Abstract
Background
Probiotic capsules have been shown to reduce the incidence of antibiotic-associated diarrhoea in a number of settings. If probiotic yogurt were equally efficacious then it would provide a simple and cost-effective means of preventing antibiotic-associated diarrhoea.
Aim
To investigate whether eating live bio yogurt at the time of taking oral antibiotics can prevent antibiotic-associated diarrhoea.
Design of study
This study was a three-arm (bio yogurt, commercial yogurt, no yogurt) randomised controlled trial with double blinding between the two yogurt arms.
Setting
A single primary care general practice surgery in Hingham, Norfolk. The study population included all ages except babies.
Method
Patients aged over 1 year who required a 1-week course of antibiotics were included in the study. There was complete follow up for 369 patients. The intervention was the consumption of 150 ml of live strawberry-flavoured yogurt for 12 days, starting on the first day of taking the antibiotic. Diarrhoea was defined as ‘three or more loose stools per day over at least 2 consecutive days’ within 12 days of starting the antibiotics.
Results
Of the 120 patients in the no-yogurt group, 17 (14%, 95% confidence interval [CI] = 9.0 to 21.5) developed diarrhoea. Of the 118 given commercial yogurt, 13 (11%, 95% CI =6.6 to 17.9) developed diarrhoea; nine of the 131 patients (7%; 95% CI = 3.7 to 12.5) given bio yogurt developed diarrhoea (P = 0.17).
Conclusion
Overall, this study failed to demonstrate that yogurt has any effect on antibiotic-associated diarrhoea.
Keywords: adverse effects, antibiotic, diarrhoea, probiotic, yogurt
INTRODUCTION
Antibiotic-associated diarrhoea occurs in 11–26% of children given antibiotics in an outpatient setting.1–3 Among adult hospital inpatients the rate of antibiotic-associated diarrhoea was 18% in the US.4 Studies have shown that consuming probiotic capsules while taking antibiotics can reduce the risk of antibiotic-associated diarrhoea by up to 70%.1,2
A meta-analysis of probiotics capsules and antibiotic-associated diarrhoea gave an odds ratio (OR) of 0.37 in favour of active treatment over placebo.5 However, a meta-analysis of probiotics for paediatric antibiotic-associated diarrhoea demonstrated that the apparent benefit of probiotics did not withstand intention-to-treat analysis.6 Furthermore, although Lactobacillus GG capsules have been shown to be effective in children, a large trial (n = 302) on US adult inpatients found the same Lactobacillus GG capsules had no effect on antibiotic-associated diarrhoea rates.7
Probiotics have been defined as ‘a live microbial feed supplement that is beneficial to health’.8 Yogurt is a probiotic.9 The majority of ordinary, commercial yogurts are ‘live’ in that they contain living bacteria (Dairy Training and Development Council [DTDC], personal communication, 2004). The bacteria in commercial yogurts are usually Lactobacillus delbrueckii subspecies bulgaris and Streptococcus salivarius subsp. thermophilus in an equal mixture; it is unclear as to whether or not these bacteria can survive in the human gut.10,11 Also, there is a concern that there may be insufficient viable cells in most yogurts at the point of consumption; less than 5×106 colony forming units/gram (cfu/g) is probably insufficient to provide a therapeutic effect.12
How this fits in
In this general practice setting the incidence of antibiotic-associated diarrhoea was only 10%. Commercial yogurt at a daily dose of 227g has been shown to reduce risk of antibiotic-associated diarrhoea in adults in a hospital setting but this is the first substantial study on the effects of yogurt in primary care. It failed to show that consumption of 150ml of strawberry yogurt could prevent antibiotic-associated diarrhoea.
There is no agreed definition of ‘bio’ yogurt but these yogurts usually contain specific Lactobacilli and Bifidus species that have been shown capable of surviving in the human gut. ‘Bio’ yogurts are becoming more popular, at least partly because of a supposed beneficial effect on health: they command about 10% of the UK market (DTDC, personal communication, 2004).
To date, the only substantial studies using yogurt to prevent antibiotic-associated diarrhoea have been with adult hospital inpatients.4,13 Beniwal4 used vanilla-flavoured commercial yogurt (n = 202) and found a statistically significant 50% reduction in the occurrence of diarrhoea (P = 0.04). A small (n = 40) Italian study of yogurt in children treated with amoxicillin showed a reduction in daily stool count from 2.7 to 2.0.14 The only other work with yogurt and antibiotic-associated diarrhoea has been on healthy volunteers.
METHOD
This study was a pragmatic three-arm randomised controlled trial with double blinding of the two yogurt arms. The study was conducted in a single rural general practice with a population of approximately 4000 in Norfolk, England. The two yogurts were bio and commercial; the third arm comprised a group who agreed to eat no yogurt during the trial.
The study ran for 21 weeks during the period November 2002 and March 2004. During the trial period those in the two yogurt arms were asked to eat no other yogurts or probiotic products. The trial length was 12 days; this was chosen because the antibiotic course given was of a week's duration and most antibiotic-associated diarrhoea occurs either while the patient is taking the antibiotics or within a few days of treatment cessation
The main outcome studied was incidence of diarrhoea. All patients attending the surgery aged 1 year or older and who required a standard 1-week course of antibiotics were eligible to participate.
Intervention
The characteristics of the yogurts used in this study are shown in Table 1. Participants were given a simple symptom diary and asked to record for the 12-day study period their bowel frequency and stool consistency and whether or not they had abdominal pain, flatulence, or self-reported thrush. Patients were given a stamped, addressed envelope in which to return their symptom diaries. They also received a telephone call using a standardised questionnaire to enquire about their symptoms after they finished their antibiotics. The call was from the practice nurse, a trained receptionist, or the main investigator. The caller was blind to the patient's treatment allocation.
Table 1.
Characteristics of the two types of yogurt used in this study.
| Characteristic | Bio yogurt | Commercial yogurt |
|---|---|---|
| Quantity | 150 ml per day | 150 ml per day |
| Duration of intervention | 12 days | 12 days |
| Flavour | Strawberry | Strawberry |
| Fat content | 3.2 g per 100ml | 9 g per 100ml |
| Streptococcus thermophillus | 8×108 cfu/ga | 8×108 cfu/ga |
| Lactobacillus acidophilus | 3×106 cfu/ga | Nil |
| Bifidobacteria anamalis subsp. lactus | 5×106 cfu/ga | Nil |
| Lactobacillus delbrueckii subsp. bulgaris | Nil | 3×106 cfu/ga |
| Total daily dose of bacteria | 109 cfu13 | 109 cfu13 |
Both yogurts were organic and were produced by Yeo Valley Organics, Somerset, UK.
cfu/g = colony forming units per gram. This was measured at point of manufacture but Yeo Valley expect these counts to be maintained for at least 2 weeks after manufacture assuming a reasonable cold chain. Data supplied by Yeo Valley Organics, Somerset, UK.
The main outcome measure was the presence or absence of diarrhoea. Diarrhoea was defined as three or more loose stools per day over at least 2 consecutive days during the 12-day follow-up period.1 Secondary outcome measures included abdominal pain, flatulence, stool counts, self-reported thrush, and whether the antibiotics failed to work. This last outcome was defined as the patient either needing a second course of antibiotics or not being better by day 12.
SPSS and CIA15 were used for analysis. The χ2 test was used for categorical variables and logistic regression was used for adjusted analyses. This study was designed to be powered at 90% to detect an absolute difference between any two arms of 11 % (equivalent to a 71% relative risk reduction1) with a two-tailed α value of 0.05, that is, at 5% significance. The standard formula for binary endpoints16 and the event rates given by Arvola et al's1 study — 16% in the placebo group and 5% in the active treatment group — suggested that this study needed to have 158 patients in each of the three arms, giving a total study population of 474.
Enrolment was by the three doctors at the practice. Randomisation was performed by the receptionists sequentially opening numbered, sealed opaque envelopes and then assigning the patients to group A, B, or ‘no yogurt’ as dictated by the slip inside the envelope. The contents of each envelope were determined by random number tables and concealed block randomisation undertaken by the authors outside the practice. Blinding was achieved by the yogurts being in pots identical except for the letter A or B being printed on the lids. Allocation of A and B to yogurt type randomly varied each week. Whether yogurt A was bio or commercial was only revealed to the investigator some months after all data had been collected and filed for that week.
RESULTS
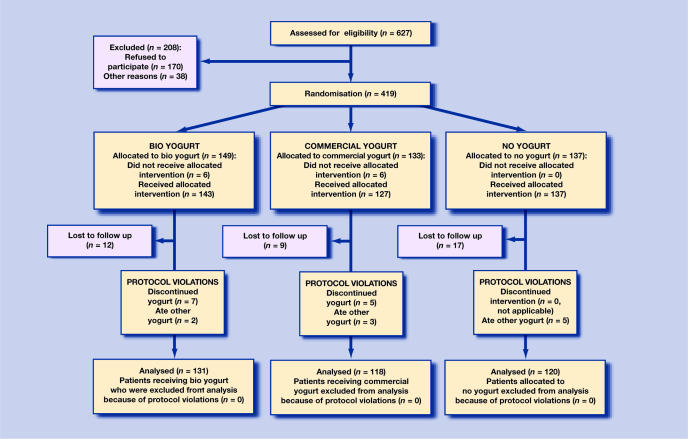
Figure 1 charts the recruitment and follow up of participants. Of the 627 potential participants to the study, 38 had contraindications to recruitment (10 had diarrhoea at presentation, eight had milk or yogurt intolerance and 20 had various other contraindications), 170 declined to participate and 419 were recruited. As such, 67% (419/627) of potential participants were recruited. The 419 patients were recruited to join the study during 21 weeks spread between November 2002 and March 2004. This fell short of the target sample size (n = 474) due to saturation within the practice, that is, a shortage of patients not already randomised. A separate record was kept of those who were either excluded or who declined to participate. The average age (35 years) and sex balance (47% male) in those who declined to participate was very similar to those who did participate.
Figure 1.
CONSORT flowchart
A total of 407 patients received their intended treatment; their characteristics are detailed in Table 2. Twelve patients did not receive one of the two study yogurts due to administrative error. Thirty-eight of 407 patients (9%) were lost to follow up. Of those 38, 12 had bio yogurt, nine had commercial yogurt and 17 had no yogurt. The total number of patients for whom there was complete follow up was 369 (91 % of 407).
Table 2.
Baseline characteristics of those patients who received their allocated intervention (n = 407).
| Bio yogurt (n = 143) | Commercial yogurt (n = 127) | No yogurt (n = 137) | |
|---|---|---|---|
| Age, years (SD)a | 37.8 (25.3) | 37.1 (23) | 38.2 (23.5) |
| Children (%, aged 1–14 years) | 29 | 26 | 23 |
| Male patients (%) | 41 | 44 | 40 |
| Past history of antibiotic-associated diarrhoea (%) | 21 | 18 | 12 |
| Past history of thrush associated with antibiotics (%) | 14 | 17 | 12 |
All analysis was on an intention-to-treat basis and patients who discontinued or stopped their yogurt were included in the analysis. Twelve patients found the yogurt unacceptable (seven in the bio group and five in the commercial-yogurt group) and stopped it for that reason; 10 patients ate other yogurt during the course of the study. In all, 75% of patients receiving yogurt were unable to guess which type they had received. Of those who felt able to say whether they had bio or commercial yogurt, 50% of their guesses were correct and 50% were incorrect.
Diarrhoea
The rates of diarrhoea were not statistically different in the three arms (P = 0.20): 7% in the bio-yogurt group, 11 % in the commercial-yogurt group, and 14% in the no-yogurt group. Sub-group analysis showed that for patients who had previously had antibiotic-associated diarrhoea, the rate of diarrhoea was 38% in patients not consuming yogurt, 20% in those having bio yogurt, and 4% in those having commercial yogurt (P = 0.03, Fisher's Exact test).
Age, sex, indication for antibiotic, type of antibiotic, and history of previous thrush with antibiotics appeared to have no effect on rates of diarrhoea. Those with a previous history of antibiotic-associated diarrhoea had a diarrhoea rate of 13 out of 64 (20%), compared with only 26 out of 305 (9%) in those who did not (P = 0.001).
Thrush
Overall, yogurt had no effect on rates of thrush (P = 0.59). Sub-group analysis showed that in those patients with a previous history of thrush the rates of thrush in this study were six out of 19 (32%), two out of 20 (10%), and eight out of 17 (47%) in the bio, commercial, and no-yogurt groups respectively (P = 0.04). The rate of thrush in those without a history of thrush was low at only 10 out of 323 (3%); yogurt had no effect on the rate.
Thrush was self-reported and not subject to objective verification. The diagnosis includes oral thrush. However, 22 out of the 26 cases were in women so it is assumed that most cases would have been vaginal thrush.
Persistence of symptoms and abdominal pain and wind
Overall, yogurt had no effect on rates of persistence of symptoms or treatment failure (P = 0.31). Persistence of symptoms was more common in the older participants (aged ≥60 years) (17/83 [20%]), compared with adults (18–59 years) (30/181 [17%]) and children (1–17 years) (6/105 [6%]). The incidences of abdominal pain at 44% and wind at 63% showed little difference between the three arms.
Logistic regression
Three logistic regression models were developed in this analysis; diarrhoea as the outcome, thrush as the outcome, and persistence of symptoms as the outcome. These models controlled for baseline differences in potentially prognostic variables that occurred despite randomisation.
With the diarrhoea model, previous antibiotic-associated diarrhoea predicted a threefold increase in risk for further diarrhoea odds ratio ([OR] = 3.13, 95% CI = 1.46 to 6.71, P = 0.003). The OR for consuming bio yogurt compared with no yogurt was 0.4 (95% CI = 0.16 to 0.96), which was also statistically significant (P = 0.04). Looking at the subgroup of patients with a previous history of antibiotic-associated diarrhoea, those on the commercial yogurt had an OR of 0.05 (95% CI = 0.01 to 0.60, P = 0.008) compared with those in the no-yogurt arm.
In the thrush model the only significant OR was for previous thrush associated with antibiotics, which predicted a 10-fold increase in risk (OR = 10.78, 95% CI = 4.00 to 29.03, P<0.001). The ORs for consuming yogurt were not statistically significant.
The logistic regression for persistence of symptoms had only one significant finding: compared with older people, children aged 1–17 years had one-quarter the rate of persistence of symptoms at 5% versus 20% (OR = 0.22, 95% CI = 0.08 to 0.61, P = 0.004). The ORs for consuming yogurt were not statistically significant.
Sub-group analysis of those aged 60 years or older, using the interaction test, showed a significant interaction (P = 0.004): those aged 60 years or over in the bio-yogurt arm had an OR of 0.07 (95% CI = 0.01 to 0.60, P = 0.004) compared with those not eating yogurt. This indicates that older patients who did not receive any yogurt were 14 times as likely to experience persistence of symptoms as those who received bio yogurt. Similarly, those in the commercial arm had an OR of 0.17 (95% CI = 0.04 to 0.70, P = 0.004) compared with those not having yogurt.
Data for the proportion of participants who experienced the main outcomes are given in Table 3, while data relating to various other outcomes are detailed in Table 4.
Table 3.
Outcomes, showing proportions of patients who experienced the main outcomes.
| Outcome | Bio yogurt (%, 95% CI) | Commercial yogurt (%, 95% CI) | No yogurt (%, 95% CI) | P-value |
|---|---|---|---|---|
| Participants suffering diarrhoea during this study | 9/131 (7, 3.7 to 12.5) | 13/118 (11, 6.6 to 179) | 17/120 (14 9.0 to 21.5) | 0.20a |
| Adjusted ORa for diarrhoea using output from logistic regression and comparing each type of yogurt with no yogurt | (0.40b, 0.16 to 0.96) P = 0.04 | (0.66b, 0.29 to 1.46) P = 0.30 | 1 | |
| Previous history of antibiotic-associated diarrhoea experiencing diarrhoea during this study | 6/30 (20, 10 to 37) | 1/23 (4, 1 to 21) | 6/16 (38, 19 to 61) | 0.03c |
| Patients developing severe diarrhoea requiring stool culture, specific treatment, or hospital admission, or any other adverse event | 0 (−) | 0 (−) | 0 (−) | |
χ2.
odds ratio.
Fisher Exact test.
Table 4.
Outcomes, showing proportions of patients experiencing the various outcomes.
| Outcome | Bio yogurt (%, 95% CI) | Commercial yogurt % (95% CI) | No yogurt % (95% CI) | P-value |
|---|---|---|---|---|
| Trial participants suffering thrush during this study | 10/131 (8, 4 to 12) | 6/118 (5, 2 to 10) | 10/120 (8, 4 to 13) | 0.590 |
| Previous history of thrush associated with antibiotics and experiencing thrush during this study (n = 58) | 6/19 (32, 14 to 52) | 2/20 (10, 3 to 30) | 8/17 (47, 31 to 74) | 0.040 |
| Participants experiencing persistence of symptoms during this study | 17/131 (13, 8 to 19) | 14/118 (12, 7 to 18) | 22/120 (18, 11 to 23) | 0.310 |
| Older patients (aged ≥60 years) experiencing persistence of symptoms during this study (n = 83) | 4/35 (11, 5 to 26) | 1/20 (5, 1 to 24) | 12/28 (43, 27 to 61) | 0.001 |
| Participants suffering abdominal pain on at least 1 day during this study | 66/131 (46, 38 to 54) | 46/118 (39, 35 to 52) | 60/120 (50, 48 to 64) | 0.100 |
| Participants suffering wind (flatulence) on at least 1 day during this study | 86/131 (65, 57 to 73) | 71/118 (60, 51 to 69) | 77/120 (64, 55 to 72) | 0.700 |
| Mean total stool count over the 12 days for individuals participating in this study | 16.1 (14.5 to 17.7) | 16.1 (4.9 to 17.3) | 16.0 (15.2 to 16.9) | 0.996 |
P-values are from χ2 tests except stool counts where a simple ANOVA was used. n = 369 unless stated.
DISCUSSION
Summary of main findings
Yogurt had no effect in this study. The reasons for this could be that yogurt and probiotics generally have little effect except in children and frail, older hospital inpatients. In this study, eating yogurt while taking antibiotics did not prevent antibiotic-associated diarrhoea as tested by the χ2 test. Although the logistic regression model suggested an effect, the protocol gave preference to the χ2 test and the level of evidence would not stand a correction for multiple testing.
Sub-group analysis suggests that patients with a previous history of either antibiotic-associated diarrhoea or thrush may benefit from consumption of yogurt while on antibiotics.
Strengths and limitations of the study
The lack of a true placebo was the main weakness of this study. At the time when the study was designed, it seemed likely that commercial yogurt was biologically inactive. It was impossible to obtain pasteurised yogurt so commercial yogurt was used as a proxy for pasteurised yogurt. The main difference between bio and commercial yogurt is the presence of supposed probiotic bacteria. Both types of yogurt contained Strep. thermophillus and a Lactobacillus species and similar bacterial counts (Table 1). With hindsight, it seems likely that this study compared two equivalent interventions to no intervention and that the careful blinding was irrelevant.
In addition, the three-arm design meant that the available patients were split between three groups rather than two, thus reducing the study's power.
A further weakness is that a relatively small dose of yogurt was used; 150ml of strawberry yogurt was chosen on the pragmatic basis that most people would be happy to consume this on a daily basis, thereby increasing the study's clinical relevance. However, 150ml of yogurt was possibly sub-therapeutic. The fruit in fruit yogurt may also cause mild diarrhoea. It is possible that probiotics are simply not particularly effective and that it required a larger trial to refute an impression of effectiveness created by small trials and positive publication bias.
The study failed to use block randomisation for previous antibiotic-associated diarrhoea. Given that the bio-yogurt arm included twice as many patients with a previous history of antibiotic-associated diarrhoea compared with the no-yogurt arm, and that previous antibiotic-associated diarrhoea was the strongest predictor for antibiotic-associated diarrhoea, this may have distorted the main outcome result.
Finally, this study turned out to be underpowered. Only 419 out of a target of 474 patients were recruited and the post-hoc power of this study (calculated using the relatively low observed frequencies of diarrhoea in this study) was just 35%.
Strengths of this study include good rates of participation and follow up with a clearly defined and clinically relevant main outcome. The intervention would be easily replicated in everyday practice. This is the largest trial to date on probiotics and was also based in primary care.
Comparison with existing literature
Antibiotic-associated diarrhoea
The comparable studies to this are Beniwal et al's4 and Hickson et al's13 in that both used different types of yogurt. However, both studies took place in largely elderly hospital in-patient populations.4,13
Beniwal's daily dose of 227g of commercial vanilla-flavoured yogurt yielded a total daily dose of bacteria of 5×108cfu.4 The event rate in the no-treatment arm of this unblinded study was 24%. It was an open study with no placebo. Follow-up rates (100% in the no-yogurt arm and 92% in the yogurt arm), were excellent. However, patients who did not continue eating the yogurt were excluded and not followed up, which effectively transformed this study to a ‘treatment-received’ analysis rather than intention to treat. If two of the eight excluded patients had diarrhoea (as is quite possible with an event rate of 24% in the control group), then P-value for this study would have missed 0.05 (calculated using CIA).15
Hickson et al used a probiotic drink yielding 2×1010 cfu per day.5 Their study was of limited generalisability as recruitment was only 8% (135 recruited out of 1760 patients screened for possible recruitment). Diarrhoea rates were high in their study with 34% of patients in the placebo group experiencing diarrhoea.
The diarrhoea rate in the current primary care study was low at 14% in the no-yogurt group. The low event rate may be the main reason for yogurt failing to have a statistically significant effect in this study despite total bacterial doses of approximately 109 cfu per day.
Thrush
Although a recent study of probiotics for the prevention of thrush in Melbourne showed no effect,17 an earlier small study of yogurt from New York showed a benefit for women eating commercial yogurt in preventing thrush over a 6-month period.18 The risk of thrush for patients without a history of thrush with antibiotics is low and this study hints at a benefit only in those patients who are susceptible to thrush when they have antibiotics.
Persistence of symptoms
The finding of greater persistence of symptoms in older patients who did not receive yogurt was a surprise and there is no previous work to substantiate this. It may be an a (type I) error, although there has previously been speculation that probiotics exert some sort of immunological priming effect.19
Implications for future research and clinical practice
The question as to whether consuming live yogurt can prevent antibiotic-associated diarrhoea remains unanswered. Resolving this issue requires further studies. Future studies must try to use a better placebo; this could be either pasteurised yogurt or chemically-treated milk.20 Natural yogurt at higher doses (for example 250ml) would probably be the best active intervention to use. Further research in populations at high risk of antibiotic-associated diarrhoea would be helpful. Alternatively, if a heterogeneous population is used then the design should include stratified randomisation or minimisation.
This study provides insufficient evidence to inform clinicians on whether to routinely advise patients to consume yogurt while they are taking antibiotics, but the evidence is compatible with the possibility that those patients with a previous history of antibiotic-associated diarrhoea could benefit from eating yogurt while they are taking antibiotics.
Acknowledgments
Thanks to Yvette Belham, Shelia Ward, Heather Hosier, Lawson Baxter, Sarah Holland, Steve Thorne, and Gerard Hayes at Yeo Valley; Rosemary Stark, Professor Hamilton-Miller, Andrew Whitby, Nick Steel, and Tony Avery. Thanks to all the patients of Hingham who so generously participated in this study
Funding body
Yeo Valley Organics kindly supplied the yogurt. An Enterprise Award from Eastern Region NHS Executive covered locum and other costs
Ethics committee
The Norwich Ethics Committee gave approval to the study's initial design on 10 January 2001 (reference LREC 2001/001)
Competing interests
The authors have stated that there are none
Discuss this article
Contribute and read comments about this article on the Discussion Forum: http://www.rcgp.org.uk/bjgp-discuss
REFERENCES
- 1.Arvola T, Laiho K, Torkkeli S, et al. Prophylactic Lactobacillus GG reduces antibiotic-associated diarrhea in children with respiratory infections: a randomized study. Pediatrics. 1999;104(5):e64. doi: 10.1542/peds.104.5.e64. [DOI] [PubMed] [Google Scholar]
- 2.Vanderhoof JA, Whitney DB, Antonson DL, et al. Lactobacillus GG in the prevention of antibiotic-associated diarrhoea in children. J Pediatr. 1999;135(5):564–568. doi: 10.1016/s0022-3476(99)70053-3. [DOI] [PubMed] [Google Scholar]
- 3.Turck D, Bernet JP, Marx J, et al. Incidence and risk factors of oral antibiotic-associated diarrhoea in an outpatient pediatric population. J Pediatr Gastroenterol Nutr. 2003;37(1):22–26. doi: 10.1097/00005176-200307000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Beniwal RS, Arena VC, Thomas L, et al. A randomised trial of yogurt for prevention of antibiotic-associated diarrhoea. Dig Dis Sci. 2003;48(10):2077–2082. doi: 10.1023/a:1026155328638. [DOI] [PubMed] [Google Scholar]
- 5.D'Souza AL, Rajkumar C, Cooke J, Bulpitt CJ. Probiotics in prevention of antibiotic associated diarrhoea: meta-analysis. BMJ. 2002;324:1361–1364. doi: 10.1136/bmj.324.7350.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnston BC, Supina AL, Vohra S. Probiotics for pediatric antibiotic-associated diarrhea: a meta-analysis of randomized placebo-controlled trials. CMAJ. 2006;175(4):377–383. doi: 10.1503/cmaj.051603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas MR, Litin SC, Osmon DR, et al. Lack of effect of Lactobacillus GG on antibiotic-associated diarrhoea: a randomized, placebo-controlled trial. Mayo Clin Proc. 2001;76(9):883–889. doi: 10.4065/76.9.883. [DOI] [PubMed] [Google Scholar]
- 8.Salminen S, Ouwehand AC, Isolauri E. Clinical application of probiotic bacteria. Int Dairy J. 1998;8:563–572. [Google Scholar]
- 9.Guarner F, Perdigon G, Corthier G, et al. Should yoghurt cultures be considered probiotic? Br J Nutr. 2005;93(6):783–786. doi: 10.1079/bjn20051428. [DOI] [PubMed] [Google Scholar]
- 10.Del Campo R, Bravo D, Cantón R, et al. Scarce evidence of yogurt lactic acid bacteria in human feces after daily yogurt consumption by healthy volunteers. Appl Environ Microbiol. 2005;71(1):547–549. doi: 10.1128/AEM.71.1.547-549.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mater DD, Bretigny L, Firmesse O, et al. Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus survive gastrointestinal transit of healthy volunteers consuming yogurt. FEMS Microbiol Lett. 2005;250(2):185–187. doi: 10.1016/j.femsle.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 12.Scotti C, Sutherland AH, Varnam AH. Quality of fermented milks in relation to claims concerning numbers and types of starter bacteria. Br J Nutr. 2002;88(Suppl 1):S117. [Google Scholar]
- 13.Hickson M, D'Souza AL, Muthu N, et al. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ. 2007;335:80–83. doi: 10.1136/bmj.39231.599815.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Contardi I. Batteriterapia orale quale prevenzione della diarrea da antibiotici in eta pediatria. La Clinical Terapeutica. 1991;136:409–413. [PubMed] [Google Scholar]
- 15.Altman DG, Machin D, Bryant TN, Gardner MJ, editors. Statistics with confidence. London: BMJ Books; 2000. [Google Scholar]
- 16.Pocock SJ. Clinical trials: a practical approach. Chichester: John Wiley & Sons; 1983. [Google Scholar]
- 17.Pirotta M, Gunn J, Chondros P, et al. Effect of Lactobacillus in preventing post-antibiotic vulvovaginal candidiasis: a randomised controlled trial. BMJ. 2004;329(7465):548–551. doi: 10.1136/bmj.38210.494977.DE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hilton E, Isenberg HD, Alperstein P, et al. Ingestion of yogurt containing Lactobacillus acidophilus as prophylaxis for candidal vaginitis. Ann Intern Med. 1992;116(5):353–357. doi: 10.7326/0003-4819-116-5-353. [DOI] [PubMed] [Google Scholar]
- 19.Saavedra JM. Probiotics plus antibiotics: regulating our bacterial environment. J Pediatr. 1999;135(5):535–537. doi: 10.1016/s0022-3476(99)70046-6. [DOI] [PubMed] [Google Scholar]
- 20.Boudraa G, Benbouabdellah M, Hachelaf W, et al. Effect of feeding yogurt versus milk in children with acute diarrhea and carbohydrate malabsorption. J Pediatr Gastroenterol Nutr. 2001;33:307–313. doi: 10.1097/00005176-200109000-00015. [DOI] [PubMed] [Google Scholar]