Abstract
We have constructed a replication competent, γ134.5-deleted Herpes Simplex Virus type 1 (HSV-1) vector (J200) that expresses the gag gene from Human Immunodeficiency Virus type-1, primary isolate 89.6 (HIV-189.6), as a candidate vaccine for HIV-1. J200 replicates in vitro, resulting in abundant Gag protein production and accumulation in the extracellular media. Immunization of Balb/c mice with a single intraperitoneal injection of J200 elicited strong Gag-specific CD8 responses, as measured by intracellular IFN-γ staining and flow cytometry analysis. Responses were highest between 6 weeks and 4 months, but persisted at 9 months post-immunization, the last time-point evaluated. These data highlight the potential utility of neuroattenuated, replication-competent HSV-1 vectors for delivery of HIV-1 immunogens.
Keywords: HSV-1 vaccine vector, Gag, HIV-1 vaccine
1. Introduction
During early attempts in the development of a vaccine for Human Immunodeficiency Virus type-1 (HIV-1) antibody responses were elicited by immunization with recombinant HIV-1 envelope glycoproteins. While this approach can prevent infections with viruses containing glycoproteins closely related to the original immunogen, it has failed to generate the broadly protective response required for a successful vaccine, given the diversity of circulating HIV clades [1–6]. The current strategy for development of a vaccine has shifted to presentation of a variety of HIV-1 recombinant proteins with novel delivery vectors and/or adjuvants to elicit T-cell mediated immunity (CMI) with an emphasis on cytotoxic CD8+ lymphocyte (CTL) responses. HIV-specific CTL responses can recognize and kill HIV infected cells and are effective in controlling HIV-1 replication during the course of natural infection [7–9]. Vaccines which elicit these types of responses can also control the replication of pathogenic retroviruses and protect against the development of immunodeficiency and death in nonhuman primate (NHP) models [10–19]. The current mechanisms for induction of potent CTL mediated immunity include immunogen delivery by attenuated viral vectors, bacterial vectors, direct plasmid DNA injection, and co-delivery of vaccine adjuvants (reviewed in [20–22]).
Attenuated viral vectors have received the most attention among the current vaccine candidates that have been developed to elicit HIV-specific CTL responses. These vectors in development may not be fully effective in humans, and other approaches may be needed. Herpes simplex virus type-1 (HSV-1) with deletions in the Δγ134.5 gene is replication-competent but is aneurovirulent [23]. Attenuated HSV-1 recombinants are attractive as HIV-1 vaccine vectors for multiple reasons: i) herpesvirus vectors can infect dendritic cells and stimulate potent, long-lasting CMI responses [24–28]; ii) attenuated HSV can establish latency and reactivate with the potential to prolong an immune response [29,30]; iii) several nonessential genes can be deleted and replaced with multiple foreign gene inserts (up to 30 kb) [31]; vi) HSV Δγ134.5 viruses have been safe during human use in clinical trials, including intracranial injection at high doses [32–35]; and v) although prior immunity to HSV has a variable effect on vector efficacy in gene therapy applications, it does not impair either antibody or CMI responses elicited against immunogens expressed from HSV vectors [36–39].
Replication competent HSV-1 attenuated by a deletion in the thymidine kinase gene tk-) has been tested as an SIV vaccine vector in the NHP model. Immunized animals had measurable SIV specific CMI and reduced viral loads after infection with a pathogenic SIV [40]. However, recombinant HSV attenuated by the tk- phenotype alone remain neurovirulent and are not suitable as a vaccine vectors in humans [41].
More recently, a replication defective HSV-1 vector with multiple immediate-early gene deletions has been characterized as an SIV vaccine vector [39,42]. This virus boosts immune responses to DNA vectors in rhesus macaques and results in decreased plasma viral loads after SIV challenge [42]. HSV-1 amplicon vectors have induced potent Gag-specific T cell responses in BALB/c mice, giving further support for the use of HSV-1 as a vector for HIV-1 vaccine delivery [43]. However, difficulties in achieving high titers of amplicon vectors may prove to be a limiting factor for vaccine production. Moreover, replication competent HSV-1 vectors have the potential to elicit enhanced immune responses versus those elicited by immunization with replication defective vectors. This is due to their ability to replicate in the host, resulting in increased immunogen production. In order to develop a replication competent vector without neurovirulent properties, we have constructed a Δγ134.5 HSV-1 that expresses the HIV-189.6 gag gene (J200) and tested the ability of this vector to elicit CMI responses in mice. These data demonstrate that HSV vectors continue to be promising candidates for HIV vaccine development and warrant further studies, including the introduction of additional HIV-1 antigens capable of eliciting a broad and potent cellular immune response.
2. Materials and Methods
2.1 Cells
Vero cells (American Type Culture Collection {ATCC}, Rockville, MD) were grown and maintained in Minimal Essential Medium (Mediatech Inc, Herndon, VA) containing 7% fetal bovine serum. The human 143 thymidine kinase minus cells (143tk-, ATCC) were grown in Dulbecco’s modified Eagle’s medium (DMEM) (Mediatech) supplemented with 10% fetal bovine serum. Rabbit skin cells (originally acquired from Dr. J. McClaren, University of New Mexico, Albuquerque, NM, USA) were maintained in DMEM supplemented with 5% fetal bovine serum.
2.2 Plasmids and Viruses
HSV-1 (F) strain is a low passage clinical isolate used as a prototype HSV-1 strain [44,45]. The HIV-189.6 proviral clone in pUC19 was obtained from the NIH AIDS Research and Reference Reagent Program. The entire HIV-189.6 gag gene and part of the pol gene containing the viral protease (referred to herein as gag-pro gene) was amplified by PCR using the following primers: 5’ primer (89.6-763LKZ), 5’ - GACTCGAGGAGGCTAGAAGGAGAACCATGG-3’ and 3’ primer (89.6-2576R) 5’ - CCGTCTAGACTGTGACAGTTTCAATAGG-3’ and subcloned into the TOPO TA cloning vector (Invitrogen, Corp.). The gag-pro gene was removed from this vector by digestion with EcoRI and XhoI, and subcloned into identical sites in plasmid pCIM to generate pCMGP89.6. pCIM, originally derived from pSI (Promega, Corp), was constructed as follows: the SV40 late polyadenylation signal was removed from pSI by digestion with EcoRI and ClaI, and replaced with the Mason-Pfizer monkey virus polyadenylation sequence (MPMVpA) and cytoplasmic transport element (CTE) [46]. The MPMVpA/CTE was PCR-amplified from pSR2 (E. Hunter, unpublished) with primers that contained unique restriction sites for EcoR1 (5’ end) and ClaI (3’ end). The SV40 promoter was removed by restriction digestion with BglII and PstI and replaced with the CMV immediate early promoter, from pCI (Promega, Corp). To construct the shuttle plasmid pJP200, the entire 3.6 kb gag-pro expression cassette, including the CMV promoter and intron just upstream of the gag-pro gene, was removed from pCMGP89.6 by digestion with BamHI and BglII, the ends blunted using DNA polymerase Klenow fragment, and subcloned into the HSV-1 shuttle plasmid pRB4878 [47] (kind gift of B. Roizman, University of Chicago). pRB4878 was digested with BstEII and StuI to remove the Egr-1 promoter and hepatitis B polyadenylation sites, and the overhanging sequences were filled in as above. This shuttle plasmid contains γ134.5 flanking sequences which facilitate recombination into this site when co-transfected with HSV DNA.
The parent recombinant HSV-1 virus R3659 has been described previously [48]. In R3659, the native thymidine kinase (tk) gene contains a 500 bp deletion, and both copies of the γ134.5 neurovirulence gene have been replaced with the tk gene expressed from the viral α27 promoter for use as a drug selection marker. The recombinant HSV-1 virus J200 was constructed using a previously described strategy [47,49]. Briefly, plasmid pJP200 was co-transfected with R3659 viral DNA, and recombinant, tk negative viruses were selected using the standard tk selection method [44]. Introduction of the HIV-189.6 gag-pro expression cassette into the γ134.5 locus was confirmed by Southern blot hybridization (data not shown). Clones J200.71 and J200.91 were selected for further characterization.
2.3 Metabolic labeling of proteins and immunoprecipitation
To demonstrate production of HIV-1 Gag polyproteins, Vero cells were seeded in T-25 tissue culture flasks one day prior to infection with J200 clones 71, 91, or R3659 (negative control without Gag expression) virus using a multiplicity of infection (MOI) of 10. Three hours post infection (hpi), cells were starved for 30 min in growth medium lacking methionine and cysteine (Met−Cys−), then 3 mls of Met−Cys− medium containing 100 µCi of [35S] Express Protein Labeling Mix (Perkin Elmer NEN, Boston, MA) was added to each flask. Flasks were returned to 37°C, 5% CO2 incubator for an additional 14 h. At 17 hpi, cell culture media was removed and infected cells were treated on ice with lysis buffer A (1% Triton X-100, 1% sodium deoxycholate, 0.15 M NaCl, 0.05 M Tris, pH 7.5) and the lysate was centrifuged in a microfuge at 14,000 rpm for 5 minutes. The supernates were transferred to new tubes, PMSF and SDS were added to achieve final concentrations of 0.1 mg/ml and 0.1%, respectively. The samples were precleared by addition of 1 µl normal human serum, 60 µl of formalin-fixed Staphylococcus aureus Protein A, and incubating for 1 h at room temperature on a rocker. After centrifugation at 20,000 × g for 10 min, supernates were transferred to new tubes, and 1 µl of a 1:1000 dilution of HIV-1 infected patient serum was added for immunoprecipitation of labeled Gag proteins. Immune complexes were precipitated using 30 µl of Protein A, followed by centrifugation as before. Complexes were washed three times in lysis buffer A containing 0.1% SDS, once in 20 mM Tris, pH 8.0, and pellets were resuspended in 30 µl protein loading buffer (50 mM Tris [pH 6.8], 100 mM dithiothreitol, 2% SDS, 0.1% bromophenol blue, and 10% glycerol). Proteins were separated by electrophoresis on a 10% SDS-polyacrylamide gel. The Prestained Benchmark Marker (Life Technologies) ladder was used as a visual marker. After electrophoresis, gels were stained in Coomassie Blue, destained in 10% (vol/vol) acetic acid and 15% (vol/vol) methanol, and soaked in Enhance (Dupont, NEN) for 30 minutes. Gels were dried and exposed to Kodak XAR film for 3 days (−70°C).
2.4 Western blot detection of Gag polyproteins
Vero cells were seeded in 6-well plates one day prior to infection with J200 or R3659 virus in MEM with 1% FBS for 2 hours using a multiplicity of infection (MOI) of 5. The cells were then fed with MEM with 7% FBS and harvested at 6, 12, 18, or 24 hpi and lysed with SDS-PAGE gel loading buffer (20% (vol/vol) glycerol, 10% (vol/vol) 2-mercaptoethanol, 6% (weight/vol) SDS, 62.5 mM Tris-HCl pH 6.8, 0.02% (weight/vol) bromophenol blue). The cell culture media was collected at each time point and centrifuged in a microfuge at 5,000 rpm for 5 min. Supernates were loaded onto a 20% weight/volume solution of sucrose in phosphate buffered saline in a SW41 rotor and centrifugation at 27,000 rpm for 16 h. The pellets were resuspended in SDS-PAGE gel loading buffer. Following SDS-PAGE, separated proteins were transferred to nitrocellulose filter paper (Protran 0.45 micron pore size, Schleicher and Schuell, Keene, NH) at 250 mA for 90 minutes in a transfer buffer of 50 mM Tris-HCl, 400 mM glycine, 0.1% SDS, 20% methanol using the Bio-Rad Mini Trans-Blot Electrophoretic Transfer Cell (Bio-Rad Laboratories, Hercules, CA). Following blocking (10% dried milk in 10mM Tris-HCl pH 8.0, 150 mM NaCl, with 0.1% Tween 20, or TBST), the filter was then incubated in TBST containing HIV-Ig (NIH AIDS Research & Reference Reagent Program) at a dilution of 1:5000. The HRP-labeled secondary antibody (goat anti-human IgG, PerkinElmer) was then added at a dilution of 1:20,000 in TBST. The secondary antibody was detected with SuperSignal West Pico Chemiluminescent Substrate (Pierce, Rockford, IL) and autoradiography.
2.5 In vitro and in vivo replication assays
Replication assays were conducted in vitro in Vero cells as described previously [47,49] at 12, 24, 48 and 72 hpi using an MOI of 1.0. For in vivo replication, 8 week old Balb/c mice were first anesthetized, then injected intramuscularly into the right hind gastrocnemius with 1 × 107 pfu of either R3659 or J200 (clone 71), in 50 µl total volume in the presence of dextran sulfate (100 µg/ml). At days 2, 4, and 7, two mice/group were sacrificed and the infected gastrocnemius removed, weighed and diluted to 20% wt/vol in a 50:50 mixture of sterile milk:DMEM/1% FBS. Samples were homogenized and subjected to three freeze/thaw (−80°C/37°C) cycles. Homogenates were sonicated prior to titering on Vero cells to determine viral recovery.
2.6 Mice and immunization
Female, 5–6 week old pathogen-free Balb/c mice were obtained from the Frederick Cancer Research and Development Center, National Cancer Institute. All animal studies were conducted in accordance with guidelines for animal use and care established by The University of Alabama at Birmingham Animal Resource Program and the Institutional Animal Care and Use Committee (protocol #010704285). Mice were immunized by intraperitoneal (i.p.) injection, using 200 µl of the desired virus dose (diluted in saline), or saline only. For assessment of Gag-specific CD8 responses, mice were challenged i.p. five days prior to sacrifice with 2.5 × 107 pfu of either vDK-1, a recombinant vaccinia virus that expresses HIV-1 Gag (vDK-1, NIH AIDS Research & Reference Reagent Program), or vSC11, a recombinant vaccinia virus constructed by homologous recombination with the shuttle plasmid pSC11 [50], which does not contain the gag gene.
2.7 Intracellular IFN-γstaining and flow cytometry analysis
Splenocytes freshly harvested at sacrifice were resuspended to a final concentration of 2 × 107 cells/ml in DMEM containing 10% FBS and 50 µM 2-mercaptoethanol. To assess intracellular gamma interferon production, splenocytes were cultured in 96 well flat-bottom tissue culture dishes with or without the addition of a major histocompatibility complex class I-restricted HIV-1 p24 peptide (AMQMLKETI) at 200ng/ml for 6 hours at 37°C in growth medium containing 10% T-STIM and GolgiPlug (BD Pharmingen, San Diego, CA), which blocks cytokine secretion from the Golgi apparatus. As a positive control, naïve splenocytes were stimulated with 50 ng/ml PMA and 1 µM ionomycin. After stimulation, cells were chilled on ice, transferred to round-bottom 96 well plates and pelleted by centrifugation at 600 rpm for 5 minutes at 4°C. Cells were first stained for CD8 expression by incubation with phycoerythrin-conjugated monoclonal rat anti-mouse CD8 antibody (0.06 µg/sample, BD Pharmingen), diluted in FACS buffer (PBS containing 2% bovine serum albumin, 0.2% sodium azide) for 30 minutes on ice in the dark. Cells were then washed 3 times in FACS buffer, resuspended in 100 µl of Cytofix/Cytoperm (BD Pharmingen), incubated as before for 20 minutes and washed three times with 1X Perm/Wash buffer (BD Pharmingen). Cells were resuspended in a 1:100 dilution of FITC-conjugated rat anti-mouse gamma interferon (IFN-γ) antibody (BD Pharmingen) in Perm/Wash buffer and incubated as before for 30 minutes. Cells were washed three times in 1X Perm/Wash buffer, twice in FACS buffer, and resuspended in 200 µls PBS containing 2% w/v paraformaldehyde. Stained cells were acquired using a FACSCalibur flow cytometer (Becton Dickinson) and analyzed using FCS Express (De Novo Software, Ontario, Canada).
3. RESULTS
3.1 Construction of a conditionally replication competent HSV-1 that expresses HIV-189.6 Gag
To maximize expression of HIV-1 antigens from our Δγ134.5 HSV-1 vaccine vector, we first constructed a new vector (pCIM) for expression of HIV-1 genes that contained the CMV immediate early promoter and the Mason-Pfizer monkey virus (M-PMV) polyadenylation signal and cytoplasmic transport element (CTE). The M-PMV CTE has previously been shown to mediate transport of unspliced viral transcripts out of the nucleus for protein synthesis in the absence of Rev protein and its cis-acting response element (RRE) [46]. The gag-pro sequence from the primary isolate HIV-189.6 [51] was cloned into pCIM, and the entire expression cassette was removed and introduced into a γ134.5 targeting vector to generate pJP200, as described in Materials and Methods. Two candidate viruses, J200.71 and J200.91, were selected following homologous recombination between pJP200 and R3659 viral DNA. Figure 1 illustrates the genomic organization of R3659 and J200. The presence of the HIV-189.6 gag-pro expression cassette within the γ134.5 site was confirmed by restriction digest analysis of the viral DNA and Southern blot hybridization (data not shown).
Figure 1.
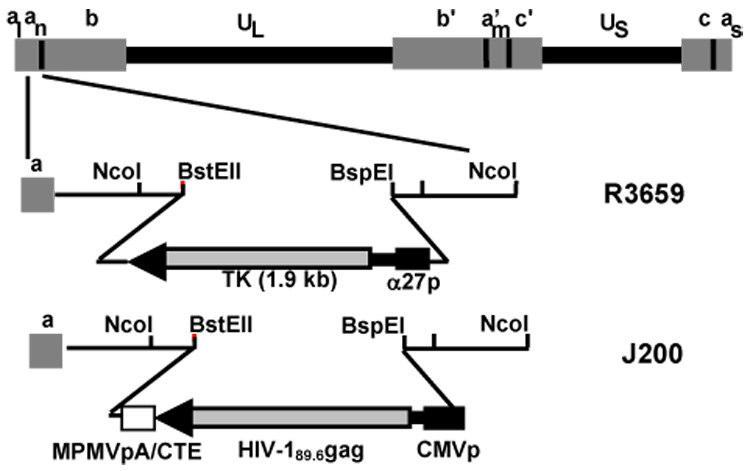
Schematic diagram of γ134.5-deleted HSV-1. Both copies of the HSV-1 γ134.5 gene were replaced with the CMV-driven HIV-189.6 Gag expression cassette (only terminal repeat location is indicated on diagram). UL, unique long; US, unique short; a,a’, b,b’, c,c’, inverted repeat sequences; MPMVpA/CTE, Mason-Pfizer monkey virus polyadenylation signal/cytoplasmic transport element; TK, thymidine kinase; α27p, HSV alpha 27 gene promoter; CMVp, cytomegalovirus immediate early promoter; R3659, parent virus of J200. Not shown schematically: the native tk gene in R3659 has been deleted.
3.2 HIV-189.6 gag-pro is efficiently expressed in J200-infected Vero cells
To demonstrate Gag protein production within J200-infected Vero cells, metabolic labeling studies were performed. At 3 hpi, cells were continuously labeled for 14 hours and HIV specific proteins were extracted from cell lysates by immunoprecipitation with serum from HIV-1 infected patients, and analyzed by SDS-PAGE. As shown in Figure 2A, the HIV-1 p55 Gag polyprotein, as well as the p41 Gag product, were detected in cell lysates from Vero cells infected with J200 clones 71 and 91, but not in cells infected with R3659 as a negative control. The p24 major capsid protein, a Gag protease cleavage product, was also visible in infected cell lysates. The p41 band represents a truncated Gag product in which an internal downstream initiation codon was utilized. An additional band present in the J200-infected cell lysates, but not the R3659-infected cell lysates, at ~47kD is presumed to be a partial Gag cleavage product.
Figure 2.
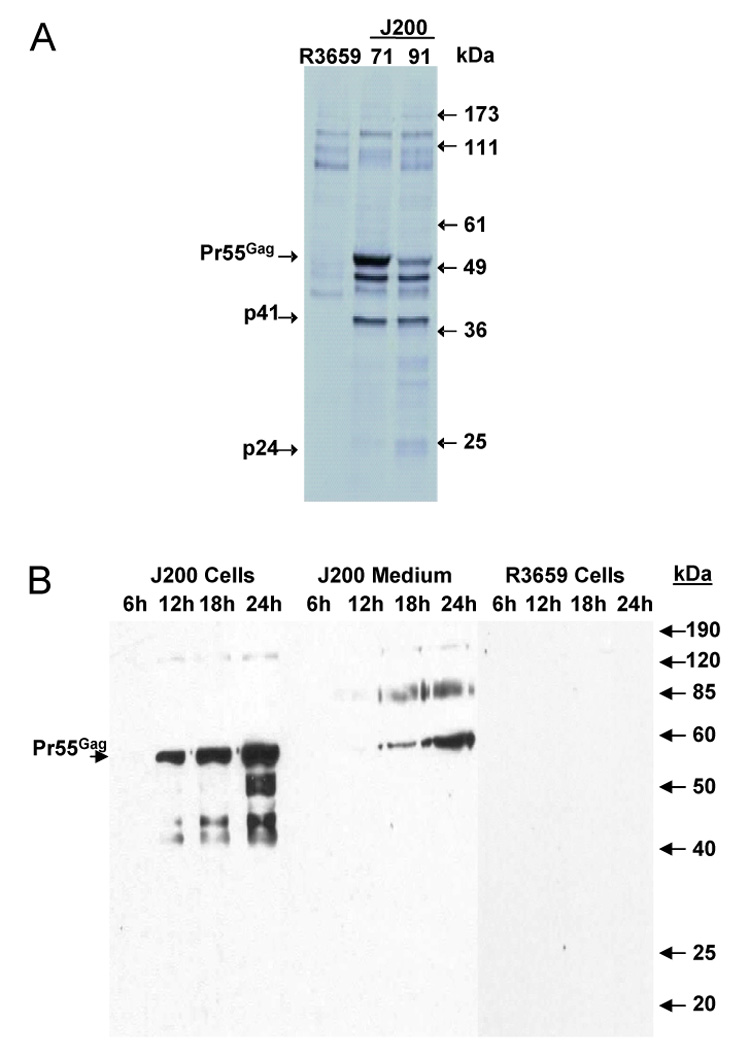
Expression of HIV-189.6 Gag from J200-infected Vero cells. Vero cells were infected with either J200 or R3659, and protein expression was assessed by metabolic labeling and immunoprecipitation (panel A) or Western blot (panel B). Pr55Gag, the p55 precursor Gag polyprotein; p41, protein product as a result of transcriptional initiation at a downstream ATG; p24, Gag cleavage product, the major HIV-1 capsid protein; kDa, protein standard size markers. 71 and 91 are individual clones of J200.
To demonstrate the release of Gag proteins over time, Vero cells were infected with J200 or R3659 (as a negative control) and cell lysates and culture media were collected at 6, 12, 18, and 24 hpi. HIV Pr55Gag polyproteins were detected by Western blot starting at 12 hpi in the J200 cell lysate and 18 hpi in the cell culture media after centrifugation through 20% (weight/volume) sucrose (Figure 2B). The absence of both Pr55Gag cleavage products in the culture media and mature or immature Gag virus-like particles after imaging with electron microscopy (data not shown) suggests that viral budding with proteolytic processing does not proceed with efficiency in this system. The mechanism for the release of Pr55Gag into the cell culture media is not clear, but may be a consequence of HSV cytopathic effect and cell lysis.
3.3 In vitro and in vivo replication of J200
The efficiency of J200 replication in vitro was compared to its parent Δγ134.5 HSV-1, R3659, and to wild type HSV-1 (F) strain, using Vero cells, which are permissive to HSV-1 infection. At 12, 24, 48, and 72 hours after infection (MOI=1) with wild type, R3659, or J200 clones 71 or 91, cells were harvested and viral recovery determined. No significant differences were seen in replication over time of either J200 clone as compared to HSV-1 (F) and R3659 (data not shown). Clone 71 was selected for all subsequent studies and is herein referred to as J200.
To assess in vivo replication efficiency over time, 1 × 107 pfu of either J200 or R3659 was injected intramuscularly into the hind leg gastrocnemius of Balb/c mice. At days 2, 4, or 7, mice (2/virus/timepoint) were sacrificed and the injected gastrocnemius was dissected and homogenized, and the homogenate titered on Vero cells. At day 2, the average titer from R3659 was 7.1 × 10³ pfu/ml, whereas the average titer of J200 was 5.5 × 105 pfu/ml. No virus was recovered from R3659-infected muscle at either day 4 or day 7 following injection. For J200, the average titer recovered at day 4 was 6.3 × 105 pfu/ml from mice infected with this virus. However, by day 7, no virus was recovered from either of the J200-infected samples.
3.4 Gag-specific CD8+ T cell responses in mice following immunization with J200
The ability of J200 to elicit Gag-specific CD8+ T cell responses was evaluated in mice by intracellular IFN-γ analysis. Balb/c mice were primed by a single i.p. administration of 1 × 107 or 5 × 107 pfu of J200 or R3659 virus, whereas control animals received saline only. At 11 weeks post-immunization, responses were boosted by i.p. injection with either a recombinant vaccinia virus (vv) that expresses HIV-1 Gag (vDK-1, referred to here as vv-Gag) or a vaccinia virus control (SC11, referred to as vv-only). Vaccinia virus replicates well in mice, and has been previously used to boost CTL responses after priming with HIV-1 Gag expressed from vaccine vectors in mice [52,53]. Splenocytes were harvested and Gag-specific CD8+ T cell responses were analyzed five days following vaccinia boost by stimulation with the MHC Class I-restricted Gag peptide AMQMLKETI and assessing IFN-γ production by intracellular cytokine staining. As shown in Figure 3 (right panels), 3.1% and 2.9% of CD8+ T cells isolated from mice immunized with 5×107 and 1×107 pfu of J200, respectively, were producing intracellular IFN-γ following vv-Gag challenge and peptide stimulation. The interferon CTL response is Gag-specific: only 0.3–0.4% of splenocytes demonstrated intracellular IFN-γ production in the absence of Gag peptide (Figure 3, left panels). In R3659-immunized, vv-Gag challenged mice, 0.3–0.4% of CD8+ T cells were positive for intracellular IFN-γ with or without peptide stimulation (data not shown).
Figure 3.
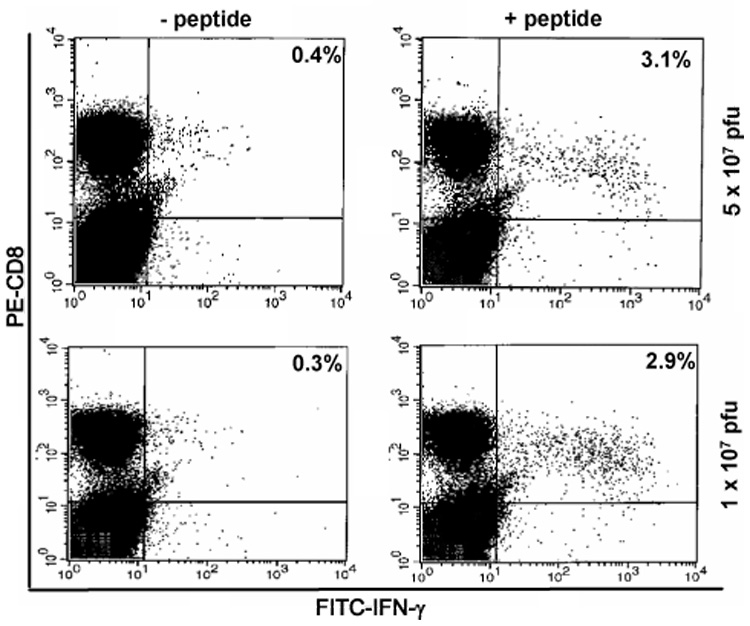
Intracellular cytokine staining and flow cytometry analysis of splenocytes stained for CD8 (PE-labeled) and intracellular IFN-γ (FITC-labeled) production at 11 weeks post-immunization. Top panels: immunization dose was 5 × 107 pfu J200. Bottom panels: immunization dose was 1 × 107 pfu J200. For details, see text.
To determine the timing of onset for Gag-specific CD8+ T cell responses elicited in response to J200 immunization, as well as the persistence of these responses, mice were immunized as above with J200 or R3659, or received saline injection only. At 3, 9, 26 and 39 weeks post-immunization, mice were sacrificed and their spleens isolated as described above. Five days prior to sacrifice at each time point, mice were boosted as above with either vv-Gag or vv-only. For all but the last time point, four J200-immunized mice received vv-Gag and two received vv-only as a control for effects of vaccinia challenge on CD8+ T cells. For the last time point, three J200-immunized mice received vv-Gag and one received vv-only as control for effects of vaccinia challenge. Splenocytes were harvested and analyzed individually for each mouse and the proportion of IFN-γ positive CD8+ cells was averaged for each treatment group. At 3 weeks following J200 immunization, the earliest time point assessed, more than 5.0% of CD8+ T cells (as determined by FACS analysis) on average were producing intracellular IFN-γ in response to vv-Gag boost in vivo and stimulation with the Gag-specific peptide in vitro. In the absence of peptide stimulation, the mean percent of CD8+ cells stained positive for IFN-γ was 0.5% in 3 out of 4 samples. Administration of vv-Gag boost after a control primary immunization with R3659 did not elicit a comparable response. J200 primary immunization without a vv-Gag boost was also insufficient to elicit a substantial response. The average vv-Gag boosted and Gag peptide-specific responses dropped to 2.5% by 9 weeks following J200 immunization (Figure 4), consistent with the first experiment (Figure 3), and remained elevated as compared to responses in the R3659 immunized controls. Even at 39 weeks, the last time point assessed, the average Gag-specific CD8+ T cells producing IFN-γ from J200-immunized mice was 2.2%, versus 0.4% for R3659 controls. In contrast, none of the other immunization groups showed increased levels of Gag-specific CD8+ T cells in response to Gag-peptide stimulation, as compared to control samples which were not peptide-stimulated. Plots included in Figure 4A are from one of the samples for each time point and are representative for results of the samples in the given treatment group. The values indicated within each plot in Figure 4A represent the means of all the samples included for that timepoint. The mean Gag-specific CD8+ T cell responses detected for each treatment group over time as compared to the control groups are shown in Figure 4B.
Figure 4.
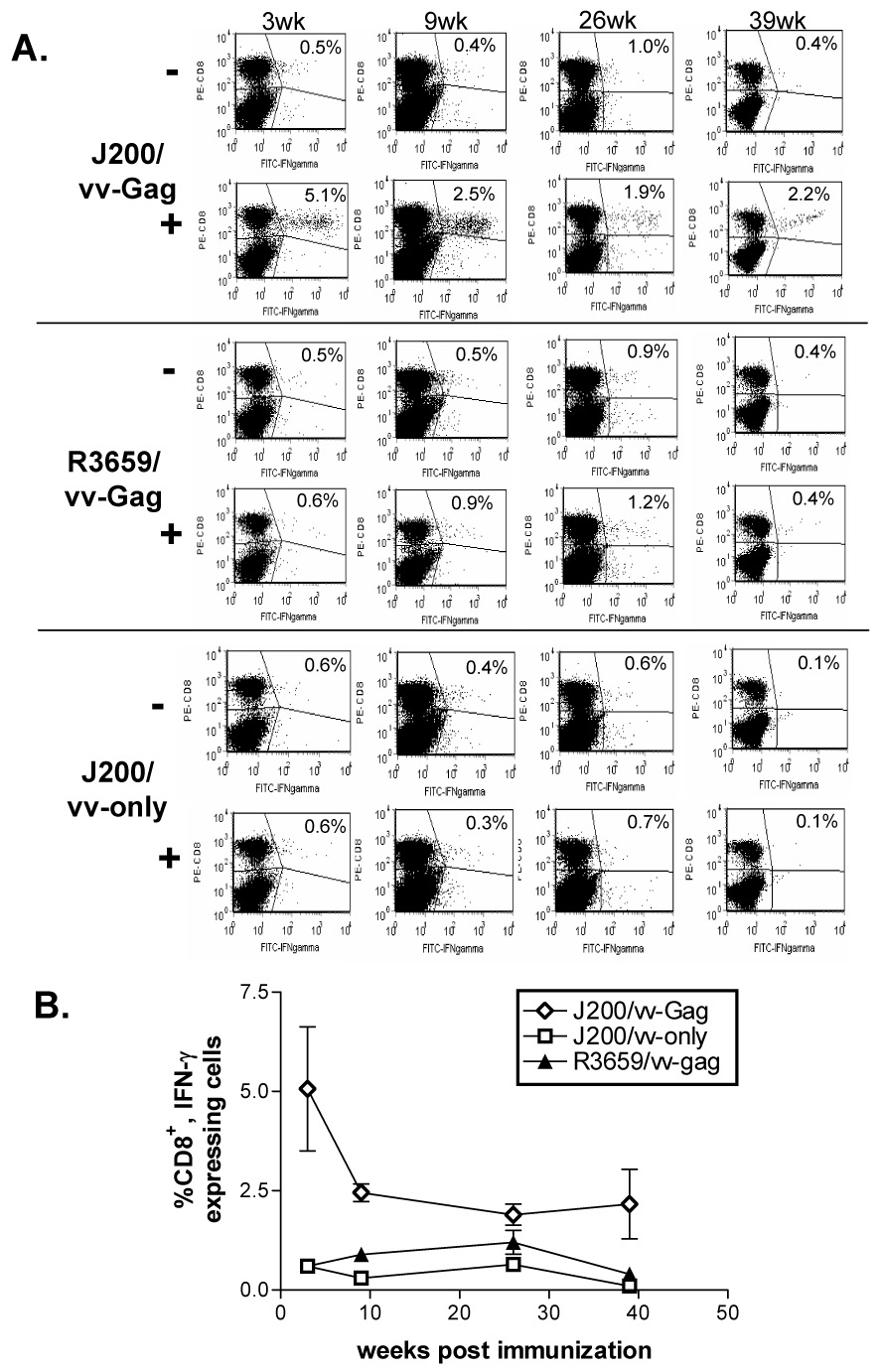
Intracellular cytokine staining and flow cytometry analysis of Gag-specific CD8+ responses over time in Balb/c mice immunized with 1 × 107 J200 i.p. Splenocytes were isolated from mice challenged 5 days prior i.p. with either vvGAG (vDK1) or vvonly (SC11), as indicated in Materials and Methods. Splenocytes were cultured in the presence (+) or absence (−) of the MHC class I-restricted HIV-1 p24 peptide (AMQMLKETI) for 6 hours prior to staining for both surface CD8+ (PE-CD8, vertical axis) and intracellular IFN-γ (FITC-IFNgamma, horizontal axis). A. Representative plots from one of the samples for each time point is shown. The average percent of positive Gag-specific IFN- γ-secreting CD8+ T cells is indicated in the upper right hand corner of each plot. B. Line graph indicating the average responses for all of the mice within a given immunization group over time. Only the samples stimulated with p24 peptide are shown.
4. DISCUSSION
An ideal HIV vaccine would elicit a broadly neutralizing antibody response which would protect the vast majority of individuals from infection. Immunization strategies designed to elicit neutralizing antibody responses are effective in protecting against an infection with a viral strain closely related to the immunogen. However, these antibody responses have failed to protect against challenge with heterologous strains, and thus have not yet reached the threshold that will be required for a universally effective vaccine. In contrast, while vaccines that elicit CMI responses do not prevent infection in animal models, they are effective against a variety of viral strains by reducing viral loads and preventing disease associated with viral infection. Predictive correlates of protection have not yet been established for CMI elicited by an HIV vaccine. However, HIV and/or SIV-specific CD8+ responses are associated with viral suppression in the course of natural infection and protection of immunized NHPs from advanced disease, following pathogenic retrovirus challenge [12–17,54]. As long as vaccines with neutralizing immunity remain elusive, the best hope for the field lies with the development of vaccines effective at reducing viral loads in the infected host. Vaccines that elicit broadly effective CMI responses are anticipated to significantly prolong survival, and inhibit transmission. The latter is crucial to curtail the rate of new infections in developing nations that lack access to antiretroviral therapy.
A diverse set of recombinant, attenuated viral vectors have been selected for their ability to stimulate CMI to heterologous antigens, and explored as vectors for HIV vaccines (reviewed in [11,21]. Some of these candidates have not yet been tested in humans but are non-toxic and elicit significant CMI responses in animal models. These include alphaviruses [55–57], polioviruses [58], and rhabdoviruses [52]. Candidate vaccine vectors in more advanced stages of development are under evaluation in human clinical trials. Canarypox vectors are well tolerated, but their immunogenicity has been disappointing: HIV-specific CD8+ CTL responses measured at any one timepoint after vaccination are present in only 20–30% of human subjects [59,60]. While these phase I/II trials were not designed to test vaccine efficacy, breakthrough HIV infections have occurred with equal frequency in the vaccinated and unvaccinated control subjects [61]. Nevertheless, since the significance of the CTL responses as a correlate of protection is not entirely clear, a large Phase III trial is underway to measure the efficacy of a canarypox vector as part of an HIV vaccine regimen. While awaiting the canarypox Phase III results, modified Vaccinia Ankara (MVA) [62–64] and adenovirus vaccine vectors[24,65] are being evaluated in both preclinical and Phase I and II patient trials. Both vectors stimulate potent CMI responses in primates and a majority of human subjects. However, prior immunity to each vector may potentially interfere with initial vaccine responses and/or booster doses required to maintain host immunity. Given the potential limitations for leading vector candidates, and the lack of adequate assays to identify correlates of immunity in humans, there is a clear need for the development of novel vaccines.
Recombinant attenuated Δγ134.5 HSV-1 has been studied as an oncolytic agent that is safe for use in humans and also has several characteristics of an attractive vaccine vector candidate. These unique properties include low-level replication in animal tissue which may elicit long lasting CMI responses after single dose administration and expression of foreign genes even in the presence of pre-existing immunity to the vector [38] combined with the complete absence of neurovirulence [23].
In this pilot study, the J200 virus replicates in animal tissue following intramuscular injection and produces significant levels of Gag protein. A single dose of J200 elicits CD8+ CMI following challenge with a heterologous viral vector (vv-Gag) up to 9 months later. The magnitude of the cellular immune response to J200 prime/vv-Gag challenge is similar in magnitude to prior observations with the most immunogenic attenuated viral vectors [52,66] and thus may also be effective at preventing advanced disease following pathogenic challenge. Further development of these Δγ134.5 HSV vaccine vectors will include the incorporation of HIV-1 env with accessory genes and measuring the breadth of the immune response against a spectrum of CTL epitopes across the entire range of vaccine immunogens, rather than the single Gag epitope used in this study.
A persistent state of immune memory and subsequent response to boost immunizations or pathogenic challenge are fundamentally essential for a successful vaccine. Although CTL activity is not measurable after the J200 primary injection, the vigorous response following vv-Gag boost clearly demonstrates the induction of a durable immune memory state which persists through an interval of at least 39 weeks. Local replication of the J200 vector likely contributes to the persistent effects observed after a single injection. Despite the inability to demonstrate prolonged replication of J200 following i.m. administration, persistent CD8 responses were elicited. This supports the hypothesis that the level of protective immune responses elicited by an antigen can be influenced by the delivery vector; in this case, an attenuated HSV. Persistent effect after a single dose has also been observed with other attenuated, replication competent vaccine vectors and this property may translate into enhanced vaccine efficacy [52,67,68].
Pre-existing host immunity has interfered with immune responses elicited by both of the viral-based vectors of the advanced HIV-1 vaccine candidates in humans, with adenoviral vectors affected more heavily than the poxvirus vectors as a group. The issue of pre-existing immunity towards HSV-derived vectors has also been investigated in murine models, with conflicting results. Neither antibody nor cellular proliferative responses elicited in mice immunized with a replication-defective HSV vector were affected by prior exposure to HSV [38]. Additionally, treatment of tumor-bearing mice with oncolytic HSV was not adversely effected even in mice with prior HSV-1 immunity [36]. Other studies in mice seropositive for HSV-1 have indicated that humoral and cell-mediated immune responses are significantly reduced following immunization with replication-defective HSV vectors as compared to immunization of HSV-1 naïve mice[69]. In humans, the response to HSV oncolytic therapy for glioma in clinical trials has not been correlative with pre-existing HSV-1 seropositivity [35].
In summary, we have provided new evidence supporting the use of HSV-based vectors for delivery of HIV-1 antigens as vaccines. We report for the first time an attenuated but replication competent HSV vector (J200) engineered to express HIV-1 Gag protein. This virus elicits potent Gag-specific CD8+ T cell responses following a single intraperitoneal immunization. The CD8+ responses elicited from this immunization persist at least 9 months. Further, prior studies with the parent virus of J200 have demonstrated its safety in mice, nonhuman primates, and in Phase 1 trials in brain tumor patients with compromised immune systems. Future studies utilizing this vector and this murine model will test other HIV-1 immunogens and vectors expressing cytokine adjuvants. Finally, studies will also be performed to assess the role of CD4+ or other responses that may mediate a state of immune memory.
ACKNOWLEDGMENTS
The authors wish to thank Dr. Bernard Roizman (University of Chicago, Chicago, IL) for the plasmid pRB4878 and parent virus R3659, and Dr. Mark Mulligan (UAB) for the vaccinia recombinants. We also thank Lifeng Zhang, Rick Bowman and Sharon Samuel for technical assistance, and Dr. Debra Quenelle for assisting with the vaccinia challenge studies. We thank Leigh Millican for assistance with the EM studies and Drs. Paul Goepfert and Patricia Fultz for numerous helpful discussions and for reviewing the manuscript. We also acknowledge the UAB CFAR FACS Core Facility, DNA Sequencing Core Facility and the UAB Peptide Synthesis Core Facility for rendered services. This work was supported in part by NIH grants R21 AI44300 (JNP) and T32 AI 007493 (STR) and the UAB GCRC Clinical Investigator Fellowship Award (STR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Berman PW, Matthews TJ, Riddle L, Champe M, Hobbs MR, Nakamura GR, et al. Neutralization of multiple laboratory and clinical isolates of human immunodeficiency virus type 1 (HIV-1) by antisera raised against gp120 from the MN isolate of HIV-1. J Virol. 1992;66(7):4464–4469. doi: 10.1128/jvi.66.7.4464-4469.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berman PW, Gregory TJ, Riddle L, Nakamura GR, Champe MA, Porter JP, et al. Protection of chimpanzees from infection by HIV-1 after vaccination with recombinant glycoprotein gp120 but not gp160. Nature. 1990;Vol. 345:622–625. doi: 10.1038/345622a0. [DOI] [PubMed] [Google Scholar]
- 3.Girard M, Kieny M-P, Pinter A, Barre-Sinoussi F, Nara P, Kolbe H, et al. Immunization of chimpanzees confers protection against challenge with human immunodeficiency virus. Proceedings of the National Academy of Sciences. 1991;88:542–546. doi: 10.1073/pnas.88.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berman PW, Groopman JE, Gregory T, Clapham PR, Weiss RA, Ferriani R, et al. Human immunodeficiency virus type 1 challenge of chimpanzees immunized with recombinant envelope glycoprotein gp120. Proc Natl Acad Sci U S A. 1988;85(14):5200–5204. doi: 10.1073/pnas.85.14.5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flynn NM, Forthal DN, Harro CD, Judson FN, Mayer KH, Para MF. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis. 2005;191(5):654–665. doi: 10.1086/428404. [DOI] [PubMed] [Google Scholar]
- 6.Gilbert PB, Peterson ML, Follmann D, Hudgens MG, Francis DP, Gurwith M, et al. Correlation between immunologic responses to a recombinant glycoprotein 120 vaccine and incidence of HIV-1 infection in a phase 3 HIV-1 preventive vaccine trial. J Infect Dis. 2005;191(5):666–677. doi: 10.1086/428405. [DOI] [PubMed] [Google Scholar]
- 7.Borrow P, Lewicki H, Hahn BH, Shaw GM, Oldstone MB. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68(9):6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koup RA, Safrit JT, Cao Y, Andrews CA, McLeod G, Borkowsky W, et al. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68(7):4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogg GS, Jin X, Bonhoeffer S, Dunbar PR, Nowak MA, Monard S, et al. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279(5359):2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 10.Smith SM. HIV vaccine development in the nonhuman primate model of AIDS. J Biomed Sci. 2002;9(2):100–111. doi: 10.1007/BF02256020. [DOI] [PubMed] [Google Scholar]
- 11.Berzofsky JA, Ahlers JD, Janik J, Morris J, Oh S, Terabe M, et al. Progress on new vaccine strategies against chronic viral infections. J Clin Invest. 2004;114(4):450–462. doi: 10.1172/JCI22674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sadagopal S, Amara RR, Montefiori DC, Wyatt LS, Staprans SI, Kozyr NL, et al. Signature for long-term vaccine-mediated control of a Simian and human immunodeficiency virus 89.6P challenge: stable low-breadth and low-frequency T-cell response capable of coproducing gamma interferon and interleukin-2. J Virol. 2005;79(6):3243–3253. doi: 10.1128/JVI.79.6.3243-3253.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramsburg E, Rose NF, Marx PA, Mefford M, Nixon DF, Moretto WJ, et al. Highly effective control of an AIDS virus challenge in macaques by using vesicular stomatitis virus and modified vaccinia virus Ankara vaccine vectors in a single-boost protocol. J Virol. 2004;78(8):3930–3940. doi: 10.1128/JVI.78.8.3930-3940.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patterson LJ, Malkevitch N, Venzon D, Pinczewski J, Gomez-Roman VR, Wang L, et al. Protection against mucosal simian immunodeficiency virus SIV(mac251) challenge by using replicating adenovirus-SIV multigene vaccine priming and subunit boosting. J Virol. 2004;78(5):2212–2221. doi: 10.1128/JVI.78.5.2212-2221.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mooij P, Nieuwenhuis IG, Knoop CJ, Doms RW, Bogers WM, Ten Haaft PJ, et al. Qualitative T-helper responses to multiple viral antigens correlate with vaccine-induced immunity to simian/human immunodeficiency virus infection. J Virol. 2004;78(7):3333–3342. doi: 10.1128/JVI.78.7.3333-3342.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKenna PM, Aye PP, Dietzschold B, Montefiori DC, Martin LN, Marx PA, et al. Immunogenicity study of glycoprotein-deficient rabies virus expressing simian/human immunodeficiency virus SHIV89.6P envelope in a rhesus macaque. J Virol. 2004;78(24):13455–13459. doi: 10.1128/JVI.78.24.13455-13459.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matano T, Kobayashi M, Igarashi H, Takeda A, Nakamura H, Kano M, et al. Cytotoxic T lymphocyte-based control of simian immunodeficiency virus replication in a preclinical AIDS vaccine trial. J Exp Med. 2004;199(12):1709–1718. doi: 10.1084/jem.20040432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKay PF, Schmitz JE, Barouch DH, Kuroda MJ, Lifton MA, Nickerson CE, et al. Vaccine protection against functional CTL abnormalities in simian human immunodeficiency virus-infected rhesus monkeys. J Immunol. 2002;168(1):332–337. doi: 10.4049/jimmunol.168.1.332. [DOI] [PubMed] [Google Scholar]
- 19.Boyer JD, Maciag PC, Parkinson R, Wu L, Lewis MG, Weiner DB, et al. Rhesus macaques with high levels of vaccine induced IFN-gamma producing cells better control viral set-point following challenge with SIV239. Vaccine. 2006;24(21):4498–4502. doi: 10.1016/j.vaccine.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 20.Schultz AM, Bradac JA. The HIV vaccine pipeline, from preclinical to phase III. Aids. 2001;15 Suppl 5:S147–S158. doi: 10.1097/00002030-200100005-00018. [DOI] [PubMed] [Google Scholar]
- 21.Hurwitz JL. HIV vaccine discovery and development. Curr Drug Targets Infect Disord. 2005;5(2):81–83. doi: 10.2174/1568005054201526. [DOI] [PubMed] [Google Scholar]
- 22.Letvin NL, Barouch DH, Montefiori DC. Prospects for vaccine protection against HIV-1 infection and AIDS. Annu Rev Immunol. 2002;20:73–99. doi: 10.1146/annurev.immunol.20.081501.094854. [DOI] [PubMed] [Google Scholar]
- 23.Chou J, Kern ER, Whitley RJ, Roizman B. Mapping of herpes simplex virus-1 neurovirulence to gamma 134.5, a gene nonessential for growth in culture. Science. 1990;250(4985):1262–1266. doi: 10.1126/science.2173860. [DOI] [PubMed] [Google Scholar]
- 24.Casimiro DR, Chen L, Fu TM, Evans RK, Caulfield MJ, Davies ME, et al. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J Virol. 2003;77(11):6305–6313. doi: 10.1128/JVI.77.11.6305-6313.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hocknell PK, Wiley RD, Wang X, Evans TG, Bowers WJ, Hanke T, et al. Expression of human immunodeficiency virus type 1 gp120 from herpes simplex virus type 1-derived amplicons results in potent, specific, and durable cellular and humoral immune responses. J Virol. 2002;76(11):5565–5580. doi: 10.1128/JVI.76.11.5565-5580.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kruse CA, Lamb C, Hogan S, Smiley WR, Kleinschmidt-Demasters BK, Burrows FJ. Purified herpes simplex thymidine kinase retroviral particles. II. Influence of clinical parameters and bystander killing mechanisms. Cancer Gene Ther. 2000;7(1):118–127. doi: 10.1038/sj.cgt.7700097. [DOI] [PubMed] [Google Scholar]
- 27.Mikloska Z, Cunningham AL. Alpha and gamma interferons inhibit herpes simplex virus type 1 infection and spread in epidermal cells after axonal transmission. J Virol. 2001;75(23):11821–11826. doi: 10.1128/JVI.75.23.11821-11826.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gorantla S, Santos K, Meyer V, Dewhurst S, Bowers WJ, Federoff HJ, et al. Human dendritic cells transduced with herpes simplex virus amplicons encoding human immunodeficiency virus type 1 (HIV-1) gp120 elicit adaptive immune responses from human cells engrafted into NOD/SCID mice and confer partial protection against HIV-1 challenge. J Virol. 2005;79(4):2124–2132. doi: 10.1128/JVI.79.4.2124-2132.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spivack JG, Fareed MU, Valyi-Nagy T, Nash TC, O'Keefe JS, Gesser RM, et al. Replication, establishment of latent infection, expression of the latency-associated transcripts and explant reactivation of herpes simplex virus type 1 gamma 34.5 mutants in a mouse eye model. J Gen Virol. 1995;76(Pt 2):321–332. doi: 10.1099/0022-1317-76-2-321. [DOI] [PubMed] [Google Scholar]
- 30.Whitley RJ, Kern ER, Chatterjee S, Chou J, Roizman B. Replication, establishment of latency, and induced reactivation of herpes simplex virus gamma 1 34.5 deletion mutants in rodent models. J Clin.Invest. 1993;91(6):2837–3843. doi: 10.1172/JCI116527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shih M-F, Arsenakis M, Tiollais P, Roizman B. Expression of hepatitis B virus S gene by herpes simplex virus 1 vectors carrying α and β regulated gene chimeras. Proc. Natl. Acad. Sci. USA. 1984;81:5867–5870. doi: 10.1073/pnas.81.18.5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rampling R, Cruickshank G, Papanastassiou V, Nicoll J, Hadley D, Brennan D, et al. Toxicity evaluation of replication-competent herpes simplex virus (ICP 34.5 null mutant 1716) in patients with recurrent malignant glioma. Gene Ther. 2000;7:859–866. doi: 10.1038/sj.gt.3301184. [DOI] [PubMed] [Google Scholar]
- 33.Markert JM, Medlock MD, Rabkin SD, Gillespie GY, Todo T, Hunter WD, et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther. 2000;7(10):867–874. doi: 10.1038/sj.gt.3301205. [DOI] [PubMed] [Google Scholar]
- 34.Papanastassiou V, Rampling R, Fraser M, Petty R, Hadley D, Nicoll J, et al. The potential for efficacy of the modified (ICP 34.5(-)) herpes simplex virus HSV1716 following intratumoural injection into human malignant glioma: a proof of principle study. Gene Ther. 2002;9(6):398–406. doi: 10.1038/sj.gt.3301664. [DOI] [PubMed] [Google Scholar]
- 35.Harrow S, Papanastassiou V, Harland J, Mabbs R, Petty R, Fraser M, et al. HSV1716 injection into the brain adjacent to tumour following surgical resection of high-grade glioma: safety data and long-term survival. Gene Ther. 2004;11(22):1648–1658. doi: 10.1038/sj.gt.3302289. [DOI] [PubMed] [Google Scholar]
- 36.Chahlavi A, Rabkin S, Todo T, Sundaresan P, Martuza R. Effect of prior exposure to herpes simplex virus 1 on viral vector- mediated tumor therapy in immunocompetent mice. Gene Ther. 1999;6(10):1751–1758. doi: 10.1038/sj.gt.3301003. [DOI] [PubMed] [Google Scholar]
- 37.Herrlinger U, Kramm CM, Aboody-Guterman KS, Silver JS, Ikeda K, Johnston KM, et al. Pre-existing herpes simplex virus 1 (HSV-1) immunity decreases, but does not abolish, gene transfer to experimental brain tumors by a HSV-1 vector. Gene Ther. 1998;5(6):809–819. doi: 10.1038/sj.gt.3300643. [DOI] [PubMed] [Google Scholar]
- 38.Brockman MA, Knipe DM. Herpes simplex virus vectors elicit durable immune responses in the presence of preexisting host immunity. J Virol. 2002;76(8):3678–3687. doi: 10.1128/JVI.76.8.3678-3687.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watanabe D, Brockman MA, Ndung'u T, Mathews L, Lucas WT, Murphy CG, et al. Properties of a herpes simplex virus multiple immediate-early gene-deleted recombinant as a vaccine vector. Virology. 2007;357(2):186–198. doi: 10.1016/j.virol.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 40.Murphy CG, Lucas WT, Means RE, Czajak S, Hale CL, Lifson JD, et al. Vaccine protection against simian immunodeficiency virus by recombinant strains of herpes simplex virus. J Virol. 2000;74(17):7745–7754. doi: 10.1128/jvi.74.17.7745-7754.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martuza RL, Malick A, Markert JM, Ruffner KL, Coen DM. Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science. 1991;252(5007):854–856. doi: 10.1126/science.1851332. [DOI] [PubMed] [Google Scholar]
- 42.Kaur A, Sanford HB, Garry D, Lang S, Klumpp SA, Watanabe D, et al. Ability of herpes simplex virus vectors to boost immune responses to DNA vectors and to protect against challenge by simian immunodeficiency virus. Virology. 2007;357(2):199–214. doi: 10.1016/j.virol.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Santos K, Duke CM, Rodriguez-Colon SM, Dakwar A, Fan S, Keefer MC, et al. Effect of promoter strength on protein expression and immunogenicity of an HSV-1 amplicon vector encoding HIV-1 Gag. Vaccine. 2007;25(9):1634–1646. doi: 10.1016/j.vaccine.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Post LE, Roizman B. A generalized technique for deletion of specific genes in large genomes: alpha gene 22 of herpes simplex virus 1 is not essential for growth. Cell. 1981;25(1):227–232. doi: 10.1016/0092-8674(81)90247-6. [DOI] [PubMed] [Google Scholar]
- 45.Jenkins F, Roizman B. Herpes simplex virus 1 recombinants with noninverting genomes frozen in different isomeric arrangements are capable of independent replication. J Virol. 1986;59(2):494–499. doi: 10.1128/jvi.59.2.494-499.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bray M, Prasad S, Dubay JW, Hunter E, Jeang K-T, Rekosh D, et al. A small element from the Mason-Pfizer monkey virus genome makes human immunodeficiency virus type 1 expression and replication Rev-independent. Proceedings of the National Academy of Sciences, USA. 1994;91:1256–1260. doi: 10.1073/pnas.91.4.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andreansky S, He B, van Cott J, McGhee J, Markert JM, Gillespie GY, et al. Treatment of intracranial gliomas in immunocompetent mice using herpes simplex viruses that express murine interleukins. Gene Ther. 1998;5(1):121–130. doi: 10.1038/sj.gt.3300550. [DOI] [PubMed] [Google Scholar]
- 48.Lagunoff M, Roizman B. The regulation of synthesis and properties of the protein product of open reading frame P of the herpes simplex virus 1 genome. J Virol. 1995;69(6):3615–3623. doi: 10.1128/jvi.69.6.3615-3623.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parker JN, Gillespie GY, Love CE, Randall S, Whitley RJ, Markert JM. Engineered herpes simplex virus expressing IL-12 in the treatment of experimental murine brain tumors. Proc Natl Acad Sci U S A. 2000;97(5):2208–2213. doi: 10.1073/pnas.040557897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chakrabarti S, Brechling K, Moss B. Vaccinia virus expression vector: coexpression of beta-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985;5(12):3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Collman R, Balliet JW, Gregory SA, Friedman H, Kolson DL, Nathanson N, et al. An infectious molecular clone of an unusual macrophage-tropic and highly cytopathic strain of human immunodeficiency virus type 1. J. Virol. 1992;66:7517–7521. doi: 10.1128/jvi.66.12.7517-7521.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McGettigan JP, Sarma S, Orenstein JM, Pomerantz RJ, Schnell MJ. Expression and immunogenicity of human immunodeficiency virus type 1 Gag expressed by a replication-competent rhabdovirus-based vaccine vector. J Virol. 2001;75(18):8724–8732. doi: 10.1128/JVI.75.18.8724-8732.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qiu JT, Song R, Dettenhofer M, Tian C, August T, Felber BK, et al. Evaluation of novel human immunodeficiency virus type 1 Gag DNA vaccines for protein expression in mammalian cells and induction of immune responses. J Virol. 1999;73(11):9145–9152. doi: 10.1128/jvi.73.11.9145-9152.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Turnbull EL, Lopes AR, Jones NA, Cornforth D, Newton P, Aldam D, et al. HIV-1 epitope-specific CD8+ T cell responses strongly associated with delayed disease progression cross-recognize epitope variants efficiently. J Immunol. 2006;176(10):6130–6146. doi: 10.4049/jimmunol.176.10.6130. [DOI] [PubMed] [Google Scholar]
- 55.Sundback M, Douagi I, Dayaraj C, Forsell MN, Nordstrom EK, McInerney GM, et al. Efficient expansion of HIV-1-specific T cell responses by homologous immunization with recombinant Semliki Forest virus particles. Virology. 2005;341(2):190–202. doi: 10.1016/j.virol.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 56.Gupta S, Janani R, Bin Q, Luciw P, Greer C, Perri S, et al. Characterization of human immunodeficiency virus Gag-specific gamma interferon-expressing cells following protective mucosal immunization with alphavirus replicon particles. J Virol. 2005;79(11):7135–7145. doi: 10.1128/JVI.79.11.7135-7145.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schlesinger S. Alphavirus vectors: development and potential therapeutic applications. Expert Opin Biol Ther. 2001;1(2):177–191. doi: 10.1517/14712598.1.2.177. [DOI] [PubMed] [Google Scholar]
- 58.Mandl S, Sigal LJ, Rock KL, Andino R. Poliovirus vaccine vectors elicit antigen-specific cytotoxic T cells and protect mice against lethal challenge with malignant melanoma cells expressing a model antigen. Proc Natl Acad Sci U S A. 1998;95(14):8216–8221. doi: 10.1073/pnas.95.14.8216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Belshe RB, Stevens C, Gorse GJ, Buchbinder S, Weinhold K, Sheppard H, et al. Safety and immunogenicity of a canarypox-vectored human immunodeficiency virus Type 1 vaccine with or without gp120: a phase 2 study in higher- and lower-risk volunteers. J Infect Dis. 2001;183(9):1343–1352. doi: 10.1086/319863. [DOI] [PubMed] [Google Scholar]
- 60.Goepfert PA, Horton H, McElrath MJ, Gurunathan S, Ferrari G, Tomaras GD, et al. High-dose recombinant Canarypox vaccine expressing HIV-1 protein, in seronegative human subjects. J Infect Dis. 2005;192(7):1249–1259. doi: 10.1086/432915. [DOI] [PubMed] [Google Scholar]
- 61.Schillinger JA, Xu F, Sternberg MR, Armstrong GL, Lee FK, Nahmias AJ, et al. National seroprevalence and trends in herpes simplex virus type 1 in the United States, 1976–1994. Sex Transm Dis. 2004;31(12):753–760. doi: 10.1097/01.olq.0000145852.43262.c3. [DOI] [PubMed] [Google Scholar]
- 62.Amara RR, Villinger F, Altman JD, Lydy SL, O'Neil SP, Staprans SI, et al. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science. 2001;292(5514):69–74. doi: 10.1126/science.1058915. [DOI] [PubMed] [Google Scholar]
- 63.Cebere I, Dorrell L, McShane H, Simmons A, McCormack S, Schmidt C, et al. Phase I clinical trial safety of DNA- and modified virus Ankara-vectored human immunodeficiency virus type 1 (HIV-1) vaccines administered alone and in a prime-boost regime to healthy HIV-1-uninfected volunteers. Vaccine. 2006;24(4):417–425. doi: 10.1016/j.vaccine.2005.08.041. [DOI] [PubMed] [Google Scholar]
- 64.Mwau M, Cebere I, Sutton J, Chikoti P, Winstone N, Wee EG, et al. A human immunodeficiency virus 1 (HIV-1) clade A vaccine in clinical trials: stimulation of HIV-specific T-cell responses by DNA and recombinant modified vaccinia virus Ankara (MVA) vaccines in humans. J Gen Virol. 2004;85(Pt 4):911–919. doi: 10.1099/vir.0.19701-0. [DOI] [PubMed] [Google Scholar]
- 65.Shiver JW, Fu TM, Chen L, Casimiro DR, Davies ME, Evans RK, et al. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature. 2002;415(6869):331–335. doi: 10.1038/415331a. [DOI] [PubMed] [Google Scholar]
- 66.McGettigan JP, Foley HD, Belyakov IM, Berzofsky JA, Pomerantz RJ, Schnell MJ. Rabies virus-based vectors expressing human immunodeficiency virus type 1 (HIV-1) envelope protein induce a strong, cross-reactive cytotoxic T-lymphocyte response against envelope proteins from different HIV-1 isolates. J Virol. 2001;75(9):4430–4434. doi: 10.1128/JVI.75.9.4430-4434.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Belyakov IM, Moss B, Strober W, Berzofsky JA. Mucosal vaccination overcomes the barrier to recombinant vaccinia immunization caused by preexisting poxvirus immunity. Proc Natl Acad Sci U S A. 1999;96(8):4512–4517. doi: 10.1073/pnas.96.8.4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Belyakov IM, Wyatt LS, Ahlers JD, Earl P, Pendleton CD, Kelsall BL, et al. Induction of a mucosal cytotoxic T-lymphocyte response by intrarectal immunization with a replication-deficient recombinant vaccinia virus expressing human immunodeficiency virus 89.6 envelope protein. J Virol. 1998;72(10):8264–8272. doi: 10.1128/jvi.72.10.8264-8272.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lauterbach H, Ried C, Epstein AL, Marconi P, Brocker T. Reduced immune responses after vaccination with a recombinant herpes simplex virus type 1 vector in the presence of antiviral immunity. J Gen Virol. 2005;86(Pt 9):2401–2410. doi: 10.1099/vir.0.81104-0. [DOI] [PubMed] [Google Scholar]


