Abstract
A selection of glycosylated polyacrylate nanoparticles has been prepared by radical-initiated emulsion polymerization in aqueous media. Using ethyl acrylate as a co-monomer, carbohydrate acrylates were incorporated into the poly(ethyl acrylate) framework to give stable emulsions of glyconanoparticles with an average particle size of around 40 nm. Using this technique a variety of glyconanoparticles were prepared from 3-O-acryloyl-1,2:5,6-di-O-isopropylidene-α-D-glucofuranose, 1-O-acryloyl-2,3:5,6-di-O-isopropylidene-α-D-mannofuranose, 6-O-acryloyl-1,2:3,4-di-O-isopropylidene-α-D-galactopyranose, 2-N-acryloyl-1,3,4,6-tetra-O-acetyl-β-D-glucosamine, 5-O-acryloyl-2,3-isopropylidene-1-methoxy-β-D-ribofuranose and 4-N-acetyl-5’-O-acryloyl-2’,3’-O-isopropylidene cytidine. Scanning electron microscopy, dynamic light scattering and proton NMR analysis of the emulsions indicated essentially 100% incorporation of the carbohydrate acrylate monomer into the polymer with the exception of O-benzyl- and O-benzoyl-protected carbohydrate acrylates, which gave incomplete incorporation. Formation of larger glyconanoparticles of ~80nm with (unprotected) 3-O-acryloyl-D-glucose and 5-O-acryloyl-1-methoxy-β-D-ribofuranose revealed the influence of free hydroxyl groups in the monomer on the particle size during polymerization, a feature which is also apparently dependent on the amount of carbohydrate in the matrix. This methodology allows for a new, simple route to the synthesis of polymeric glyconanoparticles with potential applications in targeted drug delivery and materials development.
Keywords: Carbohydrate acrylates, Emulsion polymerization, Glyconanoparticles
1. Introduction
Polymers based on carbohydrate coatings and glycosidic frameworks offer exciting opportunities for biomaterials development and drug delivery applications. As potential biomaterials with the advantage of being biodegradable and biocompatible, polymers containing carbohydrate residues have been investigated in the field of controlled drug delivery systems (Garcia-Martin, Jimenez-Hidalgo, Al-Kass, Caraballo, de Paz & Galbis, 2000). Nanoparticles functionalized with carbohydrates constitute a good biomimetic model to intervene in carbohydrate-mediated biological processes. For this reason, glycosylated nanoparticles have been prepared and applied in a variety of biomedical applications. For instance, lactose-coated gold nanoparticles have been shown to be potential anti-adhesive tools in in vivo tumoral metastasis progression (Rojo, Diaz, de la Fuente, Segura, Barrientos, Riese, Bernad, & Penades 2004). The preparation of poly(γ-benzyl L-glutamate) (PBLG) or poly(lactic acid) (PLA) nanoparticles using a carbohydrate-carrying polystyrene (PS) amphiphilic polymer, which serves as both an emulsifier and a surface coating, has been reported as a liver-specific targeting material to deliver drugs (Cho, Jeong, Ishihara, Takei, Park, Park, Maruyama, & Akaike, 1997; Cho, Kobayashi, Takei, Ishihara, Maruyama, & Akaike, 2001). Nevertheless, much more work is needed, particularly in the synthetic area, to bring the field of glycosylated nanoparticles to further fruition and adaptability.
Recently, we reported the formation of antibacterially-active polyacrylate nanoparticles by emulsion polymerization using N-thiolated β-lactam acrylates as co-monomers in a soluble mixture of butyl acrylate-styrene. Upon radical-induced polymerization in water in the presence of a surfactant, nanoparticles are formed with a highly controlled particle diameter of about 40 nm (Turos, Shim, Wang, Greenhalgh, Reddy, Dickey, & Lim, 2006). In this paper, we describe an extension of this general emulsion polymerization procedure for the preparation of carbohydrate-bearing polyacrylate nanoparticles, using a carbohydrate acrylate as a co-monomer in ethyl acrylate (Scheme 1). For these exploratory studies, we examined as co-monomers a variety of structurally diverse monosaccharide acrylates derived from glucose, ribose, mannose, galactose and glucosamine. These compounds were synthesized and then subjected to emulsion polymerization conditions in the presence of sodium dodecyl sulfate (surfactant, 3 mol%) and potassium persulfate (radical initiator, 1 mol%) to prepare glycosylated nanoparticles directly in water. In screening these ten compounds, our objective was to ascertain whether different types of hydroxyl protecting groups on the monosaccharide may influence nanoparticle formation (efficiency, particle size), and whether unprotected (hydroxylated) monosaccharides themselves can be used directly in the polymerization process. The physicochemical properties of the resulting glycosylated polyacrylate nanoparticles, or glyconanoparticles, were examined using scanning electron microscopy (SEM), dynamic light scattering (DLS) and 1H NMR spectroscopy. As our results will show, emulsion polymerization of differentially O-protected monosaccharide monomers 1-5 showed no significant dependence on the nature of the monosaccharide or on the location of the acryloyl moiety. While the procedure seems quite general, certain hydroxyl protecting groups on the carbohydrate acrylate monomer interfered with efficient nanoparticle formation. However, the presence of free (unprotected) hydroxyl groups on the carbohydrate acrylate monomer had no deleterious effects, but did cause enlargement (doubling in size) of the nanoparticles. Thus, this study indicates that a variety of different acrylated monosaccharides can be employed as co-monomers for nanoparticle formation.
Scheme 1.
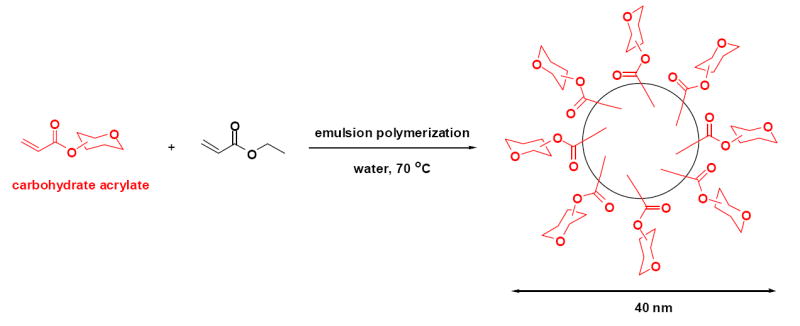
Synthesis of glyconanoparticles by emulsion polymerization
2. Experimental
2.1. Synthesis of requisite carbohydrate acrylate monomers 1-10
Solvents and chemicals were purchased from Acros Organics and used without further purification. 3-O-Acryloyl-1,2:5,6-di-O-isopropylidine-α-D-glucofuranose (1), 1-O-acryloyl-2,3:5,6-di-O-isopropylidene-α-D-mannofuranose (2) (Krishna, Kannan, Ilangovan, & Sharma, 2001), 6-O-acryloyl-1,2:3,4-di-O-isopropylidene-α-D-galactopyranose (3), 2-N-acryloyl-1,3,4,6-tetra-O-acetyl-β-D-glucosamine (4) (Garcia-Martin, Jimenez-Hidalgo, Al-Kass, Caraballo, de Paz & Galbis, 2000) and 4-N-acetyl-5’-O-acryloyl-2’,3’-O-isopropylidene cytidine (10) (Cooper, Goody, Jones, Tittensor, & Walker, 1971) were prepared following the cited literature procedures. 1-O-Acryloyl-2,3,5-tri-O-benzyl-β-D-ribofuranose (6) and 2-N-acryloyl-1,3,4,6-tetra-O-benzoyl-β-D-glucosamine (7) were prepared from 2,3,5-tri-O-benzyl-β-D-ribofuranose (Barker & Fletcher, 1961) and 1,3,4,6–tetra-O-benzoyl-β-D-glucosamine hydrochloride (McEvoy, Weiss, & Baker, 1960), respectively, following similar acrylation procedures described in the synthesis of 5-O-acryloyl-2,3-isopropylidene-1-methoxy-β-D-ribofuranose (5). 1H NMR and 13C NMR spectra were recorded on Bruker DPX 250 and Varian Inova 400 NMR spectrometers.
2.1.1. 5-O-Acryloyl-2,3-isopropylidene-1-methoxy-β-D-ribofuranose (5)
To a solution of methyl 2,3-isopropylidene-β-D-ribofuranose (Hanessian, 1997) (1.0 g, 4.9 mmol) in CH2Cl2 (5 mL) with triethylamine (1.0 mL, 7.3 mmol), acryloyl chloride (0.43 mL, 5.4 mmol) was added dropwise at 0 °C. The mixture was magnetically stirred for 30 min. at 0 °C and then for another 8 hrs at room temperature. The reaction mixture was treated with water (15 mL) and extracted into CH2Cl2 (3 × 25 mL). The combined organic layers were washed with water (2 × 50 mL), dried over anhydrous MgSO4 and evaporated under reduced pressure. The residue was purified by column chromatography (silica gel, hexane-EtOAc, 8.5:1.5) to afford the compound 5 (1.04 g, 83%) as a syrup. 1H NMR (250 MHz, CDCl3): δ 6.42 (d, 1H, J =16.3 Hz, olefinic), 6.10 (dd, 1H, J =10.3, 17.3 Hz, olefinic), 5.82 (d, 1H, J =10.3 Hz, olefinic), 4.92 (s, 1H, H-1), 4.63 (d, 1H, J =5.9 Hz, H-3), 4.55 (d, 1H, J =5.9 Hz, H-2), 4.35 (t, 1H, J=6.9 Hz, H-4), 4.11 (m, 2H, H-5,5’), 3.25 (s, 3H, OCH3), 1.42 (s, 3H, CH3), 1.26 (s, 3H, CH3); 13C NMR (250 MHz, CDCl3): δ 165.5, 131.3, 127.9, 112.4, 109.2, 85.0, 84.0, 81.7, 64.5, 54.7, 26.3, 24.8.
2.1.2. 5-O-Acryloyl-1-methoxy-β-D-ribofuranose (9)
5-O-Acryloyl-2,3-isopropylidene-1-methoxy-β-D-ribofuranose (0.50 g, 1.9 mmol) was dissolved in 80% acetic acid and stirred at 80 °C for 2 h. The solvent was then removed in vacuo and the residue was purified by column chromatography (silica gel, hexane-EtOAc, 1:6) to give compound 7 (0.28 g, 68%) as a syrup. 1H NMR (400 MHz, CDCl3): δ 6.41 (d, 1H, J =17.6 Hz, olefinic), 6.12 (dd, 1H, J =10.4,17.2 Hz, olefinic), 5.83 (d, 1H, J =10.8 Hz, olefinic), 4.79 (s, 1H, H-1), 4.40 (m, 1H), 4.15 (m, 3H), 3.97 (d, 1H, J=4.4 Hz), 3.59 (s, br, 2H, OH), 3.28 (s, 3H, OCH3);13C NMR (400 MHz, CDCl3): δ 166.1, 131.8, 128.2, 108.3, 80.9, 75.1, 72.1, 65.3, 55.2.
2.2. Glyconanoparticle preparation
Glyconanoparticles were prepared with a 9:1 (by weight) blend of ethyl acrylate and one of the monosaccharide acrylates (20% by weight) by emulsion polymerization. Briefly, ultrapure water and sodium dodecyl sulfate (SDS) were added to the mixture of monomers, and the mixture was pre-emulsified with stirring under a nitrogen atmosphere. A milky colored emulsion was prepared by adding potassium persulfate to the mixture and stirring at 70 °C for 6 h. The polymerization conditions are given in Table 1.
Table 1.
Formulation used for the emulsion polymerization
| Components | Amounts | Solid Content |
|---|---|---|
| Monosaccharide acrylate | 100 mg | 9.6% |
| Ethyl acrylate | 900 mg | 86.5% |
| Sodium dodecyl sulfate | 30 mg | 2.9% |
| Potassium persulfate | 10 mg | 1.0% |
| Water | 4 mL | |
| Reaction temperature | 70 ° C |
2.3. Glyconanoparticle characterization
2.3.1. Scanning Electron Microscopy (SEM) analysis
SEM measurements of the glyconanoparticles were captured on a Hitachi S800 SEM instrument. Samples were prepared by placing a drop of diluted emulsion (1 μL of emulsion in 30 mL of de-ionized distilled water) on the silicon wafer and evaporating the solvent by air blowing prior to coating with gold sputter under high vacuum.
2.3.2. Dynamic Light Scattering (DLS) analysis
DLS analyses were performed using a UPA 150 Honeywell MicroTrac DLS instrument equipped with a laser beam at 780 nm. The sample was diluted with de-ionized distilled water (0.05 mL of emulsion in 9.95 mL of water, polymer concentration 0.1% wt) and sonicated for 3 minutes for the analysis. The value is expressed in weight-averaged scales as unimode at a scattering angle of 180 ° (backscatter) at a temperature of 25 °C.
2.3.3. 1H NMR analysis
1H NMR spectroscopic analysis of each nanoparticle material was performed using a Bruker DPX-250 spectrometer. To prepare the sample, a film was formed by evaporation of 0.15 ml of the polymer emulsion spread on a clean glass surface. The film was dried at room temperature for 24 hrs, and then taken up (swelled) in CDCl3 to perform the NMR experiment.
3. Results and discussion
Although emulsion polymerization can be used for the synthesis of various types of nanoparticles, no reports have appeared describing the application of this technique for the preparation of glycosylated nanoparticle polymers.
The synthesis of these glycosylated polyacrylate nanoparticles by emulsion polymerization is quite simple, requiring no sophisticated equipment (magnetic stirrer) or techniques, and the emulsion is stable for months. Carbohydrate acrylates 1-5 (Figure 1) from various O-protected monosaccharides were chosen as the initial substrates for emulsion polymerization as a means to prepare differentially-glycosylated nanoparticles in water.
Figure 1.
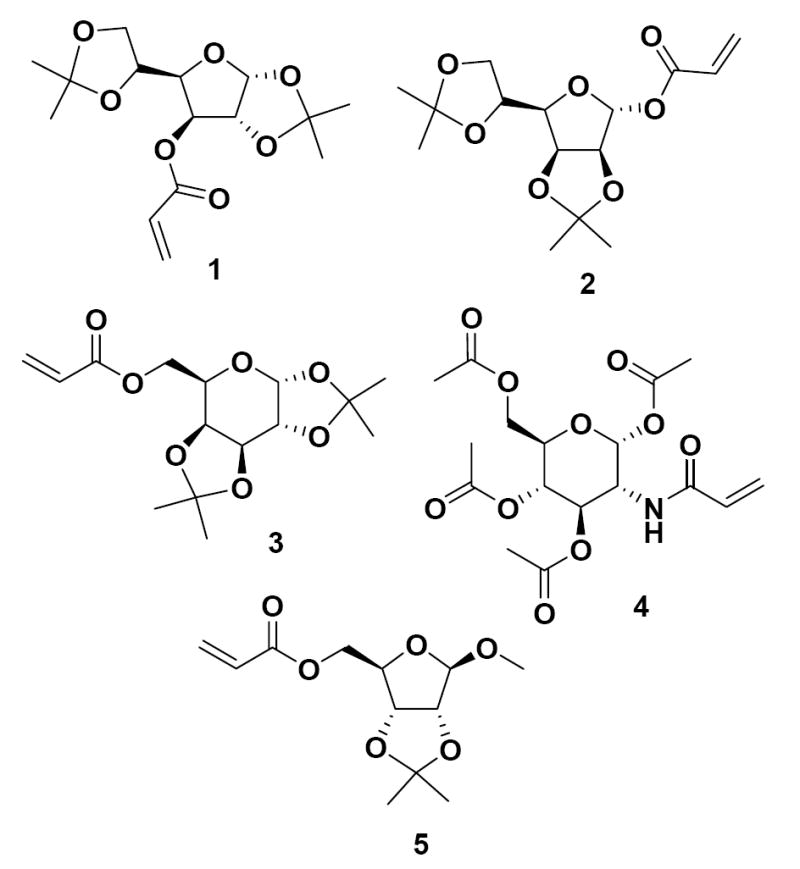
Carbohydrate monomers used for the formation of glyconanoparticles: 3-O-acryloyl-1,2:5,6-di-O-isopropylidene-α-D-glucofuranose (1), 1-O-acryloyl-2,3:5,6-di-O-isopropylidene-α-D-mannofuranose (2), 6-O-acryloyl-1,2:3,4-di-O-isopropylidene-α-D-galactopyranose (3), N-acryloyl-1,3,4,6-tetra-O-acetyl-β-D-glucosamine (4), and 5-O-acryloyl-1-methoxy-2,3-isopropylidene-β-D-ribofuranose (5).
3.1. Characterization of glyconanoparticles prepared with 3-O-acryloyl-1,2:5,6-di-O-isopropylidine-α-D-glucofuranose (1)
The morphology and the particle size of the emulsified nanoparticles were examined by scanning electron microscopy (SEM). The SEM image of glyconanoparticles prepared with 3-O-acryloyl-1,2:5,6-di-O-isopropylidene-α-D-glucofuranose (1) and ethyl acrylate (1:9 mass ratio) is shown in Figure 2a. As seen in the image, the nanoparticles were essentially perfectly spherical and measured from 20 to 70 nm in diameter, which was similar to the size distribution profile obtained by DLS analysis (Figure 2b).
Figure 2.
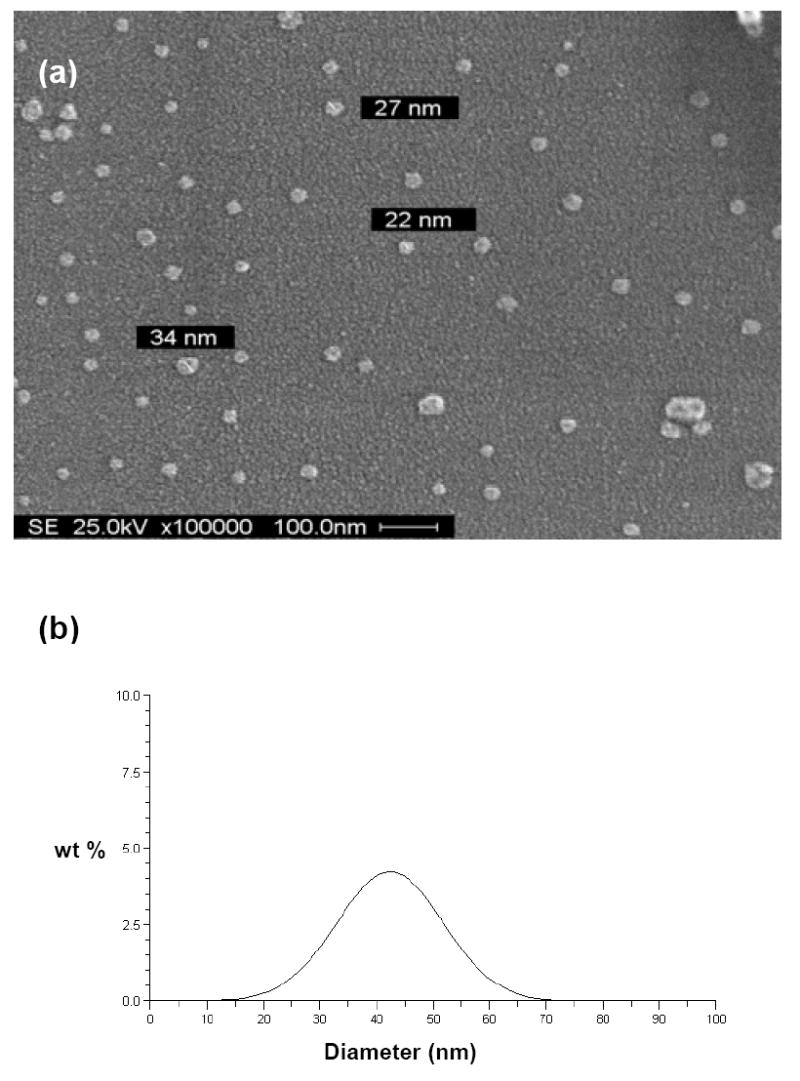
(a) SEM image and (b) particle size distribution of glyconanoparticles prepared from 3-O-acryloyl-1,2:5,6-di-O-isopropylidene-α-D-glucofuranose (1) and ethyl acrylate by emulsion polymerization.
The average diameter of the glyconanoparticles was 42 nm, with a statistical standard deviation of 9 nm. 1H NMR analysis was also preformed on the glycopolymer film obtained after evaporating the emulsion (Figure 3). Figure 3a shows the original 1H NMR spectrum of the carbohydrate acrylate co-monomer, 3-O-acryloyl-1,2:5,6-di-O-isopropylidene-α-D-glucofuranose (1), while Figure 3b shows the 1H NMR spectrum of the glycopolymer obtained after polymerization. Notably, the signals of the acryloyl protons (6.42-5.92 ppm in Figure 3a) have completely disappeared after the polymerization and broad absorptions for the protons H-1, H-3 and H-2 (5.90, 5.17 and 4.36 ppm in Figure 3b) of the monosaccharide moiety are plainly visible. This confirms the complete conversion of the carbohydrate monomer to polyacrylate within the nanoparticle matrix, without any detectible amount of residual unreacted monomer.
Figure 3.
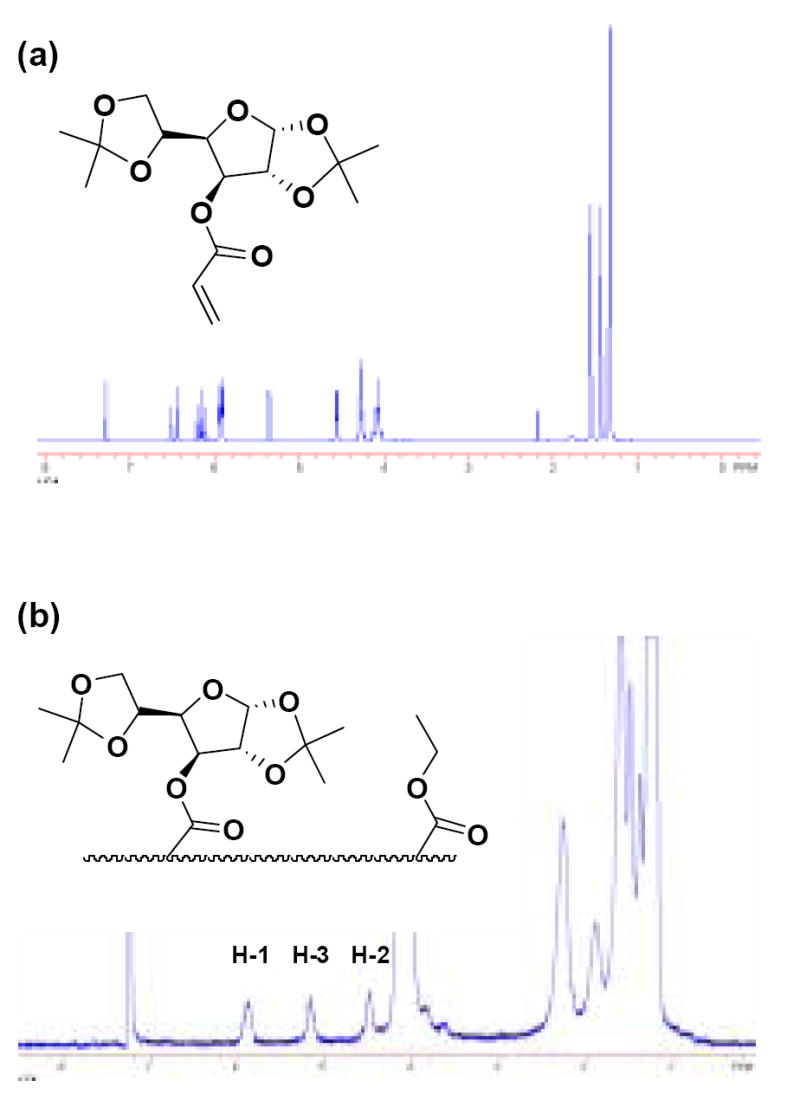
Typical 1H NMR spectra of (a) 3-O-acryloyl-1,2:5,6-di-O-isopropylidine-α-D-glucofuranose (1) and (b) the glycopolymer obtained from the emulsion after the film formation.
3.2. Influence of bulky aryl protecting groups in the carbohydrate acrylate monomer on glyconanoparticle preparation
Upon the successful execution of the radical emulsion polymerization process using protected manosaccharides 1-5, we extended these studies to two other O-protected carbohydrate monomers, O-benzyl and O-benzoyl protected monosaccharide acrylates 6 and 7 (Figure 4). To our surprise, 1H NMR analysis revealed that the use of these monomers was not as successful in the preparation of glyconanoparticles, as significant amounts of the unreacted carbohydrate acrylates remained after polymerization. The reasons for this outcome are not obvious, but the presence of these bulky aryl protecting groups on the sugar monomer may affect its reactivity or radical transfer capabilities, and thus induce low incorporation into the polymer backbone.
Figure 4.
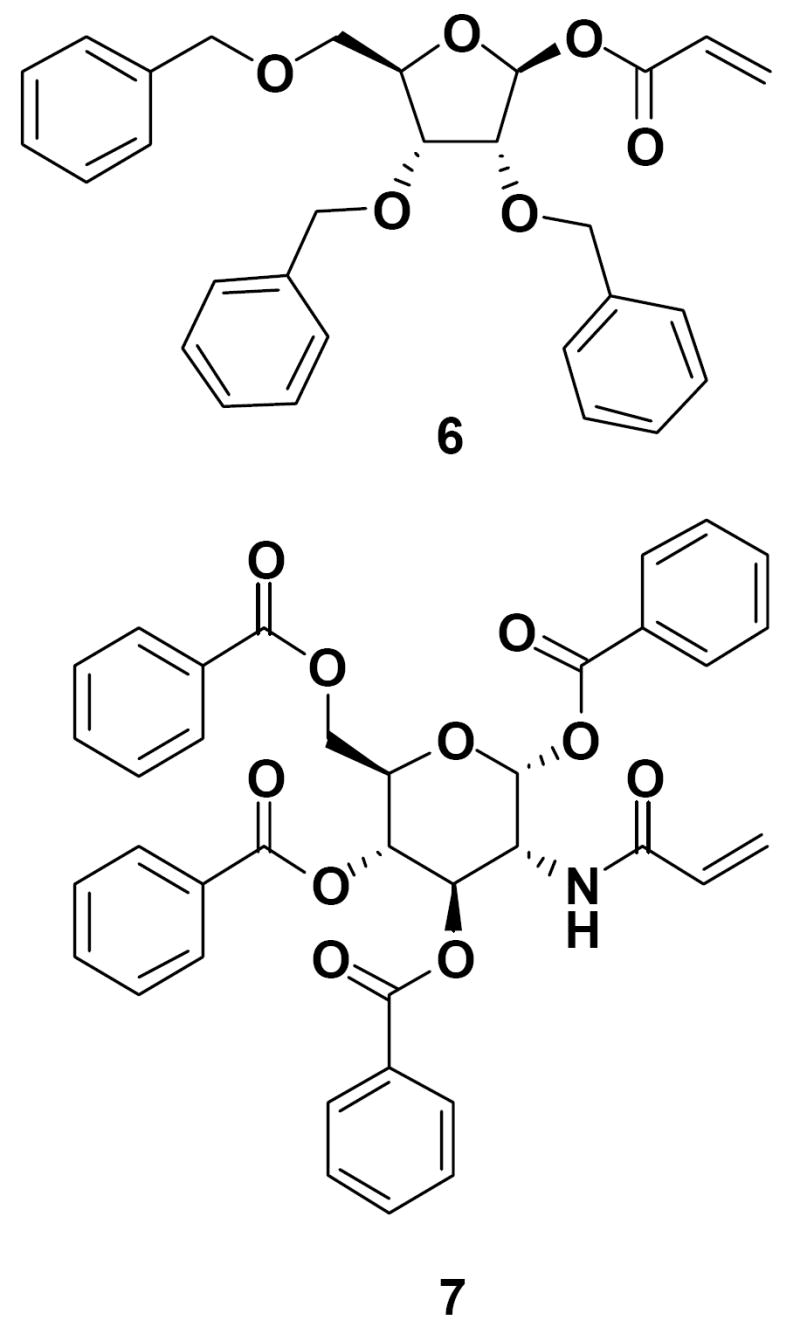
1-O-Acryloyl-2,3,5-tri-O-benzyl-β-D-ribofuranose (6) and 2-N-acryloyl-1,3,4,6-tetra-O-benzoyl-β-D-glucosamine (7).
3.3. Influence of free hydroxyl groups of the monosaccharide acrylate monomer on the formation of glyconanoparticles
In direct follow-up to this last set of experiments, we decided to investigate whether a carbohydrate acrylate having free (unprotected) hydroxyl groups could be used directly for the nanoparticle formation. This was first studied with 3-O-acryloyl-D-glucose (8) (Figure 5), the deprotected form of acrylate 1 prepared by treating compound 1 with 90% trifluoroacetic acid. The emulsion prepared with 8 and ethyl acrylate as a co-monomer appeared much less transparent to the eye than the emulsion prepared with the relevant protected monomer, 3-O-acryloyl-1,2:5,6-di-O-isopropylidene-α-D-glucofuranose (1), using the same formulation. In fact, DLS analysis of the emulsion indicated the average particle size was around 85 nm in diameter, about double the size of the particles prepared from the O-protected acrylate 1. We obtained similar results with the emulsions prepared with 5-O-acryloyl-1-methoxy-β-D-ribofuranose (9) (Figure 5) versus 5-O-acryloyl-2,3-O-isopropylidene-1-methoxy-β-D-ribofuranose (5).
Figure 5.
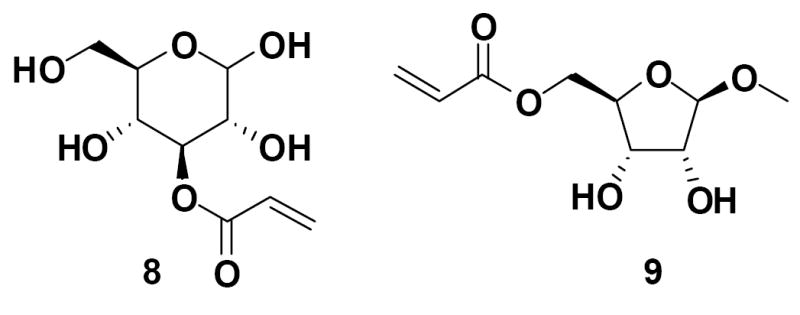
3-O-Acryloyl-D-glucose (8) and 5-O-acryloyl-1-methoxy-β-D-ribofuranose (9)
The photographic images in Fig. 6a show the increasing turbidity of the emulsions as the concentration of acrylate monomer 9 is increased 5% (E2) to 7.5% (E3) to 10% (E4) of the monomer content, relative to the appearance of the emulsion E1 prepared with fully protected monomer 5 (having 10% carbohydrate by weight relative to ethyl acrylate). Dynamic light scattering data confirms that this increasing cloudiness in the emulsions is due to larger glyconanoparticle formation (Fig. 6b) as the amount of unprotected carbohydrate monomer is increased from 5% (E2) to 10% (E4). This suggests that nanoparticle size may be dependent on the presence and the degree of hydrogen-bonding effects within the carbohydrate moiety while the polymeric matrix is being formed. Also, the extension of this preparation to nucleoside acrylates, such as for acrylated cytosine analogue 10 (Figure 7), may open potential avenues to using these nanoparticles as nucleic acid transporters.
Figure 6.
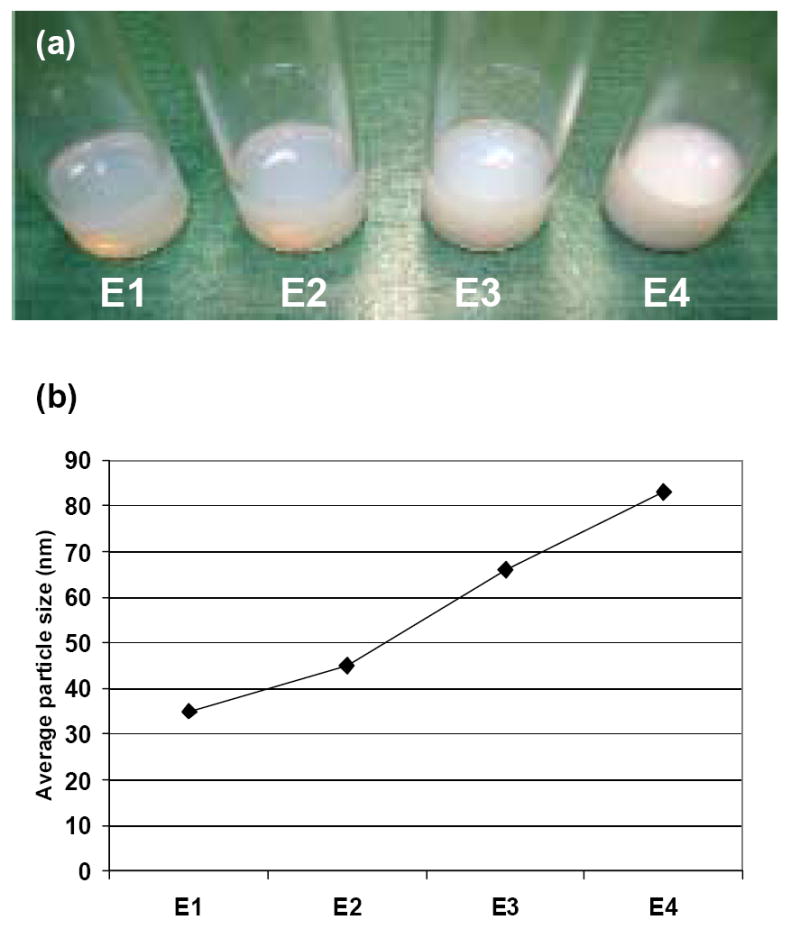
Comparison of the effects of using an O-protected versus O-unprotected monosaccharide acrylate on nanoparticle size. (a) Photographs of four different emulsion samples prepared with 20% (by weight) polymer content from 5-O-acryloyl-2,3-O-isopropylidene-1-methoxy-β-D-ribofuranose (5) and 5-O-acryloyl-1-methoxy-β-D-ribofuranose (9) as co-monomers with ethyl acrylate. E1: monomer 5 and ethyl acrylate (1:9 by weight). E2: monomer 9 and ethyl acrylate (1:19 by weight). E3: monomer 9 and ethyl acrylate (1:14 by weight). E4: monomer 9 and ethyl acrylate (1:9 by weight); (b) Average particle sizes of these formulations from DLS analysis.
Figure 7.
4-N-Acetyl-5’-O-acryloyl-2’,3’-O-isopropyl-idenecytidine (10)
4. Conclusions
In summary, we have demonstrated the preparation of polyacrylate nanoparticles bearing carbohydrates within the polymeric framework by emulsion polymerization. The glyconanoparticles displayed narrow size distributions in the range of 20-70 nm. Acetonide and acetyl protected monosaccharide acrylates were found to be well suited for this preparation, and an increase of the particle size was observed upon use of 3-O-acryloyl-D-glucose (8) and 5-O-acryloyl-1-methoxy-β-D-ribofuranose (9) as monomers bearing free hydroxyl groups. Current efforts in our laboratory are exploring the potential use of these glyconanoparticles as drug delivery vehicles for various antibiotics, and as precursors to the synthesis of new functionalized nanoparticles for applications in materials research. The results from these ongoing studies will be described in due course.
Acknowledgments
We thank Gil Brubaker (University of Florida Particle Engineering Research Center) for providing assistance with the particle analyses, and Dr. Jeung-Yeop Shim for help with SEM imaging. Financial support from the National Institutes of Heath (Grant AI05135) is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barker R, Fletcher HG. 2,3,5-Tri-O-benzyl-D-ribosyl and -L-arabinosyl bromides. J Org Chem. 1961;26:4605–4609. [Google Scholar]
- Cho CS, Jeong YI, Ishihara T, Takei R, Park JU, Park KH, Maruyama A, Akaike T. Simple preparation of nanoparticles coated with carbohydrate-carrying polymers. Biomaterials. 1997;18:323–326. doi: 10.1016/s0142-9612(96)00138-x. [DOI] [PubMed] [Google Scholar]
- Cho CS, Kobayashi A, Takei R, Ishihara T, Maruyama A, Akaike T. Receptor-mediated cell modulator delivery to hepatocyte using nanoparticles coated with carbohydrate-carrying polymers. Biomaterials. 2001;22:45–51. doi: 10.1016/s0142-9612(00)00143-5. [DOI] [PubMed] [Google Scholar]
- Cooper MJ, Goody RS, Jones AS, Tittensor JR, Walker RT. Synthetic analogues of polynucleotides. Part VII. Further syntheses of 5’-O-acryloylnucleosides and copolymers of these with other acryloyl compounds. J Chem Soc C. 1971;19:3183–3187. [Google Scholar]
- Garcia-Martin MG, Jimenez-Hidalgo C, Al-Kass SSJ, Caraballo I, de Paz MV, Galbis JA. Synthesis and characterization of some new homo- and co-poly(vinylsaccharides). Some preliminary studies as drug delivery. Polymer. 2000;41:821–826. [Google Scholar]
- Hanessian S. Preparative carbohydrate chemistry. Marcel Dekker, Inc.: New York; 1997. p. 16. [Google Scholar]
- Krishna PR, Kannan V, Ilangovan A, Sharma GVM. Asymmetric Baylis-Hillman reaction using sugar acrylates-synthesis of optically active α-methylene-β-hydroxyalkanoates. Tetrahedron: Asymmetry. 2001;12:829–837. [Google Scholar]
- McEvoy FJ, Weiss MJ, Baker BR. The synthesis of 9-(2-amino-2-deoxy-β-D-allopyranosyl)-6-dimethylaminopurine, an analog of the aminonucleoside derived from puromycin. J Am Chem Soc. 1960;82:205–209. [Google Scholar]
- Rojo J, Diaz V, de la Fuente JM, Segura I, Barrientos AG, Riese HH, Bernad A, Penades S. Gold glyconanoparticles as new tools in antiadhesive therapy. Chem Bio Chem. 2004;5:291–297. doi: 10.1002/cbic.200300726. [DOI] [PubMed] [Google Scholar]
- Turos E, Shim YJ, Wang Y, Greenhalgh K, Reddy GSK, Dickey S, Lim DV. Antibiotic-conjugated polyacrylate nanoparticles: New opportunities for development of anti-MRSA agents. Bioorg Med Chem Lett. 2006 doi: 10.1016/j.bmcl.2006.09.098. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]


