of mTOR [RRKD-mTOR] were subjected to Western blot analysis of total mTOR (top inset) or immunoprecipitated for the FLAG tag [IP: FLAG] followed by Western blot analysis of total mTOR or phosphorylated mTOR (Ser2481) [P-mTOR(2481)]. (C and D) C2C12 myoblasts stably expressing RR-mTOR (C) or RRKD-mTOR (D) were pre-incubated with 50nM Rapamycin or the solvent vehicle (0.2% DMSO) for 30min before being subjected to 20min of control or stretch conditions. Samples were subjected to Western blot analysis as described in (A). At the completion of western blotting, equal loading of protein in all lanes was verified with Coomassie Blue (Coms. Blue) staining of the transfer membranes (Images of the region containing the ≈50-35kDa proteins are shown). All western blots are representative of at least three experiments.
Numerous studies have shown that nutrients and growth factors activate p70S6k(389) phosphorylation through a rapamycin-sensitive mechanism, and that mTOR is the rapamycin-sensitive element in this pathway [11,12,17,18]. Furthermore, it has been well established that PI3K activity is required for nutrient and growth factor-induced signaling through mTOR [10,11,18]. These observations have led to the general conclusion that PI3K activity is required for mTOR signaling, and we are not aware of any studies that have directly shown otherwise. Thus, it is intriguing that mechanical stimuli can induce p70S6k(389) phosphorylation through a rapamycin-sensitive but PI3K-independent mechanism, and this observation raises questions as to whether mTOR is the rapamycin-sensitive element in this pathway.
Rapamycin inhibits mTOR signaling by forming a complex with the immunophilin FKBP12 which binds to mTOR in a region termed the FRB domain [19]. Although rapamycin is considered to be a highly specific inhibitor of mTOR, it remains possible that rapamycin can exert non-specific actions through the sequestration of the FKBP12 protein. For example, it has been shown that FKBP12 plays an important role in the function of the ryanodine receptor and signaling by the TGF-beta superfamily [20,21].
To determine whether mTOR is the rapamycin-sensitive element that confers mechanically-induced signaling to p70S6k(389), we employed C2C12 myoblasts that stably express a FLAG-tagged rapamycin-resistant mutant of mTOR (RR-mTOR) (Figure 2B). When subjected to mechanical stimulation, the RR-mTOR myoblasts showed changes in p70S6k(389) and JNK2 phosphorylation that are similar to those observed in wild type myoblasts (Figure 2A and 2C). However, unlike wild type myoblasts, p70S6k(389) phosphorylation could easily be detected in RR-mTOR myoblasts incubated in the presence of rapamycin. Furthermore, RR-mTOR myoblasts incubated in the presence of rapamycin displayed a mechanically-induced increase in p70S6k(389) phosphorylation (Figure 2C). These observations provide genetic evidence that mTOR is the rapamycin-sensitive element that confers mechanically-induced signaling to p70S6k(389).
To determine whether mTOR kinase activity was required for mechanically-induced signaling to p70S6k(389), we employed C2C12 myoblasts that stably express a FLAG-tagged rapamycin-resistant kinase-dead variant of mTOR (RRKD-mTOR). As shown in Figure 2B, this mTOR construct does not contain detectable Ser2481 phosphorylation. This observation is consistent with the hypothesis that Ser2481 is an autophosphorylation site on mTOR, and that the RRKD-mTOR construct does not contain kinase activity [22]. In these RRKD-mTOR myoblasts, we found that rapamycin completely abolished p70S6k(389) phosphorylation in both control and mechanically-stimulated conditions. This result indicates that the kinase activity of mTOR is necessary for both basal and mechanically-induced signaling to p70S6k(389).
In summary, this study first describes an in-vitro model that can be used to study the mechanisms involved in the mechanical activation of p70S6k(389) phosphorylation in skeletal muscle. Similar to what has been reported with ex-vivo and in-vivo models of mechanical stimulation, the in-vitro model of mechanical stimulation induces p70S6k(389) phosphorylation through a PI3K-independent and PLD/PA-dependent mechanism. This model was then used to demonstrate that mTOR is the rapamycin-sensitive kinase that confers mechanically-induced signaling to p70S6k(389) phosphorylation. Thus, measuring changes in p70S6k(389) phosphorylation appears to be a valid marker for measuring mechanically-induced mTOR signaling. The results of this study also demonstrate for the first time that mTOR signaling can be activated through a PI3K-independent mechanism.
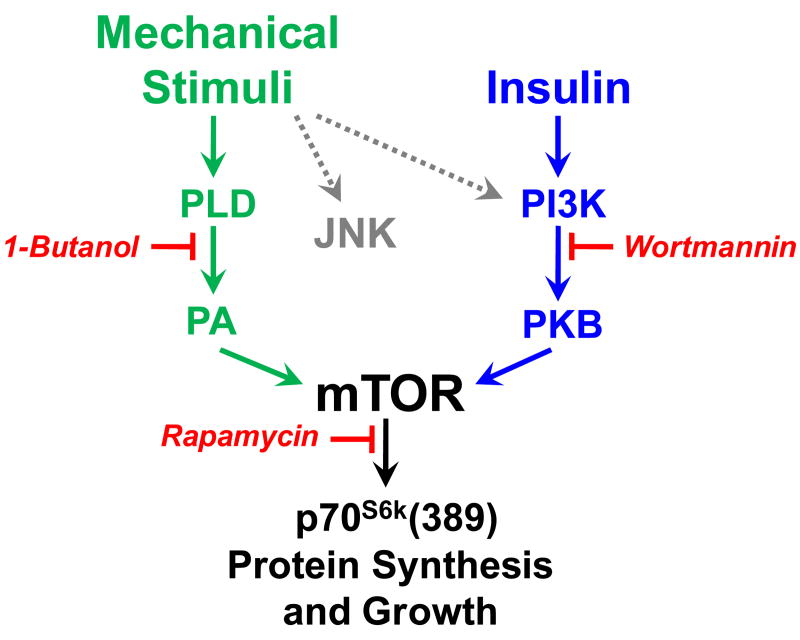
Figure 3. Schematic of the different mechanisms involved in the activation of mTOR signaling by insulin and mechanical stimuli.
Insulin and mechanical stimuli both induce phosphorylation of the hydrophobic motif Thr(389) in p70S6k through a rapamycin-sensitive mechanism, and mTOR is the kinase that confers this event. Insulin activates mTOR signaling to p70S6k(389) through a PLD-independent but PI3K-dependent mechanism. On the other hand, mechanical stimuli activate mTOR signaling to p70S6k(389) through a mechanism that is dependent on PLD but does not require PI3K activity. Additionally, inhibiting PLD activity with 1-Butanol does not block the mechanical activation of JNK or PKB phosphorylation implying that mechanical stimuli activate additional signaling pathways upstream of PLD.
Acknowledgments
The project described was supported by NIH grants F32AR052240 and 1R03AR053280 to T.A.H., R01HL64382 and R01HL80518 to S.C., and HD050837 to establish the National Center for Skeletal Muscle Research. Special thanks are extended to Jie Chen for providing technical support and the C2C12 cell lines stably expressing RR-mTOR and RRKD-mTOR.
List of Abbreviations
- JNK2
Jun N-terminal kinase 2
- mTOR
mammalian target of rapamycin
- PA
phosphatidic acid
- PLD
phospholipase D
- PI3K
phosphotidylinositol-3-kinase
- PKB
protein kinase B
- RR-mTOR
rapamycin-resistant mTOR
- RRKD-mTOR
rapamycin-resistant kinase-dead mTOR
- p70S6k
ribosomal S6 kinase
- Ser
Serine
- Thr
Threonine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goldberg AL, Etlinger JD, Goldspink DF, Jablecki C. Mechanism of work-induced hypertrophy of skeletal muscle. Med Sci Sports. 1975;7:185–98. [PubMed] [Google Scholar]
- 2.Bodine SC, et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3:1014–9. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- 3.Hornberger TA, et al. Selenoprotein-deficient transgenic mice exhibit enhanced exercise-induced muscle growth. J Nutr. 2003;133:3091–7. doi: 10.1093/jn/133.10.3091. [DOI] [PubMed] [Google Scholar]
- 4.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bodine SC. mTOR signaling and the molecular adaptation to resistance exercise. Med Sci Sports Exerc. 2006;38:1950–7. doi: 10.1249/01.mss.0000233797.24035.35. [DOI] [PubMed] [Google Scholar]
- 6.Baar K, Esser K. Phosphorylation of p70(S6k) correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol. 1999;276:C120–7. doi: 10.1152/ajpcell.1999.276.1.C120. [DOI] [PubMed] [Google Scholar]
- 7.Burry M, Hawkins D, Spangenburg EE. Lengthening contractions differentially affect p70(s6k) phosphorylation compared to isometric contractions in rat skeletal muscle. Eur J Appl Physiol. 2007;100:409–15. doi: 10.1007/s00421-007-0444-5. [DOI] [PubMed] [Google Scholar]
- 8.Haddad F, Adams GR. Aging-sensitive cellular and molecular mechanisms associated with skeletal muscle hypertrophy. J Appl Physiol. 2006;100:1188–203. doi: 10.1152/japplphysiol.01227.2005. [DOI] [PubMed] [Google Scholar]
- 9.Hornberger TA, Stuppard R, Conley KE, Fedele MJ, Fiorotto ML, Chin ER, Esser KA. Mechanical stimuli regulate rapamycin-sensitive signalling by a phosphoinositide 3-kinase-, protein kinase B- and growth factor-independent mechanism. Biochem J. 2004;380:795–804. doi: 10.1042/BJ20040274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hornberger TA, Chien S. Mechanical stimuli and nutrients regulate rapamycin-sensitive signaling through distinct mechanisms in skeletal muscle. J Cell Biochem. 2006;97:1207–16. doi: 10.1002/jcb.20671. [DOI] [PubMed] [Google Scholar]
- 11.Dann SG, Thomas G. The amino acid sensitive TOR pathway from yeast to mammals. FEBS Lett. 2006;580:2821–9. doi: 10.1016/j.febslet.2006.04.068. [DOI] [PubMed] [Google Scholar]
- 12.Foster DA. Regulation of mTOR by phosphatidic acid? Cancer Res. 2007;67:1–4. doi: 10.1158/0008-5472.CAN-06-3016. [DOI] [PubMed] [Google Scholar]
- 13.Proud CG. Signalling to translation: how signal transduction pathways control the protein synthetic machinery. Biochem J. 2007;403:217–34. doi: 10.1042/BJ20070024. [DOI] [PubMed] [Google Scholar]
- 14.Park IH, Chen J. Mammalian target of rapamycin (mTOR) signaling is required for a late-stage fusion process during skeletal myotube maturation. J Biol Chem. 2005;280:32009–17. doi: 10.1074/jbc.M506120200. [DOI] [PubMed] [Google Scholar]
- 15.Sotoudeh M, Jalali S, Usami S, Shyy JY, Chien S. A strain device imposing dynamic and uniform equi-biaxial strain to cultured cells. Ann Biomed Eng. 1998;26:181–9. doi: 10.1114/1.88. [DOI] [PubMed] [Google Scholar]
- 16.Hornberger TA, Chu WK, Mak YW, Hsiung JW, Huang SA, Chien S. The role of phospholipase D and phosphatidic acid in the mechanical activation of mTOR signaling in skeletal muscle. Proc Natl Acad Sci U S A. 2006;103:4741–6. doi: 10.1073/pnas.0600678103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hornberger TA, Sukhija KB, Chien S. Regulation of mTOR by mechanically induced signaling events in skeletal muscle. Cell Cycle. 2006;5:1391–6. doi: 10.4161/cc.5.13.2921. [DOI] [PubMed] [Google Scholar]
- 18.Harris TE, Lawrence JC., Jr TOR signaling. Sci STKE 2003. 2003:re15. doi: 10.1126/stke.2122003re15. [DOI] [PubMed] [Google Scholar]
- 19.Lorenz MC, Heitman J. TOR mutations confer rapamycin resistance by preventing interaction with FKBP12-rapamycin. J Biol Chem. 1995;270:27531–7. doi: 10.1074/jbc.270.46.27531. [DOI] [PubMed] [Google Scholar]
- 20.Lehnart SE, Huang F, Marx SO, Marks AR. Immunophilins and coupled gating of ryanodine receptors. Curr Top Med Chem. 2003;3:1383–91. doi: 10.2174/1568026033451907. [DOI] [PubMed] [Google Scholar]
- 21.Wang T, Donahoe PK. The immunophilin FKBP12: a molecular guardian of the TGF-beta family type I receptors. Front Biosci. 2004;9:619–31. doi: 10.2741/1095. [DOI] [PubMed] [Google Scholar]
- 22.Peterson RT, Beal PA, Comb MJ, Schreiber SL. FKBP12-rapamycin-associated protein (FRAP) autophosphorylates at serine 2481 under translationally repressive conditions. J Biol Chem. 2000;275:7416–23. doi: 10.1074/jbc.275.10.7416. [DOI] [PubMed] [Google Scholar]