Abstract
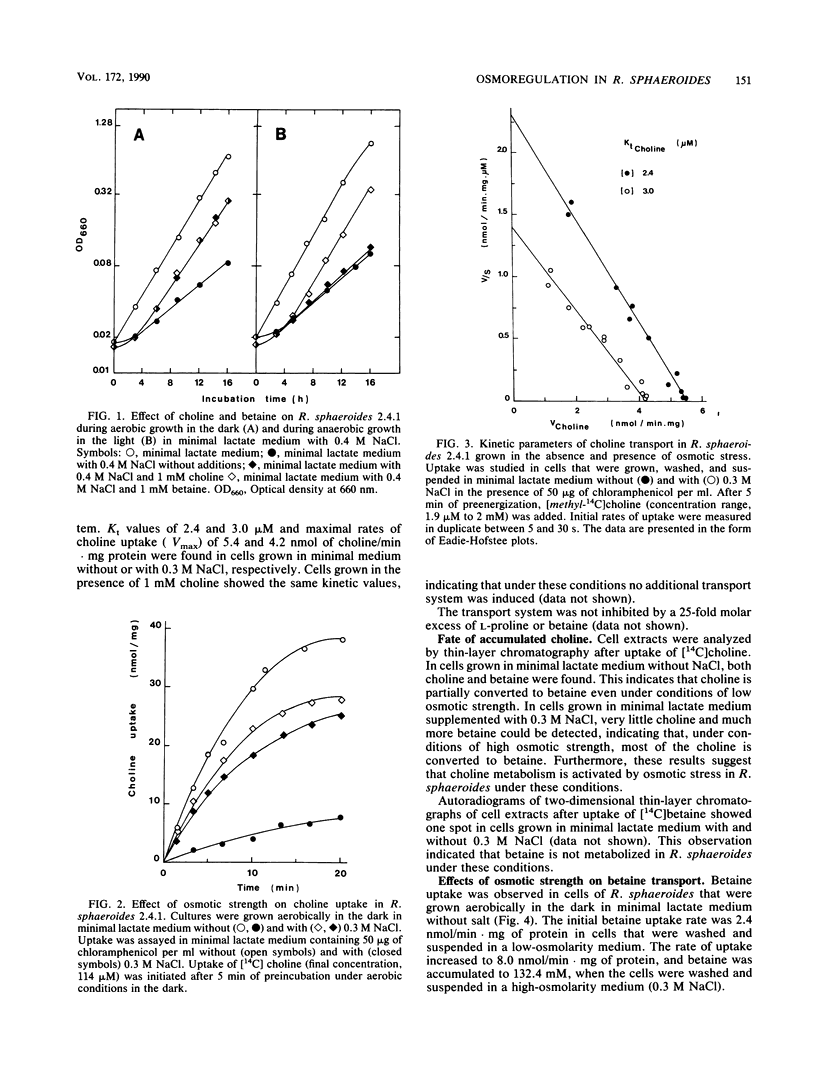
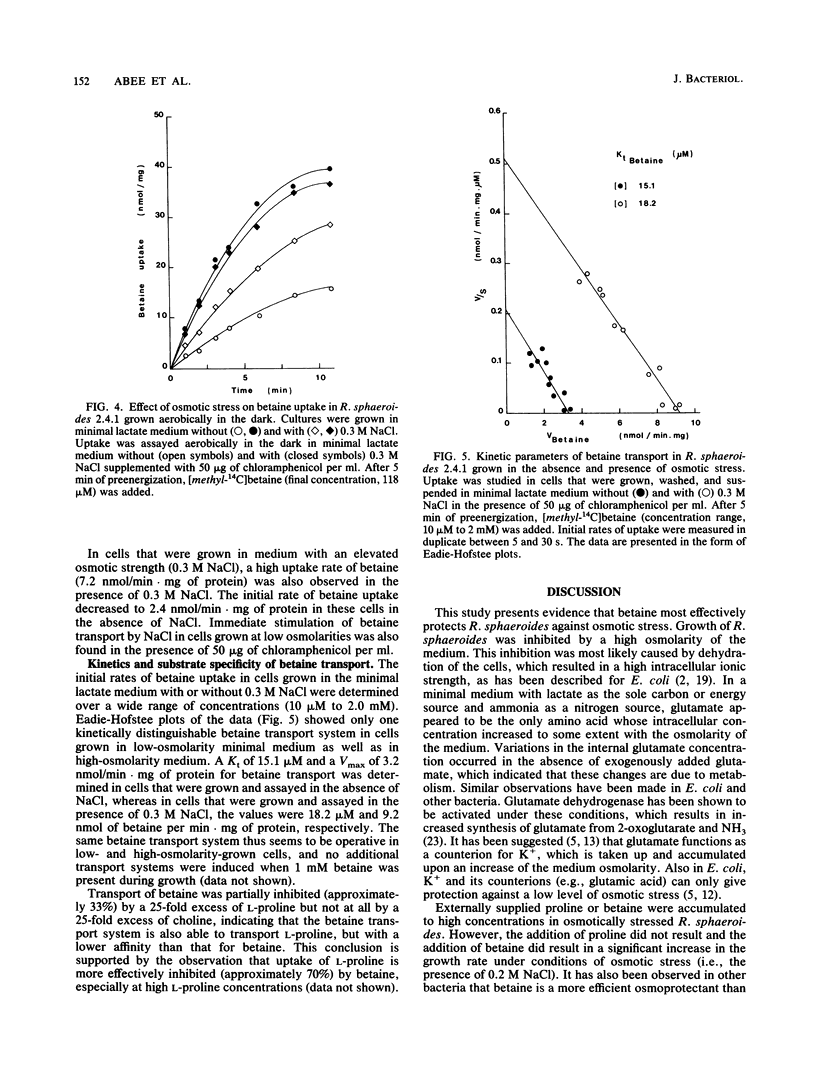
Betaine (N,N,N-trimethylglycine) functioned most effectively as an osmoprotectant in osmotically stressed Rhodobacter sphaeroides cells during aerobic growth in the dark and during anaerobic growth in the light. The presence of the amino acids L-glutamate, L-alanine, or L-proline in the growth medium did not result in a significant increase in the growth rate at increased osmotic strengths. The addition of choline to the medium stimulated growth at increased osmolarities but only under aerobic conditions. Under these conditions choline was converted via an oxygen-dependent pathway to betaine, which was not further metabolized. The initial rates of choline uptake by cells grown in media with low and high osmolarities were measured over a wide range of concentrations (1.9 microM to 2.0 mM). Only one kinetically distinguishable choline transport system could be detected. Kt values of 2.4 and 3.0 microM and maximal rates of choline uptake (Vmax) of 5.4 and 4.2 nmol of choline/min.mg of protein were found in cells grown in the minimal medium without or with 0.3 M NaCl, respectively. Choline transport was not inhibited by a 25-fold excess of L-proline or betaine. Only one kinetically distinguishable betaine transport system was found in cells grown in the low-osmolarity minimal medium as well as in a high-osmolarity medium containing 0.3 M NaCl. In cells grown and assayed in the absence of NaCl, betaine transport occurred with a Kt of 15.1 microM and a Vmax of 3.2 nmol/min . mg of protein, whereas in cells that were grown and assayed in the presence of 0.3 M NaCl, the corresponding values were 18.2 microM and 9.2 nmol of betaine/min . mg of protein. This system was also able to transport L-proline, but with a lower affinity than that for betaine. The addition of choline of betaine to the growth medium did not result in the induction of additional transport systems.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin W. W., Sheu M. J., Bankston P. W., Woldringh C. L. Changes in buoyant density and cell size of Escherichia coli in response to osmotic shocks. J Bacteriol. 1988 Jan;170(1):452–455. doi: 10.1128/jb.170.1.452-455.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron A., Jung J. U., Villarejo M. Purification and characterization of a glycine betaine binding protein from Escherichia coli. J Biol Chem. 1987 Aug 25;262(24):11841–11846. [PubMed] [Google Scholar]
- Cairney J., Booth I. R., Higgins C. F. Salmonella typhimurium proP gene encodes a transport system for the osmoprotectant betaine. J Bacteriol. 1985 Dec;164(3):1218–1223. doi: 10.1128/jb.164.3.1218-1223.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinnbier U., Limpinsel E., Schmid R., Bakker E. P. Transient accumulation of potassium glutamate and its replacement by trehalose during adaptation of growing cells of Escherichia coli K-12 to elevated sodium chloride concentrations. Arch Microbiol. 1988;150(4):348–357. doi: 10.1007/BF00408306. [DOI] [PubMed] [Google Scholar]
- Giaever H. M., Styrvold O. B., Kaasen I., Strøm A. R. Biochemical and genetic characterization of osmoregulatory trehalose synthesis in Escherichia coli. J Bacteriol. 1988 Jun;170(6):2841–2849. doi: 10.1128/jb.170.6.2841-2849.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand S. C., Somero G. N. Urea and methylamine effects on rabbit muscle phosphofructokinase. Catalytic stability and aggregation state as a function of pH and temperature. J Biol Chem. 1982 Jan 25;257(2):734–741. [PubMed] [Google Scholar]
- Higgins C. F., Sutherland L., Cairney J., Booth I. R. The osmotically regulated proU locus of Salmonella typhimurium encodes a periplasmic betaine-binding protein. J Gen Microbiol. 1987 Feb;133(2):305–310. doi: 10.1099/00221287-133-2-305. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Landfald B., Strøm A. R. Choline-glycine betaine pathway confers a high level of osmotic tolerance in Escherichia coli. J Bacteriol. 1986 Mar;165(3):849–855. doi: 10.1128/jb.165.3.849-855.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen P. I., Sydnes L. K., Landfald B., Strøm A. R. Osmoregulation in Escherichia coli by accumulation of organic osmolytes: betaines, glutamic acid, and trehalose. Arch Microbiol. 1987 Feb;147(1):1–7. doi: 10.1007/BF00492896. [DOI] [PubMed] [Google Scholar]
- Le Rudulier D., Strom A. R., Dandekar A. M., Smith L. T., Valentine R. C. Molecular biology of osmoregulation. Science. 1984 Jun 8;224(4653):1064–1068. doi: 10.1126/science.224.4653.1064. [DOI] [PubMed] [Google Scholar]
- May G., Faatz E., Villarejo M., Bremer E. Binding protein dependent transport of glycine betaine and its osmotic regulation in Escherichia coli K12. Mol Gen Genet. 1986 Nov;205(2):225–233. doi: 10.1007/BF00430432. [DOI] [PubMed] [Google Scholar]
- Milner J. L., Grothe S., Wood J. M. Proline porter II is activated by a hyperosmotic shift in both whole cells and membrane vesicles of Escherichia coli K12. J Biol Chem. 1988 Oct 15;263(29):14900–14905. [PubMed] [Google Scholar]
- Poolman B., Smid E. J., Veldkamp H., Konings W. N. Bioenergetic consequences of lactose starvation for continuously cultured Streptococcus cremoris. J Bacteriol. 1987 Apr;169(4):1460–1468. doi: 10.1128/jb.169.4.1460-1468.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth W. G., Porter S. E., Leckie M. P., Porter B. E., Dietzler D. N. Restoration of cell volume and the reversal of carbohydrate transport and growth inhibition of osmotically upshocked Escherichia coli. Biochem Biophys Res Commun. 1985 Jan 16;126(1):442–449. doi: 10.1016/0006-291x(85)90625-4. [DOI] [PubMed] [Google Scholar]
- Sutherland L., Cairney J., Elmore M. J., Booth I. R., Higgins C. F. Osmotic regulation of transcription: induction of the proU betaine transport gene is dependent on accumulation of intracellular potassium. J Bacteriol. 1986 Nov;168(2):805–814. doi: 10.1128/jb.168.2.805-814.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempest D. W., Meers J. L., Brown C. M. Influence of environment on the content and composition of microbial free amino acid pools. J Gen Microbiol. 1970 Dec;64(2):171–185. doi: 10.1099/00221287-64-2-171. [DOI] [PubMed] [Google Scholar]


