Abstract
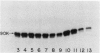
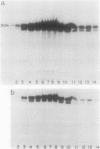
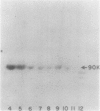
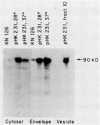
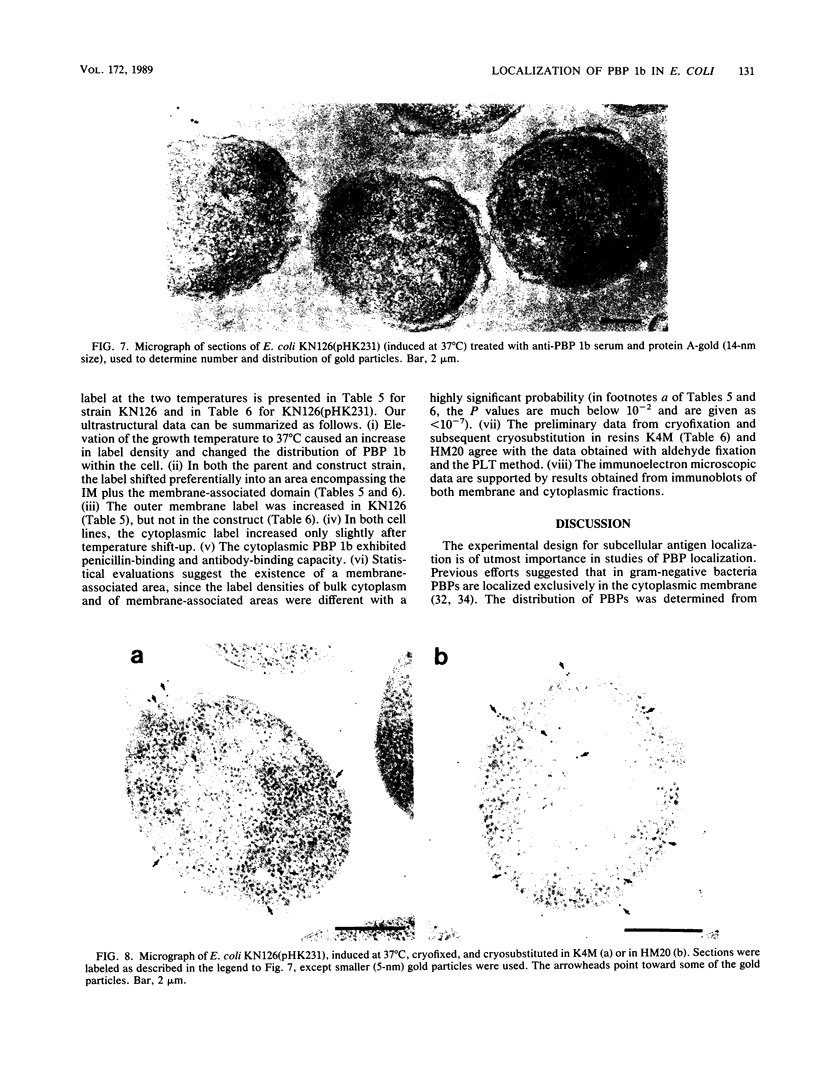
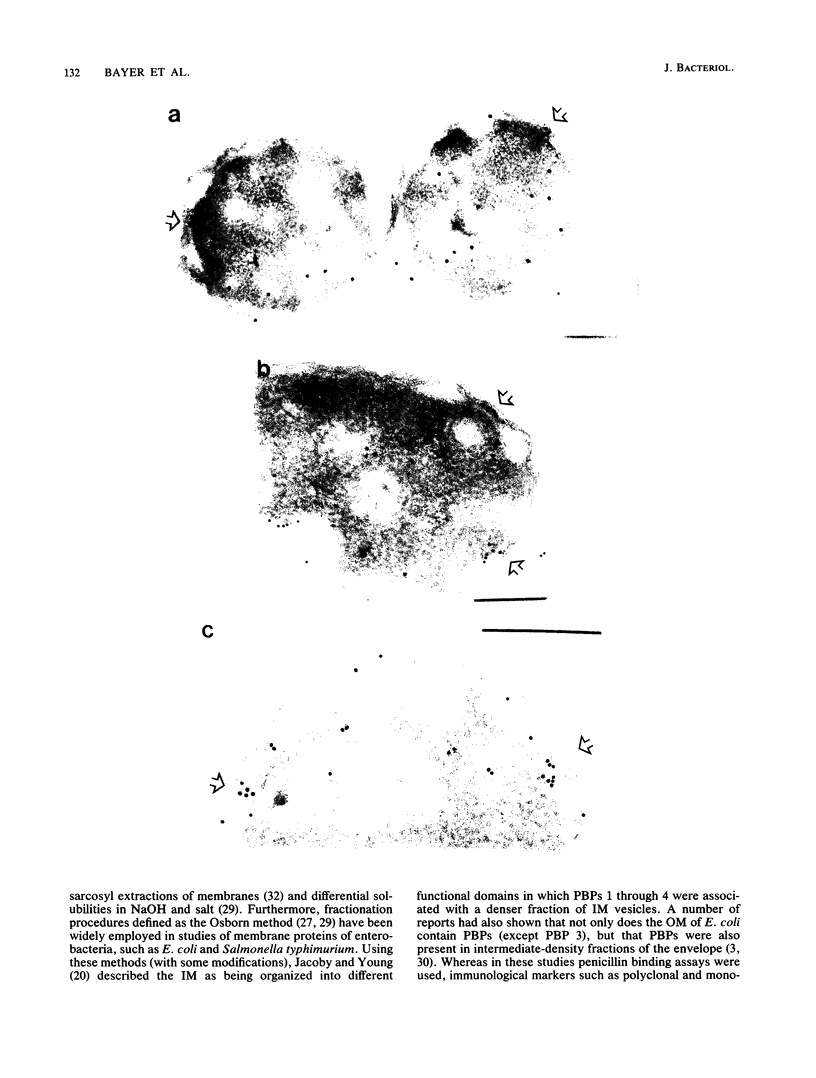
We report the localization of penicillin-binding protein 1b (PBP 1b) in Escherichia coli KN126 and in an overproducing construct containing plasmid pHK231. We used PBP 1b-specific antiserum for the immunoelectron microscopy of ultrathin sections of whole cells and for immunoelectrophoresis of cytoplasm and isolated membrane fractions. We studied ultrathin sections of both glutaraldehyde-fixed cells that had been embedded after progressively lowering the temperature and cryofixed cells that had been freeze-substituted in Lowicryl K4M and HM20. Most of the PBP 1b-specific label was observed in the inner membrane (IM) and the adjacent cytoplasm, much less was observed in the outer membrane (OM); appreciable amounts were also seen in the bulk cytoplasm. Distribution and intensity of label were both temperature dependent: temperature shift-up to 37 degrees C, causing PBP 1b overproduction in the construct, showed a statistically highly significant increase in label of the IM, including a cytoplasmic zone (of at least 30 nm in depth) adjacent to the IM, a zone we termed the membrane-associated area. Concomitant with the temperature shift-up, a decrease in label density was observed in the bulk cytoplasm. Increased label was also found in IM-OM contact areas (zones of membrane adhesion). The periplasm did not show significant label. Western blotting (immunoblotting) revealed PBP 1b in most of the isolated membrane fractions; however, the highest label density was found in membrane fractions of intermediate density, supporting the suggestion of an increased concentration of PBP 1b in the membrane adhesion zones. In summarizing, we propose that PBP 1b is present in the membrane-associated area of the cytoplasm, from where proteins (such as PBP 1b or thioredoxin) gain access to their specific insertion sites in the envelope. The use of several methods of immunoelectron microscopy provided the first unequivocal evidence for localization of PBP 1b at membrane adhesion sites. Since such sites are specifically labeled with anti-PBP 1b serum, we hypothesize that they contain parts of the machinery for assembly and growth of the murein layer.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbas J. A., Díaz J., Rodríguez-Tébar A., Vázquez D. Specific location of penicillin-binding proteins within the cell envelope of Escherichia coli. J Bacteriol. 1986 Jan;165(1):269–275. doi: 10.1128/jb.165.1.269-275.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. E., Bayer M. H. Effects of bacteriophage fd infection on Escherichia coli HB11 envelope: a morphological and biochemical study. J Virol. 1986 Jan;57(1):258–266. doi: 10.1128/jvi.57.1.258-266.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. E., Bayer M. H., Lunn C. A., Pigiet V. Association of thioredoxin with the inner membrane and adhesion sites in Escherichia coli. J Bacteriol. 1987 Jun;169(6):2659–2666. doi: 10.1128/jb.169.6.2659-2666.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. H., Costello G. P., Bayer M. E. Isolation and partial characterization of membrane vesicles carrying markers of the membrane adhesion sites. J Bacteriol. 1982 Feb;149(2):758–767. doi: 10.1128/jb.149.2.758-767.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch D., Leunissen J., Verbakel J., de Jong M., van Erp H., Tommassen J. Periplasmic accumulation of truncated forms of outer-membrane PhoE protein of Escherichia coli K-12. J Mol Biol. 1986 Jun 5;189(3):449–455. doi: 10.1016/0022-2836(86)90316-5. [DOI] [PubMed] [Google Scholar]
- Burman L. G., Park J. T. Molecular model for elongation of the murein sacculus of Escherichia coli. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1844–1848. doi: 10.1073/pnas.81.6.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burn P. Amphitropic proteins: a new class of membrane proteins. Trends Biochem Sci. 1988 Mar;13(3):79–83. doi: 10.1016/0968-0004(88)90043-6. [DOI] [PubMed] [Google Scholar]
- Carlemalm E., Villiger W., Hobot J. A., Acetarin J. D., Kellenberger E. Low temperature embedding with Lowicryl resins: two new formulations and some applications. J Microsc. 1985 Oct;140(Pt 1):55–63. doi: 10.1111/j.1365-2818.1985.tb02660.x. [DOI] [PubMed] [Google Scholar]
- Den Blaauwen T., Wientjes F. B., Kolk A. H., Spratt B. G., Nanninga N. Preparation and characterization of monoclonal antibodies against native membrane-bound penicillin-binding protein 1B of Escherichia coli. J Bacteriol. 1989 Mar;171(3):1394–1401. doi: 10.1128/jb.171.3.1394-1401.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira L. C., Schwarz U., Keck W., Charlier P., Dideberg O., Ghuysen J. M. Properties and crystallization of a genetically engineered, water-soluble derivative of penicillin-binding protein 5 of Escherichia coli K12. Eur J Biochem. 1988 Jan 15;171(1-2):11–16. doi: 10.1111/j.1432-1033.1988.tb13751.x. [DOI] [PubMed] [Google Scholar]
- Freudl R., Schwarz H., Stierhof Y. D., Gamon K., Hindennach I., Henning U. An outer membrane protein (OmpA) of Escherichia coli K-12 undergoes a conformational change during export. J Biol Chem. 1986 Aug 25;261(24):11355–11361. [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Ishino F., Mitsui K., Tamaki S., Matsuhashi M. Dual enzyme activities of cell wall peptidoglycan synthesis, peptidoglycan transglycosylase and penicillin-sensitive transpeptidase, in purified preparations of Escherichia coli penicillin-binding protein 1A. Biochem Biophys Res Commun. 1980 Nov 17;97(1):287–293. doi: 10.1016/s0006-291x(80)80166-5. [DOI] [PubMed] [Google Scholar]
- Jacoby G. H., Young K. D. Unequal distribution of penicillin-binding proteins among inner membrane vesicles of Escherichia coli. J Bacteriol. 1988 Aug;170(8):3660–3667. doi: 10.1128/jb.170.8.3660-3667.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato J., Suzuki H., Hirota Y. Dispensability of either penicillin-binding protein-1a or -1b involved in the essential process for cell elongation in Escherichia coli. Mol Gen Genet. 1985;200(2):272–277. doi: 10.1007/BF00425435. [DOI] [PubMed] [Google Scholar]
- Keck W., Wientjes F. B., Schwarz U. Comparison of two hydrolytic murein transglycosylases of Escherichia coli. Eur J Biochem. 1985 May 2;148(3):493–497. doi: 10.1111/j.1432-1033.1985.tb08866.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Low M. G. Biochemistry of the glycosyl-phosphatidylinositol membrane protein anchors. Biochem J. 1987 May 15;244(1):1–13. doi: 10.1042/bj2440001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Pratt J. M., Jackson M. E., Holland I. B. The C terminus of penicillin-binding protein 5 is essential for localisation to the E. coli inner membrane. EMBO J. 1986 Sep;5(9):2399–2405. doi: 10.1002/j.1460-2075.1986.tb04510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Tébar A., Barbas J. A., Vázquez D. Location of some proteins involved in peptidoglycan synthesis and cell division in the inner and outer membranes of Escherichia coli. J Bacteriol. 1985 Jan;161(1):243–248. doi: 10.1128/jb.161.1.243-248.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G., Jobanputra V. Mutants of Escherichia coli which lack a component of penicillin-binding protein 1 are viable. FEBS Lett. 1977 Jul 15;79(2):374–378. doi: 10.1016/0014-5793(77)80824-7. [DOI] [PubMed] [Google Scholar]
- Spratt B. G., Pardee A. B. Penicillin-binding proteins and cell shape in E. coli. Nature. 1975 Apr 10;254(5500):516–517. doi: 10.1038/254516a0. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Properties of the penicillin-binding proteins of Escherichia coli K12,. Eur J Biochem. 1977 Jan;72(2):341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- Tamaki S., Nakajima S., Matsuhashi M. Thermosensitive mutation in Escherichia coli simultaneously causing defects in penicillin-binding protein-1Bs and in enzyme activity for peptidoglycan synthesis in vitro. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5472–5476. doi: 10.1073/pnas.74.12.5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorhout W., De Kroon T., Leunissen-Bijvelt J., Verkleij A., Tommassen J. Accumulation of LamB-LacZ hybrid proteins in intracytoplasmic membrane-like structures in Escherichia coli K12. J Gen Microbiol. 1988 Mar;134(3):599–604. doi: 10.1099/00221287-134-3-599. [DOI] [PubMed] [Google Scholar]