Abstract
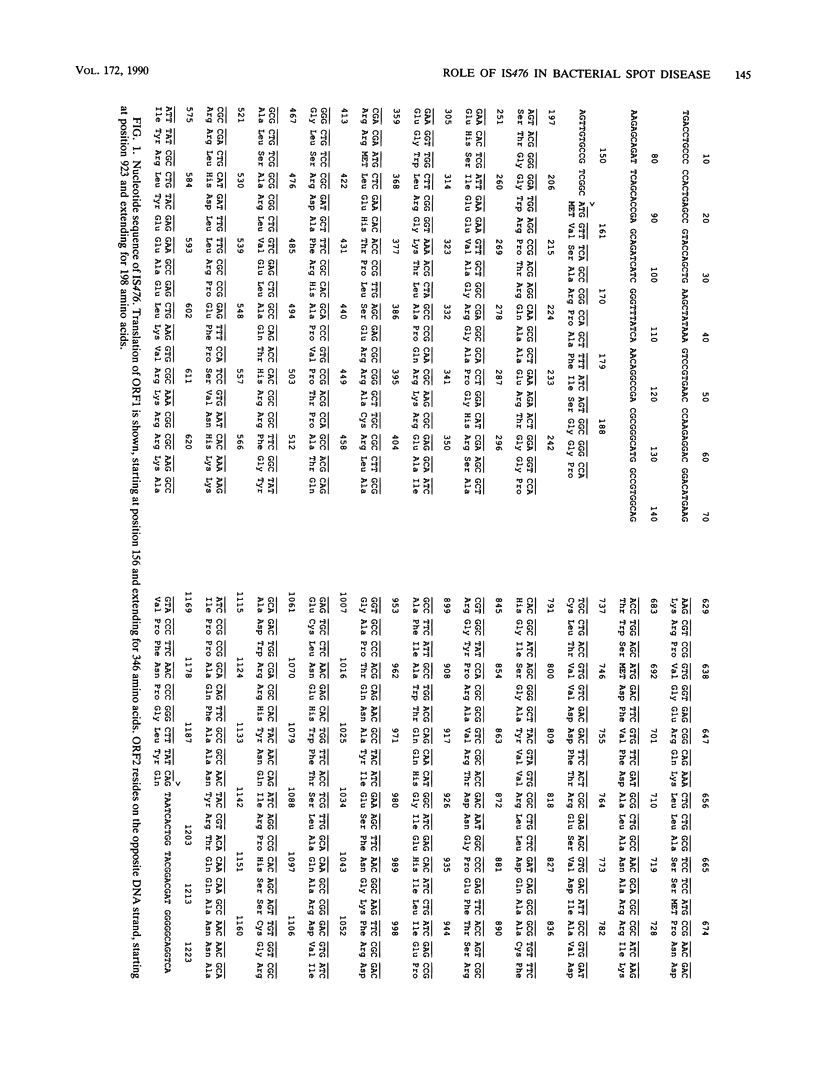
IS476 is an endogenous insertion sequence present in copper-tolerant strains of Xanthomonas campestris pv. vesicatoria. Sequence analysis has revealed that the element is 1,225 base pairs in length, has 26-base-pair inverted repeats, and causes a 4-base-pair target site duplication upon insertion into the avirulence gene avrBs1. Comparison of the full-length sequence with sequences in the National Biomedical Research Foundation and National Institutes of Health data bases showed that one of the predicted IS476 proteins is partially homologous to the putative transposase of IS3 from Escherichia coli, and the inverted repeats of IS476 have significant homology to the inverted repeats of the IS51 insertion sequence of Pseudomonas syringae pv. savastanoi. A transposition assay based on the insertional inactivation of the sacRB locus of Bacillus subtilis was used to demonstrate that one of the three copies of IS476 residing on the 200-kilobase copper plasmid pXVCU1 is capable of transposition in several strains of Xanthomonas campestris. The position of IS476 insertion in several avrBs1 mutants was established and was shown to influence both induction of hypersensitivity and bacterial growth in planta.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campbell A., Starlinger P., Berg D. E., Botstein D., Lederberg E. M., Novick R. P., Szybalski W. Nomenclature of transposable elements in prokaryotes. Plasmid. 1979 Jul;2(3):466–473. doi: 10.1016/0147-619x(79)90030-1. [DOI] [PubMed] [Google Scholar]
- Comai L., Kosuge T. Transposable element that causes mutations in a plant pathogenic Pseudomonas sp. J Bacteriol. 1983 Jun;154(3):1162–1167. doi: 10.1128/jb.154.3.1162-1167.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gay P., Le Coq D., Steinmetz M., Berkelman T., Kado C. I. Positive selection procedure for entrapment of insertion sequence elements in gram-negative bacteria. J Bacteriol. 1985 Nov;164(2):918–921. doi: 10.1128/jb.164.2.918-921.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay P., Le Coq D., Steinmetz M., Ferrari E., Hoch J. A. Cloning structural gene sacB, which codes for exoenzyme levansucrase of Bacillus subtilis: expression of the gene in Escherichia coli. J Bacteriol. 1983 Mar;153(3):1424–1431. doi: 10.1128/jb.153.3.1424-1431.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzy-Treboul G., Chambert R., Dedonder R. Levansucrase of Bacillus subtilis : reexamination of some physical and chemical properties. Biochimie. 1975;57(1):17–28. doi: 10.1016/s0300-9084(75)80105-2. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Ronald P. C., Staskawicz B. J. The avirulence gene avrBs1 from Xanthomonas campestris pv. vesicatoria encodes a 50-kD protein. Mol Plant Microbe Interact. 1988 May-Jun;1(5):191–198. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. H., Brutlag D., Friedland P., Kedes L. H. BIONET: national computer resource for molecular biology. Nucleic Acids Res. 1986 Jan 10;14(1):17–20. doi: 10.1093/nar/14.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staskawicz B., Dahlbeck D., Keen N., Napoli C. Molecular characterization of cloned avirulence genes from race 0 and race 1 of Pseudomonas syringae pv. glycinea. J Bacteriol. 1987 Dec;169(12):5789–5794. doi: 10.1128/jb.169.12.5789-5794.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmerman K. P., Tu C. P. Complete sequence of IS3. Nucleic Acids Res. 1985 Mar 25;13(6):2127–2139. doi: 10.1093/nar/13.6.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen M. C., Stall R. E., Staskawicz B. J. Characterization of a gene from a tomato pathogen determining hypersensitive resistance in non-host species and genetic analysis of this resistance in bean. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6743–6747. doi: 10.1073/pnas.85.18.6743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T., Lee P. D., Kosuge T. Insertion sequence elements of Pseudomonas savastanoi: Nucleotide sequence and homology with Agrobacterium tumefaciens transfer DNA. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8263–8267. doi: 10.1073/pnas.83.21.8263. [DOI] [PMC free article] [PubMed] [Google Scholar]