Abstract
The present work examines chemical and structural response in B. anthracis spores killed by a mixture of supercritical carbon dioxide (SCCO2) and hydrogen peroxide (H2O2). Deactivation of 6-log of B. anthracis spores by SCCO2+H2O2 was demonstrated, but changes in structure were observed in only a small portion of spores. Results from phase contrast microscopy proved that this treatment is mild and does not trigger germination-like changes. TEM imaging revealed mild damage in a portion of spores while the majority remained intact. Dipicolinic acid (DPA) analysis showed that <10% of the DPA was released from the spore core into the external milieu, further demonstrating only modest damage to the spores. Confocal fluorescent microscopy, assessing uptake of DNA-binding dyes, directly demonstrated compromise of the permeability barrier. However, the magnitude of uptake was small compared to spores that had been autoclaved. This work suggests that SCCO2+H2O2 is quite mild compared to other sterilization methods, which has major implications in its application. These results provide some insight on the possible interactions between spores and the SCCO2+H2O2 sterilization process.
Keywords: integrity, sterilization, spores, supercritical carbon dioxide, hydrogen peroxide
Introduction
A gentler but effective alternative to the current standard sterilization methods (e.g. autoclaving, gamma-irradiation, and ethylene oxide) is sorely needed, due to the limitations of these methods and the development of novel polymeric biomaterials which are usually sensitive to these methods (Matthews et al., 2001, Premnath et al., 1996). Steam sterilization damages or destroys heat-sensitive and hydrolytically labile materials (Dempsey and Thirucote, 1989); γ-irradiation may cause changes in shear and tensile strength, elastic modulus, and transparency of polymers (Dillow et al., 1999); and ethylene oxide has significant effect on molecular weight of some biodegradable polymers (Verheyen et al., 1992). Since CO2 has a relatively low “critical temperature”, it is of particular interest as a low temperature sterilization medium. It is non-toxic, non-flammable, chemically inert, and physiologically safe (Spilimbergo and Bertucco, 2003, Zhang et al., 2006). In its supercritical state (T>31.1°C, P>1070 psi), SCCO2 has a liquid-like density (Span and Wagner, 1996) while maintaining gas-like diffusivity (McHugh and Krukonis, 1993), allowing SCCO2 to easily penetrate complex structures. Therefore, a SCCO2 sterilization technique would be a viable option for heat-sensitive and/or porous materials.
SCCO2 has been widely investigated for deactivation of vegetative bacteria, but its effect on bacterial spores has received limited attention (Zhang, Davis, Matthews, Drews, LaBerge and An, 2006). Spores are highly resistant to heat, UV radiation, free radicals, and chemicals. They are quite different, physiologically and morphologically, from vegetative cells. For example the interior of the spore (the core) is highly dehydrated (compare with the 80~90% water content in vegetative cells) and contains high concentrations of dipicolinic acid (DPA) and small acid soluble proteins (SASPs). An intact inner membrane is believed to be important in retaining these molecules. The outside of the spore contains unique layers (including exosporium and coat) not found in vegetative cells. The coat is the primary permeability barrier to entry of large molecules. Furthermore, unlike the vegetative cell, there are both inner and outer membranes (Madigan et al., 2002; Driks, 1999).
To effectively kill spores, a temperature of 121°C is commonly used in a steam autoclave, whereas many other vegetative bacteria are killed at temperatures between 60 and 100°C. The deactivation effect of SCCO2 has been evaluated on spores of numerous species (Spilimbergo and Bertucco, 2003, Zhang, Davis, Matthews, Drews, LaBerge and An, 2006). Harsh conditions (e.g. pressures up to 30 MPa, temperatures up to 100°C, and exposure up to 100 hr) are required to achieve a significant reduction in viability of spores, which may damage heat-sensitive materials and devices, and increase investments and operating costs. Synergistic effects of SCCO2 with pulse electric field up to 25 kV/cm (Spilimbergo et al., 2003) and high hydrostatic pressure up to 600 MPa (Park et al., 2002) have been reported. However, the high voltage and high hydrostatic pressure could also be damaging. In our earlier publication, it was shown that at mild temperatures (<60°C), with trace level (~200 ppm) of aqueous hydrogen peroxide, spores of B. atrophaeus ATCC 9372, B. pumilus ATCC 27142, and G. stearothermophilus ATCC 7953 can be reduced in number by more than 6-log (Hemmer et al., 2006, Zhang et al., 2006, Zhang et al., 2006).
It is important to understand the interactions between spores and SCCO2 + H2O2. Physical changes have been reported on vegetative bacteria and fungi killed by SCCO2. Cell wall rupture has been observed with E. coli (Fraser, 1951) and S. cerevisiae (Shimoda et al., 1998), resulting from fast expansion of absorbed CO2 during the depressurization stage. Changes of internal structures, such as gas between the cell wall and cytoplasm, fractures in cell membranes, were observed on L. plantarum with SEM (Hong and Pyun, 1999). As noted above, due to differences in biochemical composition of vegetative cells and spores, it is reasonable to suggest that damage to spores might be distinct. Furthermore, it may not be possible to extrapolate previous studies of aqueous H2O2 sterilization to what occurs on spore deactivation with H2O2 in an SCCO2 phase. Decades of study on H2O2 sterilization have revealed that the damage to the spores depends on concentration and whether H2O2 is in the liquid or vapor state (King and Gould, 1969, Shin et al., 1994, Tsuchida and Tsuchido, 1997).
The purpose of the current work was to study changes in morphology and permeability resulting from treatment of spores with SCCO2 supplemented with H2O2 with implications on application as a sterilizing agent.
MATERIALS AND METHODS
Chemicals
Difco™ tryptic soy agar (Becton, Dickinson and Company, Sparks, MD) and 30% H2O2 aqueous solution were obtained from Fisher Scientific (Fair Lawn, NJ). Anhydrous CO2 (purity > 99.8%) was obtained from National Specialty Gases (Durham, NC). The BacLight Bacterial Viability Kit L13152 was obtained from Invitrogen Corporation (Eugene, OR). This kit includes two transfer pipets containing SYTO 9 and propidium iodide (PI) respectively. Pall GHP Acrodisc® 25 mm syringe filters with 0.45 μm GHP membrane were used to filter-sterilize deionized water (East Hills, NY). VWR microslides and Fisherbrand® microscope cover glass were used to prepare spore slides (West Chester, PA).
Preparation of spores
Bacillus anthracis ΔSterne-1 was used as a test microorganism to determine the sporicidal ability of SCCO2+H2O2 and was a gift from Dr. S.H. Leppla (National Institutes of Health, Bethesda, MD). Generally, growth was on nutrient agar plates (Difco, Detroit, MI) at room temp. Sporulation was essentially complete after 7 to 14 days (>95%). Spores were harvested by washing agar surfaces with water. The spore suspension was then centrifuged (17,210 × g , 12 min). The supernatant was removed and spores were re-suspended in water and pelleted by centrifugation; this was repeated 3 times. Spores were then subjected to freeze-thawing by storing at -70°C for at least 12 hr overnight. The spore suspension was thawed and incubated at room temp for 2 hr. This resulted in lysis of remaining vegetative cells; preparations were >99% spores. The degree of sporulation was assessed by either malachite green staining or phase contrast microscopy. Then the spores were washed 3 times in distilled water and freeze-dried (< 1 mmHg and -100°C). Spores were either inoculated on a small piece of Schleicher & Schuell filter paper (#470) to produce “spore strips” or inoculated into small glass vial (“spore vials”).
Preparation of spore strips
Milligram quantities of lyophilized spores were weighed into a 2.0 mL Target DP™ glass vial (National Scientific Company, Rockwood, TN). The spores were then transferred from the glass vial and suspended in 5 mL of sterile water. 30 μL of the spore suspension was transferred onto steam autoclaved 0.6 cm × 4 cm Schleicher & Schuell filter paper (#470) (Whatman Inc., Florham Park, New Jersey). The filter paper strips were air-dried inside a Labconco Class II biosafety cabinet (Labconco Corporation, Kansas City, MI).
Preparation of spore vials
After SCCO2 + H2O2 treatment it is difficult to release spores from strips for biochemical or morphological studies. Thus milligram quantities of lyophilized spores were suspended in 5 mL of water. 100 μL of suspension was transferred into each of several autoclaved 2.0 mL glass vials. The septum of the cap of the vial was removed and replaced with a two-layer filter that allowed CO2 to flow in and out of the vial and prevented spores from escaping. The top layer was cut from a Pall GN-6 Metricel® membrane filter (0.45 μm pore size, 152 μm thickness, East Hills, NY) and the bottom layer was cut from a Nylon™ filter (30 μm pore size, 64 μm thickness, Spectrum Laboratories, Rancho Dominguez, CA). Nylon provides mechanical strength to the membrane filter. These glass vials were frozen in fast freeze flasks at -70°C for at least 12 hrs before they were lyophilized. Each spore vial contained 106 ~ 107 spores.
Supercritical CO2 sterilization
Spore strips were exposed to SCCO2 under controlled conditions (temperature, pressure, and time) in an ISCO SFX 2-10 two-cartridge fluid extractor (Lincoln, NE), depicted in Figure 1. This figure shows only one of the two 10 mL parallel cartridges in the SFX 2-10. The SFX 2-10 has a pre-heater to heat the CO2 to the desired temperature. CO2 is fed into one of the two high-pressure cartridges, which are sealed with hand-tight closures. The cartridges are embedded in an isothermal heat block to maintain the desired temperature. The extractor is pressurized by two ISCO D260 high-pressure syringe pumps, which are controlled by an ISCO series D pump controller. The pumps are filled from a standard gas cylinder. CO2 flows through a Valco Instruments (Houston, TX) six-port liquid injection valve (Figure 1), and then into the pressure cartridge. The valve is switched between the sample-loading position and the injecting position. The amount of delivered liquid is determined by a detachable loop connecting port 3 and port 6. A 5 μL loop was used in this work. At the end of an experiment, CO2 within the pressure cartridge is released rapidly through a 1/16 inch vent valve.
Figure 1.
Schematic of supercritical CO2 apparatus
Prior to each treatment, four spore strips or a vial were transferred aseptically into a steam autoclaved, dry, 10 mL ISCO pressure cartridge. The desired temperature was established in the SFX 2-10, then the pressure cartridge was inserted. After flushing with pure CO2 (~800 psi) for approximately five sec, the six-port valve was switched from the load to the inject position. The vent valve was closed and the system was pressurized with CO2. The hydrogen peroxide in the sample loop was thus transported quantitatively into the cartridge. The pressure was monitored with a Digiquartz portable precision pressure transducer (Paroscientific, Inc., model 740, Redmond, WA). After the desired time, the cartridge was depressurized to atmospheric pressure by opening the vent valve. The pressurization and depressurization were completed within one minute. The cartridge containing the spore strips or the vial was immediately removed from the SFX 2-10 extractor. The spores were recovered either by pulverizing the strips in a blender or suspending spores in the vial with sterile water. The degree of killing was quantified with a standard plate counting technique. The log reduction of spores on a spore strip was calculated with equation (1).
| (1) |
Phase contrast microscopy imaging
Samples of untreated, CO2 treated, and CO2+H2O2 treated spores were suspended in 0.5 mL of sterile deionized water. A small drop of spore suspension was placed on a micro slide and covered with a cover slip. The slides were imaged with a Nikon Eclipse E600 microscope (Nikon Instruments Inc., Melville, NY) with 40X objective lens in phase contrast mode. Images were captured by a Micropublisher digital camera with the Qimaging software (version 2.81.0, Quantitative Imaging Corporation, Burnaby, Canada) in auto exposure mode.
Ultra-structural characterization
Untreated spores and spores processed with CO2 or CO2 + H2O2 were observed using TEM. The spores were incubated in a mixture of 1 mL of a 2.5% glutaraldehyde, 0.1 M sodium cacodylate solution containing 0.1% ruthenium red (Electron Microscopy Sciences, Fort Washington, PA) for one hr at 37°C with gentle rocking. The suspension was then centrifuged to obtain a spore pellet. Each pellet was washed in 0.1 M sodium cacodylate buffer (pH 7.2) and fixed for 3 hr at room temp in a 2% osmium tetroxide (Electron Microscopy Sciences), 0.1 M sodium cacodylate solution containing 0.1% ruthenium red. A negative control was treated identically, but ruthenium red was omitted from these two steps. Spores were washed three times in 0.1 M sodium cacodylate buffer and embedded in 3% agar (EM Science, Gibbstown, NJ). Dehydration involved sequential treatment with 25, 50, 75, 95, and 100% ethanol (AAPER Alcohol & Chemical Co., Shelbyville, KY). Afterwards, cells were placed sequentially in propylene oxide (Electron Microscopy Sciences), propylene oxide/polybed 812 (2:1) and pure polybed 812 (Polysciences, Warrington, PA). Polymerization was carried out at 60°C in pure polybed. Specimen blocks were thin-sectioned (~120 nm thick), then collected on 200 mesh copper grids. EM sections were then stained with a 2% uranyl acetate solution (Electron Microscopy Sciences) for 40 min at 37°C. The sections were then treated with Hanaichi lead citrate (0.15 % lead nitrate, 0.15 % sodium acetate, 1% sodium citrate dissolved in 41 mL water and 9 mL of 1N sodium hydroxide (Fisher Scientific)) for 2 min. Spores were observed by transmission electron microscopy (Waller et al., 2004).
Assay for release of dipicolinic acid (DPA)
A one mM DPA solution was prepared (99%, Acros Organics) in 10 μM terbium chloride (99.9%, Acros Organics, Fair Lawn, NJ) buffered with 1M sodium acetate (>99%, Fisher Scientific, Fair Lawn, NJ) and 1M acetic acid (99.8%, Acros Organics) at a pH of 5.6 (Hindle and Hall, 1999). Standard DPA solutions in the assay solution contained concentrations between 100 and 1000 nM.
The effect of CO2 or CO2 + H2O2 on DPA release from spores was studied. It has been established that the majority of DPA is present in the core (Germaine and Murrell, 1974, Kozuka et al., 1985, Leanz and Gilvarg, 1973), although some residual may exist in the cortex. Spore vials that were not treated by CO2 or CO2+H2O2 were used as control samples. The spores were suspended in 12 mL assay solution and split into three aliquots. The first aliquot was directly filtered through a Pall GHP Acrodisc® 25 mm syringe filter (0.45 μm pore size, Pall Corporation, NY) without heat-shock (NHS); the second one was heat-shocked (HS) at 62°C before filtration; the third one was steam autoclaved (AC) before filtration to release all DPA within the spores (Janssen et al., 1958). All samples were analyzed with a SLM-Aminco (NY) spectrofluorometer. Steady-state emission and excitation spectra were collected with magic-angle polarization. Samples were excited at 280 nm, and emission spectra were collected at 545 nm. Excitation and collection bandpasses were 3 nm in all cases, and the slit widths were 8 nm. The integration time was 3 sec.
BacLight fluorescent assay of bacterial spores
The control (no treatment), CO2 processed (4000 psi, 40°C, 4 hours), CO2+H2O2 processed (4000 psi, 40°C, 4 hours, 200 ppm H2O2), or steam autoclaved (121°C, 35 min) spores were stained with BacLight following the instructions in the user manual. Spores were suspended in 1 mL filter-sterilized water. Stock solutions of BacLight reagent were prepared by dissolving the dyes in the two transfer pipets into 5-mL filter-sterilized water. The resulting concentrations were 6 μM in SYTO 9 and 30 μM in PI. Spore suspension (0.1 mL) and BacLight stock solution (0.1 mL) were transferred into a 2 mL glass vial and mixed thoroughly. The mixture was incubated in the dark for 15 min at room temp.
BacLight stained spore suspension (10 μL) was transferred to a glass microslide and air dried in a biohood. A small drop of mounting oil (included in the BacLight kit) was applied on the dried spore smear. A cover glass was used to trap the mounting oil between the microslide and the cover glass. Fingernail polish (a surrogate sealing material) was carefully brushed around the edge of the cover glass as a sealant.
The slides with BacLight stained spore samples were imaged with a Bio-Rad MRC1024 confocal scanning laser microscope (Bio-Rad Laboratories, Inc., Hercules, CA) with 60X oil lens. The microscope is equipped with a 488 nm excitation filter, a 605DF32 emission filter for the red channel (PI fluorescence), and a 522DF32 emission filter for the green channel (SYTO 9 fluorescence). Fluorescent images were acquired by LaserSharpe 2000 software. The adjustable parameters of LaserSharpe software (Krypton/Argon (laser intensity), Iris, gain, offset, and zoom) were kept constant throughout this study (Table 1).
Table 1.
Parameters for the fluorescent microscope configuration
| Laser | Iris | Gain | Offset | Zoom | |
|---|---|---|---|---|---|
| Green channel | 10 | 3.0 | 950.0 | 14.0 | 3X |
| Red channel | 10 | 2.6 | 960.5 | -3.5 | 3X |
Results
Sterilization of B. anthracis spores
Pure CO2 has been shown to be ineffective in killing dry spores (Kamihira et al., 1987), and our unpublished results with B. atrophaeus spores confirmed this. Therefore, only the results on the use of H2O2 in SCCO2 were studied . The number of viable B. anthracis spores cultured from 30 μL of aqueous spore suspension was 4.13 ± 0.36 × 106. The average number of viable spores recovered from each untreated spore strip was 2.82 ± 0.29 × 106; approximately 70% of the spores inoculated onto each spore strip. The lower number was presumably due to incomplete release. After SCCO2+H2O2 treatment at 4000 psi, 40°C for 4 hours, only an average of 12.2 ± 17.7 spores were recovered from each spore strip, which was equivalent to a 5.74 log reduction of B. anthracis spores. When “spore vials” were used, an average of 4.59±0.85×107 B. anthracis spores were recovered. After SCCO2+H2O2 treatment at 4000 psi, 40°C for 4 hr, an average of 33.3 ± 57.7 (equivalent to a 6.14 log reduction of B. anthracis spores) were cultured. Therefore, SCCO2+H2O2 treatment is sufficient to achieve high degree of deactivation at 40°C, 4000 psi for 4 hr.
Phase contrast microscopy
Phase contrast images of untreated spores, CO2 processed, and CO2+H2O2 processed spores are shown in Figure 2. Unprocessed spores were phase bright (Figure 2 a). Spores remained phase bright after the treatment with CO2 (Figure 2 b) and after complete deactivation with SCCO2+H2O2 (Figure 2 c). The results suggest that structural changes, associated with subsequent germination, were not initiated by SCCO2 + H2O2
Figure 2.
Phase contrast images of untreated, pure CO2 processed, and CO2+H2O2 processed spores
TEM imaging
The TEM images of B. anthracis after different treatments are shown in Figure 3. Spores structures, e.g. coats, cortex, membranes and the spore core, can be readily identified from the spores stained with osmium tetroxide alone. The exosporium was visible, but poorly stained (Figure 3 a). Ruthenium red staining significantly enhances the visibility of the exosporium (Figure 3 b) (Waller, Fox, Fox, Fox and Price, 2004). After CO2+H2O2 treatment, protrusions of the exosporium were observed on a minority of the spores (Figure 3 d), indicating disruption. Other ultra-structural changes were not obvious. Similar effects were observed with SCCO2 alone (Figure 3 c).
Figure 3.
TEM images of B. anthracis spores
DPA analysis
The amount of DPA released from untreated B. anthracis spores, CO2 treated spores, and CO2 + H2O2 treated spores were measured. The effect of HS on untreated and treated spores was also evaluated. The results are shown in Figure 4. Negative control (untreated) and positive control (autoclaved) samples were used to define minimum and maximum DPA release from spores.
Figure 4.
DPA release from B. anthracis spores. (a) Controls and spores treated with CO2+H2O2. (b) Controls and spores treated with pure CO2.
For untreated spores, an average of 8.9±0.4 nmol and 7.3±0.8 nmol/mg DPA was released (two independent experiments). Steam autoclaving released greatly more DPA; an average of 413.6±51.6 nmol (the first batch of vials, Figure 4 a) and 335.6±75.0 nmol (the second batch of vials, Figure 4 b) of DPA, equivalent to 6.9% and 5.6% of spore dry weight. Heat-shock is used to activate bacterial spores for germination (Curran and Evans, 1945). It was hypothesized in this study that heat-shocking of the dead spores with compromised membranes would lead to readier release of DPA due to the higher mass transfer rate at the heat-shock temperature. The increase in DPA release from HS negative control samples was barely detectable although statistically significant, increasing from 8.9±0.4 nmol (NHS) to 10.4±0.6 nmol/mg (HS).
SCCO2+H2O2 treated samples released greater amounts of DPA compared to untreated samples. Two different SCCO2+H2O2 treatments (1500 psi, 40°C, 1hr and 4000 psi, 40°C, 4hr) were performed (Figure 4 a). The gentler treatment released 17.9±4.6 nmol/mg for NHS spores. The harsher treatment released a slightly higher level (22.2±12.0 nmol/mg) but the difference between the two treatments was not statistically different. For HS spores, though the spores were dead and could not germinate, with the gentler SCCO2+H2O2 treatment 25.3±6.0 nmol/mg was released and was further increased to 41.3±17.2 nmol/mg for the harsher treatment (Figure 4 a ); results were statistically significant.
There was little effect of HS on DPA release for samples treated with CO2 alone. The average amounts of DPA released from NHS samples were 7.9±0.5 and 7.5±0.6 nmol/mg (1500 psi and 4000 psi) respectively, which was not statistically different from the 7.3±0.8 nmol DPA release from NHS untreated samples.
BacLight fluorescence assay
Autofluorescence occured only when laser intensity, iris and gain were set at their maximum values (image not shown). No auto fluorescence was detected when these parameters were lowered to values used to image stained spores (Table 1). The negative control, CO2 processed, CO2+H2O2 processed, and steam autoclaved (positive control) spores were stained with BacLight and imaged with the BioRad MRC1024 confocal scanning laser microscope. Representative images from each sample are shown in Figure 5.
Figure 5.
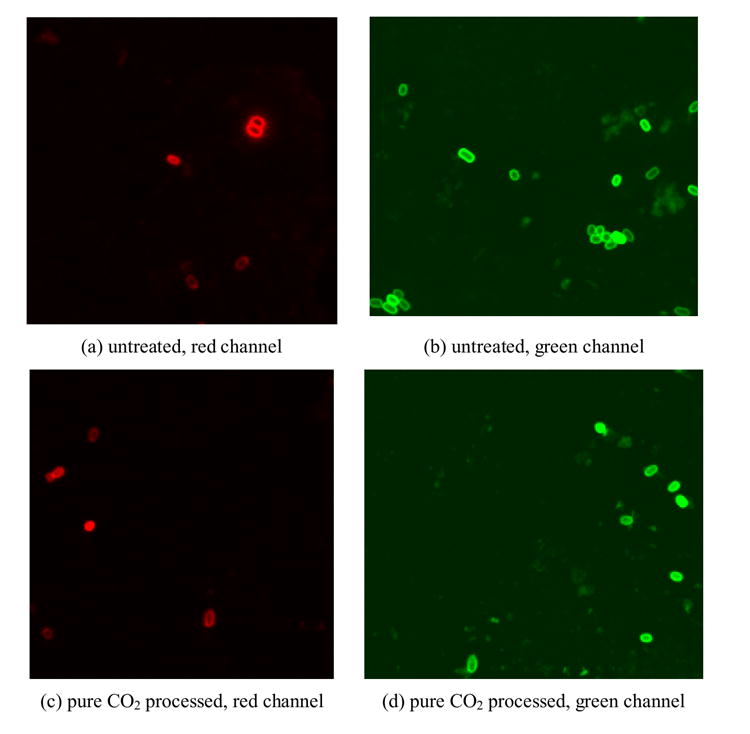
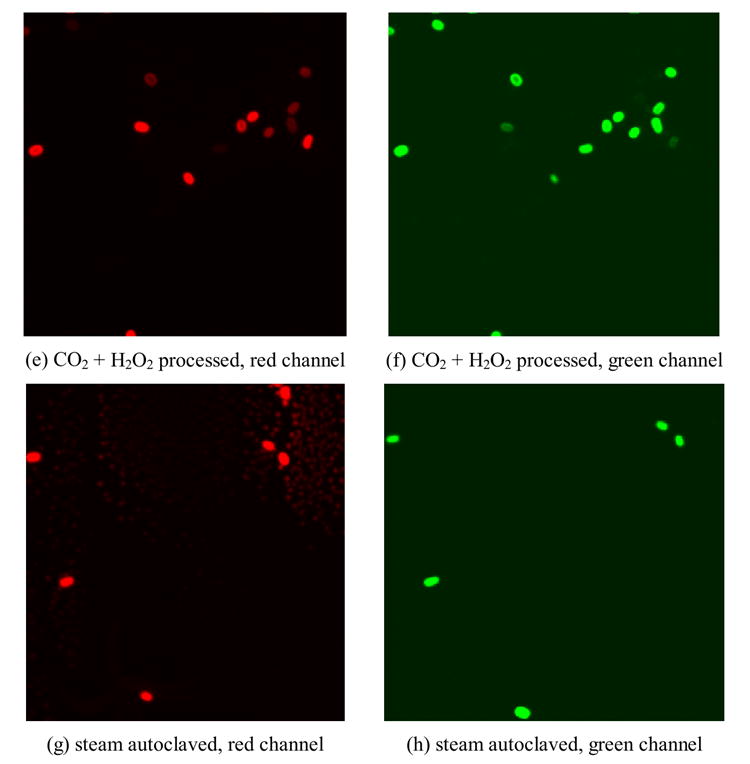
Fluorescent images of BacLight stained B. anthracis spores
Qualitatively, most of the untreated spores were only stained peripherally with either PI (red) or SYTO 9 (green) (Figure 5 a,b). After steam autoclaving in suspension for 30 min, both SYTO 9 and PI penetrated into the spore; fluorescent red or green staining respectively was observed (Figure 5 g,h). With CO2 treatment, most of the spores were still stained peripherally, as were the untreated spores. However, a higher degree of penetration of dyes was observed (Figure 5 c,d). After being treated with CO2+H2O2, more spores were stained completely by PI and SYTO 9 (Figure 5 e,f).
In order to obtain semi-quantitative results, large numbers of completely stained spores and peripherally stained spores were counted (> 100). Comparable counting results were obtained by the two independent observers. The results are shown in Figure 6. 7% and 21% of untreated B. anthracis spores were completely stained by PI and SYTO 9, respectively. 100% of autoclaved spores were completely stained with both PI and SYTO 9. After the spores were treated for 4 hr by pure CO2 at 4000 psi, 40°C, complete staining with PI or SYTO 9 increased to 15% and 53%, respectively. After the spores were treated for 4 hr with CO2 and 200 ppm H2O2 at 4000 psi, 40°C, the percentages of completely PI stained and completely SYTO 9 stained spores increased to 50% and 84%, respectively.
Figure 6.
Percentages of different staining patterns of B. anthracis spores
Discussion
The sporicidal effects of SCCO2 + H2O2 on B. anthracis spores was studied (4000 psi and 40°C). As a result, a high degree of reduction of viable spores (up to 6.14 log reduction) has been observed, confirming the sporicidal effects of this mixture at low temperatures for spores of other bacilli (Hemmer, Drews, LaBerge and Matthews, 2006, Zhang, Burrows, Matthews, Drews, LaBerge and An, 2006). This degree of log reduction meets the sterilization requirement set by the FDA.
It has been shown that spore germination or germination-like changes can be initiated with either high static pressure, pressurized CO2, or aqueous H2O2 alone. Initiation of B. subtilis spores germination was achieved with static pressures between 200 and 400 MPa (Furukawa et al., 2003). It also has been shown that treatment with pressurized CO2 helps induce spore germination, rendering the spores more susceptible to deactivation (Ballestra and Cuq, 1998). According to a recent study, gaseous CO2 at 6.5 MPa (942 psi) resulted in germination of B. coagulants and B. licheniformis spores when present in aqueous suspension (Furukawa et al., 2004). Spores treated with 0.86 M aqueous H2O2 undergo germination-like changes in the cell envelope, as evidenced by change from phase-bright to phase dark. This is due to loss of intra-cellular ions, such as DPA, and uptake of water (King and Gould, 1969, Powell, 1957). However, the phase contrast results reported by this work suggest that neither germination nor germination-like changes occure though the spores were effectively killed by SCCO2 + H2O2. In this work, the pressure (27.6 MPa) was much less than the static pressure required (hundreds of MPa) to germinate spores. It is suspected that the presence of large quantities of water in Furukawa et al. (2004)’s work may play a significant role in germinating spores. However, in this work, the spores were in a dry condition. The H2O2 concentration (ppm level) is also much lower than the 0.86 M used to cause germination-like changes. Therefore, this process is gentler than any of the other three treatments, though a high degree of killing is ensured.
TEM imaging demonstrated protrusions of exosporium on a small portion of spores after both CO2 and CO2 + H2O2 treatment. However, that many spores appeared undamaged suggest that the changes were more subtle in nature than steam autoclaving which destroys both outer and inner structures (Fonzi et al., 1999).
Results from the DPA analysis also suggested that SCCO2 + H2O2 caused only mild structural changes to spores. Statistically more DPA release was observed with spores exposed to SCCO2 and H2O2 as compared to untreated controls. The difference was more dramatic when samples were first heat-shocked. However, even with a combination of heat shock and SCCO2+H2O2, the release of DPA was quite limited compared to autoclaving (less than 10% DPA release), indicating the treatment is relatively mild.
The peripheral staining of untreated spores with either PI or SYTO 9 was clearly different from complete uptake observed for vegetative cells (Arzese et al., 2003). The peripheral staining pattern has previously been explained as the staining of the cortex and the unstained center as the core (Melly et al., 2002). However, it is possible that the dye might also be staining the exosporium or coat (the outermost layers). Autoclaving is an aggressive treatment that kills 100% of the spores and presumably causes drastic cellular changes possibly providing complete access to intra-cellular DNA. Therefore, 100% of the spores were stained with both PI and SYTO 9. The majority of spores treated with CO2 were stained peripherally, similar to the untreated spores, suggesting that CO2 does not alter the permeability of spores. After spores were treated with CO2+H2O2, penetration through the inner membrane (and other cell envelope layers) to the core is significantly increased. Since only 50% of the spores were stained with PI, half of the spores still maintained inner membrane integrity and had intact permeability barriers. This was the most dramatic change observed in this work. Under the same conditions, approximately 6 log (equivalent to 99.9999%) reduction of spores was achieved. The change in permeability (approximately 50%) was still modest compared with the 6 log killing of spores observed.
Conclusions
Up to 6 log reduction of B. anthracis spores was achieved by exposing the spores to SCCO2 + H2O2. Comparing with the high degree of killing, the SCCO2 + H2O2 process is very gentle in changing spore structure. No germination-like changes were observed with phase contrast microscopy, indicating a less harsh process than either high static pressure, high pressure CO2 with high water content, or high concentration H2O2 treatments. TEM imaging of the spores stained with ruthenium red showed mostly intact spores, indicating a much milder treatment that conventional steam autoclaving. DPA release assay demonstrated definitive while gentle alteration of the spore permeability barrier following SCCO2 + H2O2 treatment. The most dramatic change of spore structure was demonstrated by the BacLight fluorescent assay, approximately half of the spores lost membrane integrity. However, compared with the almost complete killing of spores observed, this degree of change is still mild. These observations confirm that SC CO2 + H2O2 treatment has great promise as a sterilization technique that is quite mild in nature.
Acknowledgments
The work was supported by National Institutes of Health grants R01 B0055201 (M.A. Matthews) and R21 AI05943 (G. Stewart and A. Fox). We thank Bob Price and Jeff Davis for electron microscopic characterization of spores.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arzese A, Skerlavaj B, Tomasinsig L, Gennaro R, Zanetti M. Antimicrobial activity of SMAP-29 against the Bacteroides fragilis group and clostridia. J Antimicrob Chemother. 2003;52:375–381. doi: 10.1093/jac/dkg372. [DOI] [PubMed] [Google Scholar]
- Curran HR, Evans FR. Heat Activation Inducing Germination in the Spores of Thermotolerant and Thermophilic Aerobic Bacteria. J Bacteriol. 1945;49:335–346. doi: 10.1128/jb.49.4.335-346.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey DJ, Thirucote RR. Sterilization of medical devices: A review. Journal of Biomaterials Applications. 1989;3:454–523. doi: 10.1177/088532828800300303. [DOI] [PubMed] [Google Scholar]
- Dillow AK, Dehghani F, Hrkach JS, Foster NR, Langer R. Bacterial inactivation by using near- and supercritical carbon dioxide. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:10344–10348. doi: 10.1073/pnas.96.18.10344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonzi M, Montomoli E, Gasparini R, Devanna D, Fonzi L. Morpho-structural variations of bacterial spores after treatment in steam vacuum assisted autoclave. Bull Group Int Rech Sci Stomatol Odontol. 1999;41:124–130. doi: 10.3201/eid0801.010143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser D. Bursting bacteria by release of gas pressure. Nature. 1951;167:33–34. doi: 10.1038/167033b0. [DOI] [PubMed] [Google Scholar]
- Furukawa S, Shimoda M, Hayakawa I. Mechanism of the inactivation of bacterial spores by reciprocal pressurization treatment. J Appl Microbiol. 2003;94:836–841. doi: 10.1046/j.1365-2672.2003.01913.x. [DOI] [PubMed] [Google Scholar]
- Furukawa S, Watanabe T, Tai T, Hirata J, Narisawa N, Kawarai T, Ogihara H, Yamasaki M. Effect of high pressure gaseous carbon dioxide on the germination of bacterial spores. Int J Food Microbiol. 2004;91:209–213. doi: 10.1016/S0168-1605(03)00372-6. [DOI] [PubMed] [Google Scholar]
- Germaine GR, Murrell WG. Use of Ultraviolet-Radiation to Locate Dipicolinic Acid in Bacillus cereus Spores. J Bacteriol. 1974;118:202–208. doi: 10.1128/jb.118.1.202-208.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmer JD, Drews MJ, LaBerge M, Matthews MA. Sterilization of Bacterial Spores by Using Supercritical Carbon Dioxide and Hydrogen Peroxide. J Biomed Mater Res (Part B) 2006;80B:511–518. doi: 10.1002/jbm.b.30625. [DOI] [PubMed] [Google Scholar]
- Hindle AA, Hall EAH. Dipicolinic acid (DPA) assay revisited and appraised for spore detection. Analyst (Cambridge, United Kingdom) 1999;124:1599–1604. doi: 10.1039/a906846e. [DOI] [PubMed] [Google Scholar]
- Hong SI, Pyun YR. Inactivation kinetics of Lactobacillus plantarum by high pressure carbon dioxide. J Food Sci. 1999;64:728–733. [Google Scholar]
- Janssen FW, Lund AJ, Anderson LE. Colorimetric assay for dipicolinic acid in bacterial spores. Science. 1958;127:26–27. doi: 10.1126/science.127.3288.26. [DOI] [PubMed] [Google Scholar]
- Kamihira M, Taniguchi M, Kobayashi T. Sterilization of Microorganisms with Supercritical Carbon Dioxide. Agric Biol Chem. 1987;51:407–412. [Google Scholar]
- King WL, Gould GW. Lysis of bacterial spores with hydrogen peroxide. J Appl Bacteriol. 1969;32:481–490. doi: 10.1111/j.1365-2672.1969.tb01002.x. [DOI] [PubMed] [Google Scholar]
- Kozuka S, Yasuda Y, Tochikubo K. Ultrastructural-Localization of Dipicolinic Acid in Dormant Spores of Bacillus subtilis by Immunoelectron Microscopy with Colloidal Gold Particles. J Bacteriol. 1985;162:1250–1254. doi: 10.1128/jb.162.3.1250-1254.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leanz G, Gilvarg C. Dipicolinic acid location in intact spores of Bacillus megaterium. J Bacteriol. 1973;114:455–456. doi: 10.1128/jb.114.1.455-456.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews MA, Warner LS, Kaiser H. Exploring the feasibility of using dense-phase carbon dioxide for sterilization. Medical Device & Diagnostic Industry. 2001;5:140–149. [Google Scholar]
- McHugh M, Krukonis V. Supercritical fluid extraction. Butterworth-Heinemann; USA: 1993. [Google Scholar]
- Melly E, Cowan AE, Setlow P. Studies on the mechanism of killing of Bacillus subtilis spores by hydrogen peroxide. J Appl Microbiol. 2002;93:316–325. doi: 10.1046/j.1365-2672.2002.01687.x. [DOI] [PubMed] [Google Scholar]
- Park SJ, Lee JI, Park J. Effects of a combined process of high-pressure carbon dioxide and high hydrostatic pressure on the quality of carrot juice. Journal of Food Science. 2002;67:1827–1834. [Google Scholar]
- Powell EO. The appearance of bacterial spores under phase-contrast illumination. J Appl Bacteriol. 1957;20:342–348. [Google Scholar]
- Premnath V, Harris WH, Jasty M, Merrill EW. Gamma sterilization of UHMWPE articular implants: An analysis of the oxidation problem. Biomaterials. 1996;17:1741–1753. doi: 10.1016/0142-9612(95)00349-5. [DOI] [PubMed] [Google Scholar]
- Shimoda M, Yamamoto Y, Cocunubo-Castellanos J, Tonoike H, Kawano T, Ishikawa H, Osajima Y. Antimicrobial effects of pressured carbon dioxide in a continuous flow system. J Food Sci. 1998;63:709–712. [Google Scholar]
- Shin SY, Calvisi EG, Beaman TC, Pankratz HS, Gerhardt P, Marquis RE. Microscopic and Thermal Characterization of Hydrogen Peroxide Killing and Lysis of Spores and Protection by Transition Metal Ions, Chelators, and Antioxidants. Appl Environ Microbiol. 1994;60:3192–3197. doi: 10.1128/aem.60.9.3192-3197.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Span R, Wagner W. A new equation of state for carbon dioxide covering the fluid region from the triple-point temperature to 1100 K at pressures up to 800 MPa. Journal of Physical and Chemical Reference Data. 1996;25:1509–1596. [Google Scholar]
- Spilimbergo S, Bertucco A. Non-thermal bacteria inactivation with dense CO2. Biotechnol Bioeng. 2003;84:627–638. doi: 10.1002/bit.10783. [DOI] [PubMed] [Google Scholar]
- Spilimbergo S, Dehghani F, Bertucco A, Foster NR. Inactivation of bacteria and spores by pulse electric field and high pressure CO2 at low temperature. Biotechnology and Bioengineering. 2003;82:118–125. doi: 10.1002/bit.10554. [DOI] [PubMed] [Google Scholar]
- Tsuchida Y, Tsuchido T. Sensitivities to hydrogen peroxide vapor of intact and decoated spores of Bacillus subtilis as influenced by divalent cations supplemented in sporulation medium. Biocontrol Sci. 1997;2:19–26. [Google Scholar]
- Verheyen CCPM, De Wijn JR, Van Blitterswijk CA, De Groot K. Evaluation of hydroxylapatite/poly(L-lactide) composites: Mechanical behavior. Journal of Biomedical Materials Research. 1992;26:1277–1296. doi: 10.1002/jbm.820261003. [DOI] [PubMed] [Google Scholar]
- Waller LN, Fox N, Fox KF, Fox A, Price RL. Ruthenium red staining for ultrastructural visualization of a glycoprotein layer surrounding the spore of Bacillus anthracis and Bacillus subtilis. J Microbiol Methods. 2004;58:23–30. doi: 10.1016/j.mimet.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Zhang J, Burrows S, Matthews MA, Drews MJ, LaBerge M, An YH. Sterilizing Bacillus pumilus spores using supercritical carbon dioxide. Journal of Microbiological Methods. 2006;66:479–485. doi: 10.1016/j.mimet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Zhang J, Dalal N, Gleason C, Matthews MA, Waller LN, Fox KF, Fox A, Drews MJ, LaBerge M, An YH. On the mechanisms of deactivation of Bacillus atrophaeus spores using supercritical carbon dioxide. Journal of Supercritical Fluids. 2006;38:268–273. [Google Scholar]
- Zhang J, Davis TA, Matthews MA, Drews MJ, LaBerge M, An YH. Sterilization using high-pressure carbon dioxide. Journal of Supercritical Fluids. 2006;38:354–372. [Google Scholar]


