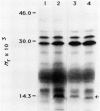
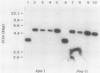
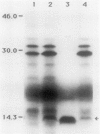
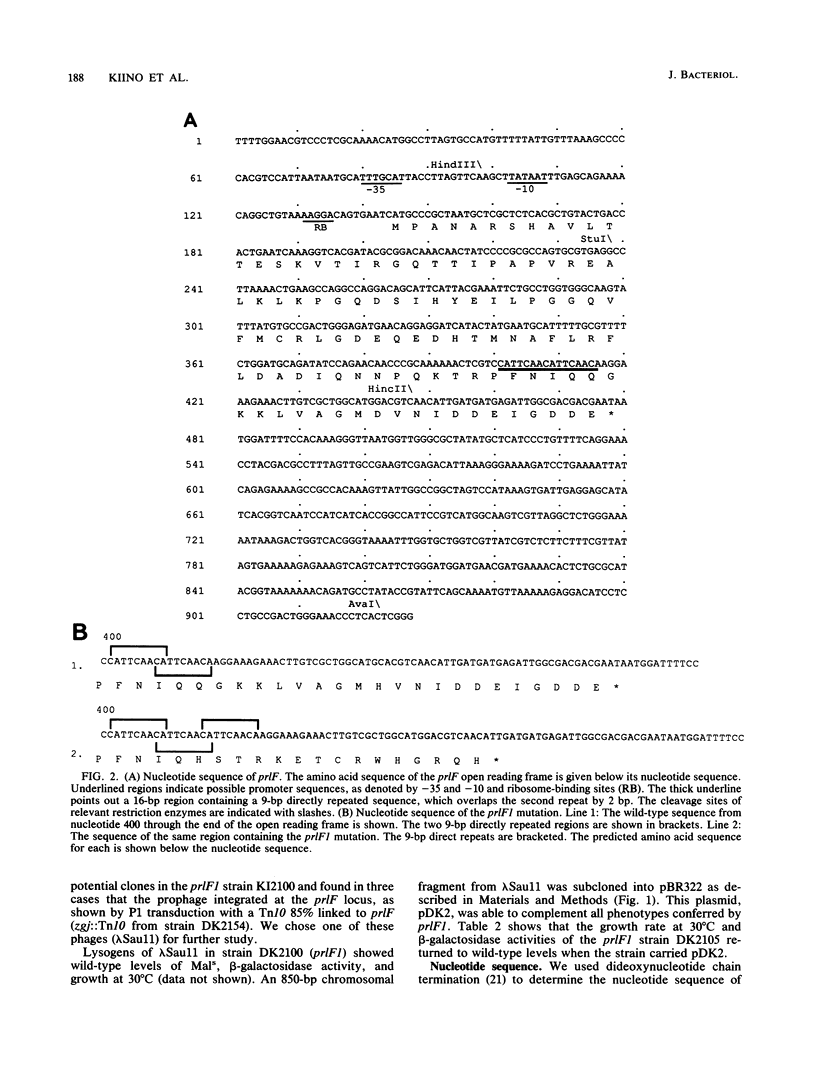
Abstract
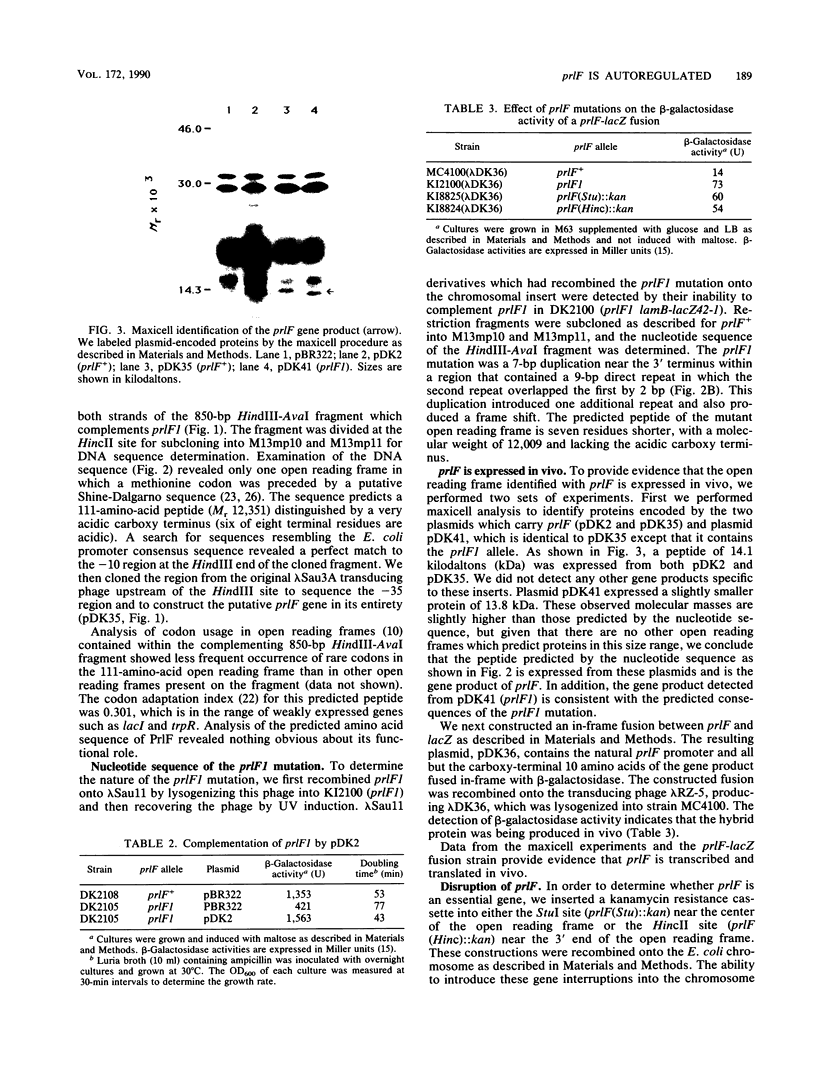
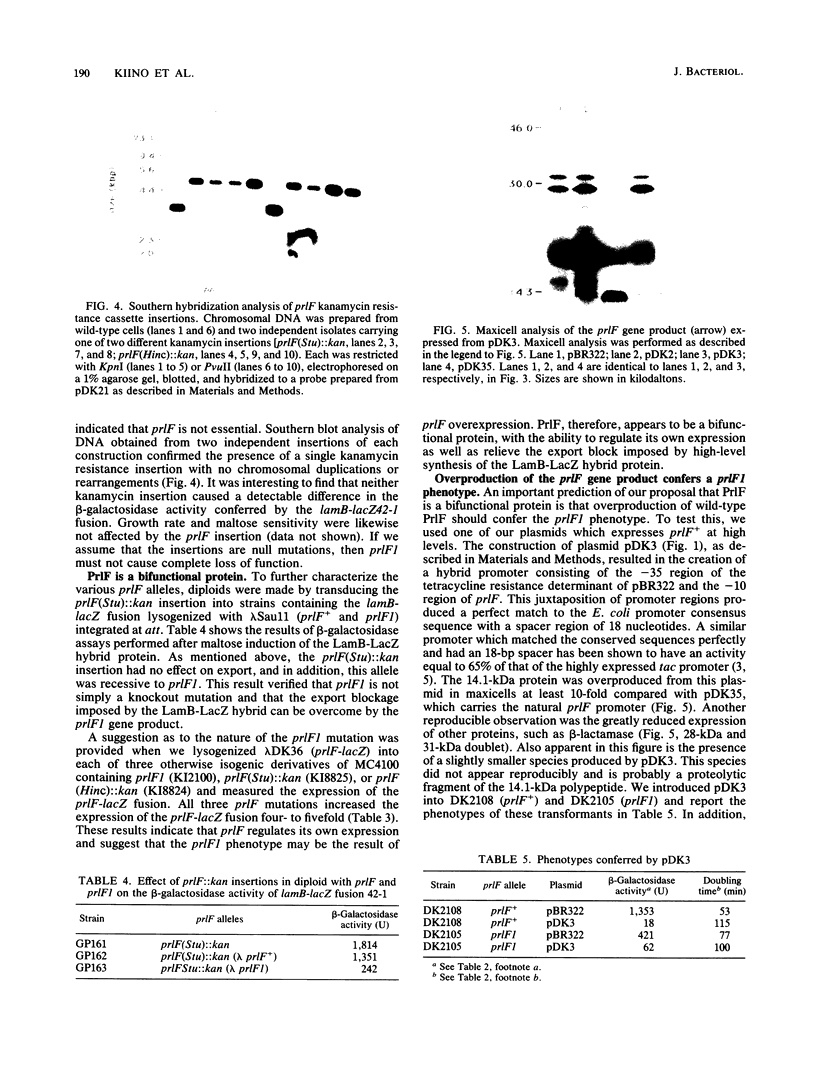
We have cloned and determined the nucleotide sequence of the prlF gene. An open reading frame predicting a 111-amino-acid protein (Mr 12,351) with an acidic carboxy terminus was identified. The DNA sequence preceding this open reading frame revealed a putative promoter and a ribosome-binding site. The nucleotide sequence of the prlF1 mutation revealed a 7-base-pair duplication resulting in a slightly smaller predicted gene product of Mr 12,009 that lacked the acidic carboxy terminus. Maxicell analysis of prlF and prlF1 subclones identified peptides of sizes similar to those predicted by the nucleotide sequences. The prlF sequence was shown to be expressed in vivo by both maxicell analysis and construction of a prlF-lacZ fusion. Two kanamycin resistance insertions within the prlF open reading frame were introduced into the chromosome, replacing the wild-type gene. In contrast to the prlF1 mutation, these insertions had no detectable effect on cell growth or on the beta-galactosidase activity or maltose sensitivity (two sensitive indicators of hybrid protein export) conferred by the lamB-lacZ42-1 gene fusion. Overproduction of the wild-type prlF gene product from a plasmid carrying an active hybrid promoter, however, conferred a prlF1 phenotype. In addition, both the prlF1 mutation and both kanamycin resistance insertions increased the beta-galactosidase activity of a prlF-lacZ fusion. These results suggest that prlF is autoregulated and that overproduction of the prlF gene product increases the export efficiency of beta-galactosidase hybrid proteins from the cytoplasm.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benson S. A., Hall M. N., Silhavy T. J. Genetic analysis of protein export in Escherichia coli K12. Annu Rev Biochem. 1985;54:101–134. doi: 10.1146/annurev.bi.54.070185.000533. [DOI] [PubMed] [Google Scholar]
- Bieker K. L., Silhavy T. J. PrlA is important for the translocation of exported proteins across the cytoplasmic membrane of Escherichia coli. Proc Natl Acad Sci U S A. 1989 Feb;86(3):968–972. doi: 10.1073/pnas.86.3.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius J., Erfle M., Storella J. Spacing of the -10 and -35 regions in the tac promoter. Effect on its in vivo activity. J Biol Chem. 1985 Mar 25;260(6):3539–3541. [PubMed] [Google Scholar]
- Casadaban M. J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976 Jul 5;104(3):541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emr S. D., Hall M. N., Silhavy T. J. A mechanism of protein localization: the signal hypothesis and bacteria. J Cell Biol. 1980 Sep;86(3):701–711. doi: 10.1083/jcb.86.3.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fandl J. P., Tai P. C. Biochemical evidence for the secY24 defect in Escherichia coli protein translocation and its suppression by soluble cytoplasmic factors. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7448–7452. doi: 10.1073/pnas.84.21.7448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardel C., Benson S., Hunt J., Michaelis S., Beckwith J. secD, a new gene involved in protein export in Escherichia coli. J Bacteriol. 1987 Mar;169(3):1286–1290. doi: 10.1128/jb.169.3.1286-1290.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribskov M., Devereux J., Burgess R. R. The codon preference plot: graphic analysis of protein coding sequences and prediction of gene expression. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):539–549. doi: 10.1093/nar/12.1part2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiino D. R., Silhavy T. J. Mutation prlF1 relieves the lethality associated with export of beta-galactosidase hybrid proteins in Escherichia coli. J Bacteriol. 1984 Jun;158(3):878–883. doi: 10.1128/jb.158.3.878-883.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto C. A., Beckwith J. Mutations in a new gene, secB, cause defective protein localization in Escherichia coli. J Bacteriol. 1983 Apr;154(1):253–260. doi: 10.1128/jb.154.1.253-260.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner S. R., Nagaishi H., Clark A. J. Indirect suppression of recB and recC mutations by exonuclease I deficiency. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1366–1370. doi: 10.1073/pnas.69.6.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marck C. 'DNA Strider': a 'C' program for the fast analysis of DNA and protein sequences on the Apple Macintosh family of computers. Nucleic Acids Res. 1988 Mar 11;16(5):1829–1836. doi: 10.1093/nar/16.5.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Mizusawa S., Ward D. F. A bacteriophage lambda vector for cloning with BamHI and Sau3A. Gene. 1982 Dec;20(3):317–322. doi: 10.1016/0378-1119(82)90200-1. [DOI] [PubMed] [Google Scholar]
- Oliver D. B., Beckwith J. E. coli mutant pleiotropically defective in the export of secreted proteins. Cell. 1981 Sep;25(3):765–772. doi: 10.1016/0092-8674(81)90184-7. [DOI] [PubMed] [Google Scholar]
- Ostrow K. S., Silhavy T. J., Garrett S. cis-acting sites required for osmoregulation of ompF expression in Escherichia coli K-12. J Bacteriol. 1986 Dec;168(3):1165–1171. doi: 10.1128/jb.168.3.1165-1171.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A., Hack A. M., Rupp W. D. Simple method for identification of plasmid-coded proteins. J Bacteriol. 1979 Jan;137(1):692–693. doi: 10.1128/jb.137.1.692-693.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. M., Li W. H. The codon Adaptation Index--a measure of directional synonymous codon usage bias, and its potential applications. Nucleic Acids Res. 1987 Feb 11;15(3):1281–1295. doi: 10.1093/nar/15.3.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. Determinant of cistron specificity in bacterial ribosomes. Nature. 1975 Mar 6;254(5495):34–38. doi: 10.1038/254034a0. [DOI] [PubMed] [Google Scholar]
- Silhavy T. J., Benson S. A., Emr S. D. Mechanisms of protein localization. Microbiol Rev. 1983 Sep;47(3):313–344. doi: 10.1128/mr.47.3.313-344.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stormo G. D., Schneider T. D., Gold L. M. Characterization of translational initiation sites in E. coli. Nucleic Acids Res. 1982 May 11;10(9):2971–2996. doi: 10.1093/nar/10.9.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock G. M., Berman M. L., Silhavy T. J. Chimeric genetics with beta-galactosidase. Gene Amplif Anal. 1983;3:27–64. [PubMed] [Google Scholar]
- Winans S. C., Elledge S. J., Krueger J. H., Walker G. C. Site-directed insertion and deletion mutagenesis with cloned fragments in Escherichia coli. J Bacteriol. 1985 Mar;161(3):1219–1221. doi: 10.1128/jb.161.3.1219-1221.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer H. A., Comstock L. J., Vasser M. The tac promoter: a functional hybrid derived from the trp and lac promoters. Proc Natl Acad Sci U S A. 1983 Jan;80(1):21–25. doi: 10.1073/pnas.80.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]