SUMMARY
The anti-tumorigenic activity of anti-oxidants has been presumed to arise from their ability to squelch DNA damage and genomic instability mediated by reactive oxygen species (ROS). Here we report that anti-oxidants inhibited three tumorigenic models in vivo. Inhibition of a MYC-dependent human B lymphoma model was unassociated with genomic instability, but was linked to diminished hypoxia inducible factor (HIF)-1 levels in a prolyl hydroxylase 2 and von Hippel-Lindau protein dependent manner. Ectopic expression of an oxygen-independent, stabilized HIF-1 mutant rescued lymphoma xenografts from inhibition by two anti-oxidants, N-acetylcysteine and vitamin C. These findings challenge the paradigm that anti-oxidants diminish tumorigenesis primarily through decreasing DNA damage and mutations and provide significant support for a key anti-tumorigenic effect of diminishing HIF levels.
SIGNIFICANCE
The potential anti-tumorigenic effects of anti-oxidants have been the driving force for large chemoprevention clinical trials as well as multiple studies in preclinical tumor models. Although the effect of anti-oxidants has been presumed mediated through squelching of reactive oxygen species and reducing genomic instability, the findings of this study indicate that the anti-tumorigenic effect of anti-oxidants is dependent on the hypoxia inducible factor, HIF-1. Our observations provide mechanistic insights and suggest a therapeutic role for anti-oxidants against HIF-1 dependent tumors.
INTRODUCTION
Tumorigenesis resulting from multi-step activation of oncogenes, loss of tumor suppressors, and adaptation to the tumor microenvironment is thought to be in part driven by genomic instability resulting from oxidative DNA damage. In fact, a number of animal models suggest that anti-oxidants could diminish tumorigenesis by suppressing DNA damage and genomic instability. For example, loss of ATM accelerates transgenic murine lymphomagenesis in a fashion that is suppressible by N-acetylcysteine (NAC), a well-established anti-oxidant that had been previously shown to diminish oxidative DNA modifications (Aitio, 2006; Reliene et al., 2004; Reliene and Schiestl, 2006). This claim has been bolstered by a study of a p53-dependent mouse lymphomagenesis model in which N-acetylcysteine (NAC) slowed tumor progression seemingly by reducing genomic instability (Sablina et al., 2005). Furthermore, NAC has been used clinically in chemoprevention trials with the assumption that it could reduce oxidative stress, which would contribute to genomic instability and tumorigenesis. In fact, oral NAC 1800 mg daily could be given to human subjects safely with associated measurable reduction in markers of oxidation (Mantovani et al., 2003). Disappointingly, however, NAC at 600 mg daily over 2 years did not appear to have a measurable impact in patients with head and neck or lung cancer (Aitio, 2006). Notwithstanding this specific outcome with NAC, antioxidants continue to be of key interest for chemoprevention (Narayanan, 2006). The combination of tumorigenic animal models that respond to anti-oxidants, the effects of anti-oxidants on oxidative DNA damage, the contribution of DNA damage to genomic instability, and the contribution of genomic instability to tumorigenesis have resulted in the paradigm that anti-oxidants could diminish tumorigenesis by decreasing oxidative DNA damage and genomic instability. This notion in part originated from the initial claim that vitamin C is an effective cancer therapeutic in the late 1970’s (Cameron et al., 1979), but experimental mechanistic evidence for the anti-tumorigenic effects of anti-oxidants have not been well-established. We have also observed that both NAC and vitamin C have significant anti-tumorigenic effects in vivo, but in stark contrast to conclusions made from earlier studies, we found that their effects are highly dependent on hypoxia inducible factor 1 (HIF-1) rather than on reduction of genomic instability in a MYC-dependent lymphoma model.
Since Myc has also been tightly linked to generation of ROS, promotion of genomic instability, and tumorigenesis (Vafa et al., 2002), we sought to determine whether NAC could diminish tumorigenesis in two MYC-dependent models and one MYC-independent model. We sought to determine the potential role of ROS in Myc-induced tumorigenesis and genomic instability and found that the anti-oxidant N-acetylcysteine (NAC) could dramatically diminish tumorigenesis of P493 human B cell xenografts in SCID mice as well as inhibiting a Myc-dependent transgenic model of hepatocellular carcinoma. We had expected that tumors arising in the xenografts would display genomic instability as seen in the transgenic murine lymphoma models, but found through the use of spectral karyotyping and 300K SNP genotyping that very few changes could be detected at this level of resolution. These findings suggest that the anti-tumorigenic effects of NAC are not due to a diminution of genomic instability and led us to seek another mechanism. Because of the dramatic phenotypic effects of NAC, we sought to determine whether tumor adaptation to the microenvironment could be affected since the stability of HIF-1 is dependent on ROS metabolism.
The activation of HIF-1, a transcription factor that is stabilized in response to hypoxia, significantly contributes to the induction of VEGF for angiogenesis and the conversion of glucose to lactate for tumor glucose metabolism (17). HIF-1 consists of an oxygen sensitive HIF-1α subunit that heterodimerizes with the HIF-1β subunit to bind DNA. In high oxygen tension, HIF-1α is hydroxylated by prolyl hydroxylases (PHDs) using α-ketoglutarate derived from the Krebs cycle. The hydroxylated HIF-1α subunit is ubiquitylated by the von Hippel Lindau protein, VHL, and destined for degradation by proteasomes, such that HIF-1α is continuously synthesized and degraded under non-hypoxic conditions (Semenza, 2003). In hypoxia HIF-1α remains unhydroxylated, thereby stabilizing HIF-1. HIF-1 in turn directly transactivates glycolytic enzyme genes and VEGF. Hence, adaptation to the hypoxic tumor microenvironment results in increased glucose uptake and lactate production and angiogenesis.
There are several isoforms of PHDs with PHD2 playing the most critical role in hydroxylating HIF-1α (Berra et al., 2003; Brahimi-Horn et al., 2007). Over the last several years the connection between mitochondrial ROS production, hypoxia and HIF-1 stabilization has emerged. In contrast to expectation, hypoxia or limited oxygen increases mitochondrial ROS rather diminishing it (Kaelin, 2005b). ROS, in turn, inactivates PHD2 and hence stabilizes HIF-1α. ROS inactivates PHDs through oxidation of the ferrous ion that is central and essential for the catalytic hydroxylation of prolines. Vitamin C has been shown to decrease HIF-1 levels by preventing the oxidation of the catalytic ferrous ion (Lu et al., 2005). Whether the anti-tumorigenic effects of vitamin C and NAC are attributable to the prevention of genomic instability or adaptation to the tumor microenvironment has not been established (Cameron et al., 1979; Pauling et al., 1985). We report here substantial evidence favoring the latter, that the anti-tumorigenic effects of NAC and vitamin C in Myc-mediated tumorigenesis are indeed HIF-1 dependent. These unexpected findings are likely to contribute significantly to our understanding of the role of anti-oxidants in tumorigenesis and perhaps insight into novel adjuvant therapies.
RESULTS and DISCUSSION
We used the human P493 B cells that conditionally express MYC upon removal of tetracycline or doxycycline (DOX) (Supplemental Figure S1A) in a xenograft model in SCID mice (Schuhmacher et al., 1999). NAC treatment administered in the drinking water dramatically decreased lymphomagenesis, which was suppressed by doxycycline-induced repression of MYC (Figure 1 A and 1B). In contrast to tetracycline, NAC neither inhibits the expression of Myc protein nor the growth of P493 cells in vitro (Supplemental Figure S1B and data not shown).
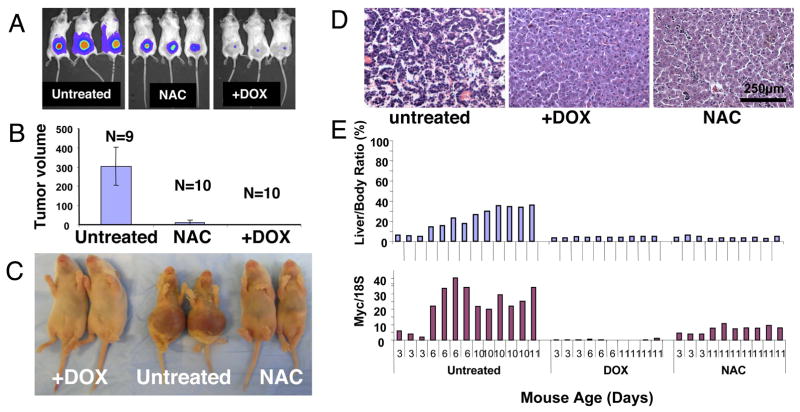
Figure 1.
A. Representative human P493 B cell tumors with tetracycline repressible MYC marked with luciferase were imaged via a Xenogen instrument at day 10. Animals received normal water (Untreated) or water containing 40 mM N-acetylcysteine (NAC) or 0.01% (w/v) doxycycline (+DOX). B. Tumor volumes (Mean ±SD) measured by caliper at 2 weeks of a cohort of animals, including ones shown in 1A. Ten animals treated with both NAC and DOX displayed no tumors (data not shown). C. Representative MYC-inducible neonatal hepatocellular carcinomas in the absence of doxycycline (Untreated) are evident through gross hepatomegaly, which was not observed in either doxycycline (+DOX) or NAC treated 11 day-old animals. D. Representative hematoxylin and eosin stained histological sections of livers from untreated, DOX or NAC treated 8-day old neonates (originally 20x magnification). E. At least 10 animals in each untreated, doxycycline (DOX) or NAC treated groups are shown with the corresponding liver to body mass ratio (upper panel) or Myc mRNA level (lower panel). Mouse age is indicated in the bottom of the lower panel.
We also used TRE-LAP MYC transgenic mice which over-express MYC in liver cells upon removal of DOX from the drinking water (Beer et al., 2004). As reported previously, we observed MYC overexpression and hepatocellular carcinoma (HCC) formation in neonates from untreated pregnant females. Neonates at 6 days of age demonstrated HCC that occupied the entire liver, whereas neonates from NAC treated pregnant females did not develop HCC by 11 days, when all untreated transgenic animals had succumbed to HCC (Figure 1C and 1D). In this model, the MYC transgene is induced nearly 10-fold in non-tumorous livers above doxycycline-treated controls; however, MYC expression is further accentuated in tumor cells through an unknown mechanism (Beer et al., 2004). The livers of day 6 and older untreated neonates have greater MYC expression (Figure 1E) because their livers were infiltrated with tumor cells that have accentuated MYC expression as compared with lower MYC expression in the NAC-treated non-tumorous livers. It is notable that at neonatal day 3, when the livers have not developed tumors, NAC does not affect the expression of MYC while DOX treatment abolished MYC expression (Figure 1E). Hence, NAC neither directly affected the expression of MYC in the human P493 B cells (Supplemental Figure S1B) nor in the MYC-dependent liver cancer model.
From the two models, which both displayed remarkable responses to NAC treatment, we selected the experimentally more tractable human P493 B cells to delineate the mechanism of NAC action. We first determined whether genomic instability is involved in P493 lymphomagenesis and tested the hypothesis that Myc induction of ROS and DNA damage (Supplemental Figure S1A and S1C) would contribute to chromosomal instability and tumor progression in vivo as seen in murine models (Karlsson et al., 2003; Limoli et al., 2003; Louis et al., 2005; Sander et al., 2005). We note that NAC diminishes MYC-induction of ROS production and phosphorylated histone H2AX, which serves as a surrogate marker of transient DNA damage (Supplemental Figure S1B and S1C). We compared the genomes of B cells grown in vitro with those recovered from the xenografts. We found no significant genomic instability through spectral karyotyping (Supplemental Figure S1D), high density SNP analysis and array comparative genomic hybridization (Supplemental Figures S1E and S1F), implying that the effect of NAC could not be attributed to diminished chromosomal instability. In fact, although p53 was implicated in ROS-induced genomic instability, a recent study suggests that p53-mediated tumor suppression is independent of its ability to guard the genome (Christophorou et al., 2006). Moreover, the low level of chromosomal instability of our in vivo human B cell tumor model (see Supplemental Information) is consistent with the low level of genomic changes in primary human Burkitt’s lymphomas, which are characterized by chromosomal translocations involving MYC, as compared with diffuse large B cell lymphomas (Hummel et al., 2006).
Earlier studies have suggested that ROS levels are essential for the stability of HIF-1, which has emerged at the crossroads of many tumorigenic pathways via its ability to regulate genes involved in angiogenesis, glycolysis and mitochondrial function (Brunelle et al., 2005; Covello and Simon, 2004; Giaccia et al., 2003; Guzy et al., 2005; Kaelin, 2005b; Pouyssegur et al., 2006; Semenza, 2003; Zhang et al., 2007). Hence, we reasoned that NAC, an ROS scavenger, could possibly inhibit HIF-1 activity by neutralizing ROS and thereby attenuate tumorigenesis. HIF-1 is a heterodimeric transcription factor composed of HIF-1β and HIF-1α. Proline residues 402 and 564 are hydroxylated by prolyl hydroxylase (PHD), a modification that marks HIF-1α for recognition by the von Hippel-Lindau (VHL) protein, ubiquitination and subsequent degradation by proteasomes (Kaelin, 2005a). Similar to the anti-oxidant ascorbate (Vitamin C), which was reported to decrease HIF-1α levels by maintaining the catalytic ferrous ion of PHD in the reduced active state, we hypothesize that NAC might also activate PHDs thereby decreasing HIF-1α levels (Knowles et al., 2003; Lu et al., 2005).
To test whether NAC could decrease HIF-1α, we studied human P493 B cells, which lack HIF-2α expression (Supplemental Figure S2). Treatment of hypoxic (1% oxygen) P493 cells with NAC diminished the level of HIF-1α protein (Figure 2A). The level of VEGF, a target of HIF-1 regulation, was also significantly diminished by NAC in four independent experiments (Figure 2C). The effect of NAC on HIF-1α levels was also observed in the human prostate carcinoma cell line PC3, which has been engineered to contain a GFP reporter responsive to HIF-1 through hypoxia response elements (Figures 2D and 2E) (Raman et al., 2006). Both HIF-1α level and the reporter activity were diminished by NAC, indicating that the effects are not restricted to human B cells. Since NAC did not affect HIF-1α mRNA levels in P493 cells (Figure 2B), we hypothesize that NAC induces HIF-1α degradation as previously observed with ascorbate (Lu et al., 2005).
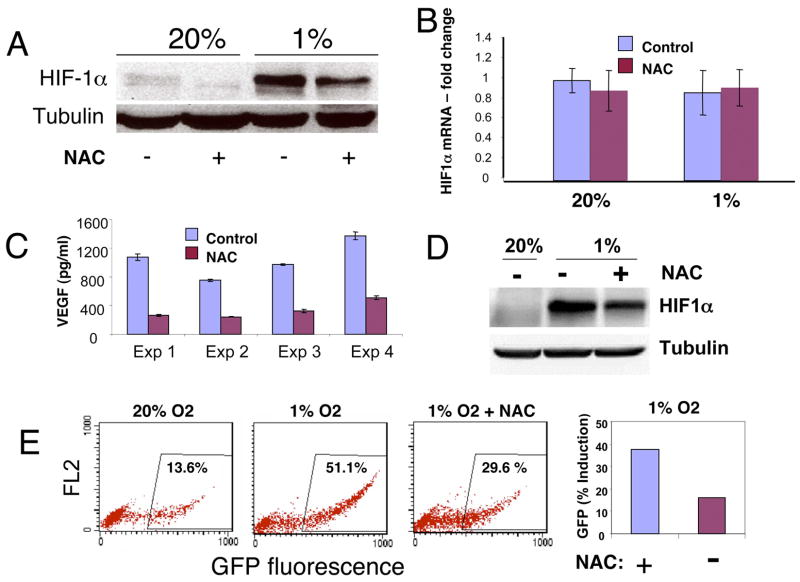
Figure 2.
A. Immunoblots of HIF-1α protein levels in human P493 B cells grown in 20% or 1% oxygen in the presence or absence of 10 mM N-acetylcysteine (NAC) and the tubulin loading control are shown. Cells were treated with NAC or subjected to hypoxia for 24 hr before analysis. B. NAC (10 mM) treatment for 24 hr does not affect HIF-1α mRNA levels (Mean ± SD) in P493 cells. C. VEGF secreted into conditioned P493 media is markedly diminished by NAC, which does not directly inhibit the VEGF immunoassay in vitro. ELISA results (Mean ± SD) for duplicate measurements from four independent experiments are shown. D. Immunoblot of HIF-1α induced in hypoxic (1% oxygen for 8 hours) human prostate carcinoma PC3 cell line bearing an HRE-driven green fluorescent protein (GFP) reporter demonstrates the inhibitory effect of NAC on HIF-1α level. E. Flow cytometric analysis of GFP expression demonstrate induction of the HRE-responsive reporter in 1% oxygen that is diminished by 10 mM NAC, illustrating the functional effect of NAC on HIF-1α level and the reporter in the same cell line. Left panel. Dot plots represented by FL.1 and FL.2 parameters indicate the negative and positive PC3 cells expressing GFP. FL.1 represents a channel over the GFP emission spectrum peak, whereas FL.2 is a channel set over higher wavelengths providing an irrelevant parameter used to complete a display of the data in 2 dimensions. Right panel. Percent GFP fluorescence detected under 1% hypoxia over that at 20% oxygen in the presence or absence of 10 mM NAC.
Because HIF-1α levels are regulated by PHDs that mark HIF-1α for recognition by VHL and target it for proteasomal degradation, we sought to determine whether the effects of NAC on HIF-1α levels are dependent on PHDs and VHL. First, we examined the effects of NAC on a HIF-1α mutant, CA5, which has been rendered resistant to proteasomal degradation via deletion of part of the oxygen-dependent domain of HIF-1α and mutation of specific prolyl residues (Kelly et al., 2003). When P493 cells that ectopically expressed CA5 were treated with NAC, under hypoxic conditions, the levels of endogenous HIF-1α levels were reduced whereas CA5 levels were unaffected (Figure 3A).
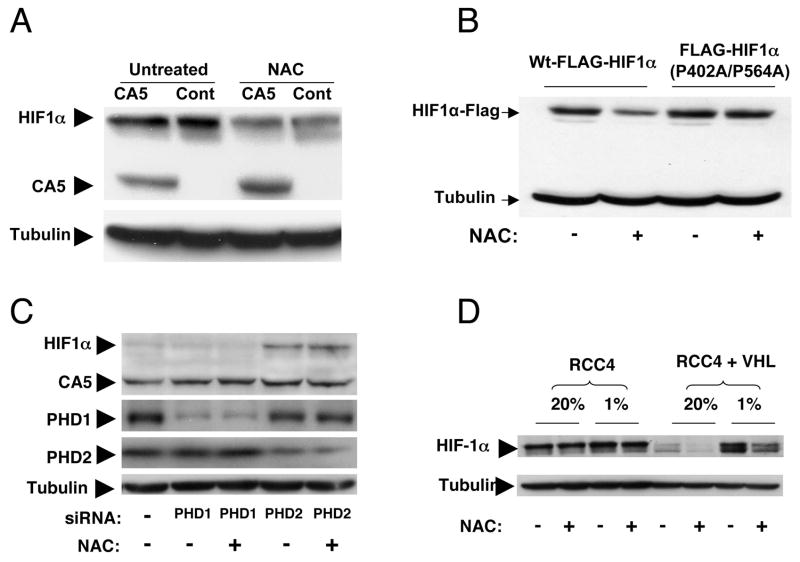
Figure 3.
A. Immunoblot of endogenous HIF-1α and the HIF-1 CA5 mutant in CA5 or control (Cont) hypoxic P493 cells untreated (Untreated) or treated with 10 mM N-acetylcysteine (NAC). Tubulin serves as a sample loading control. B. P493-6 cells were transfected with expression vectors encoding wild-type FLAG-HIF-1α or FLAG-HIF-1α (P402A/P564A) by using the Amaxa Nucleofector System. After 24h, cells were cultured with or without 10 mM NAC under 1% O2 for an additional 24h. Cells were then lysed and immunoblotted with anti-FLAG antibody. Tubulin was also immunoblotted in the same membrane as a loading control. C. The P493 cells overexpressing CA5 were untreated or treated with siRNAs against prolyl hydroxylases PHD1 or PHD2. Immunoblots of HIF-1 (HIF-1α and CA5), PHD1, PHD2 and tubulin are shown. Note that CA5 is constitutively expressed while endogenous HIF-1α is expressed only in PHD2 siRNA treated cells in a manner that is not affected by NAC. D. RCC4 cells lacking VHL or reconstituted with VHL (RCC4 + VHL) were grown in 20% or 1% oxygen in the presence or absence of 10 mM NAC.
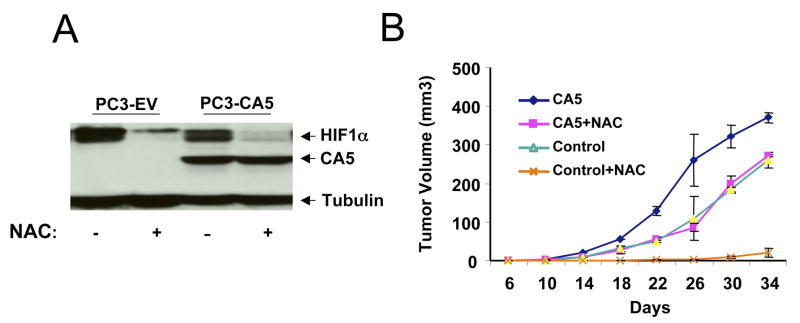
To determine the role of prolyl hydroxylation in the sensitivity of HIF-1α to NAC, we tested a FLAG-tagged HIF-1α with prolyl mutations P402A and P564A in comparison to the tagged wild-type HIF-1α (Liu et al., 2007). As shown in Figure 3B, the HIF-1α P402A/P564A mutant was resistant, while the wild-type HIF-1α was sensitive to NAC treatment. Furthermore, cells were transfected with siRNA targeting either PHD1 or PHD2. The expression of HIF-1α was increased in PHD2 siRNA treated cells but not affected in PHD1 siRNA treated cells under non-hypoxic condition (Figure 3C and Supplemental Figure S3), confirming earlier reports that PHD2 but not PHD1 is involved in HIF-1α degradation (Berra et al., 2003). Importantly, NAC did not decrease the expression of HIF-1α in PHD2 knock-down cells, indicating that NAC effect is dependent on PHD2. We also examined the effect of NAC on HIF-1α levels in the RCC4 renal carcinoma cell line that lacks VHL and a derivative line with reconstituted VHL expression (Figure 3D) (Hu et al., 2003). While the constitutively elevated level of HIF-1α in the VHL-deficient parental RCC4 cells was not sensitive to NAC, hypoxia-induced HIF-1α (and HIF2α; see Supplemental Figure S2) levels in the VHL-reconstituted RCC4 cells was fully sensitive to NAC treatment. These observations support the hypothesis that NAC diminishes HIF-1α levels by stimulating HIF-1α degradation in a PHD2 and VHL dependent manner.
To determine whether the anti-tumorigenic effect of NAC in vivo is HIF-1α dependent, we tested the tumorigenic potential of P493 cells ectopically expressing the stabilized HIF-1α mutant, CA5 (Kelly et al., 2003). As compared with control transduced P493 cells, the CA5 expressing cells display a significant degree of resistance to NAC treatment with only a slight decrease in tumor size (Figure 4A and 4B), which may be due to effects of NAC on tumor stromal cells that do not express CA5. The resistance of the CA5 tumors to NAC is not due to the inability of NAC to permeate the tumors, since the levels of endogenous HIF-1α in the tumors are dramatically reduced by NAC treatment (Figure 4C). In addition to the effects of NAC, ascorbate also effectively inhibited P493 lymphomagenesis whereas P493 cells overexpressing CA5 showed significant resistance to the effects of ascorbate (Figure 5).
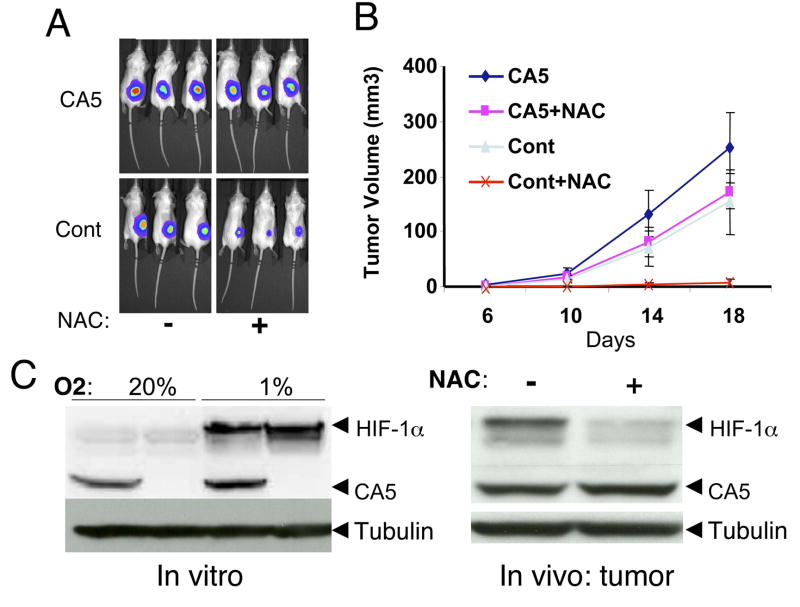
Figure 4.
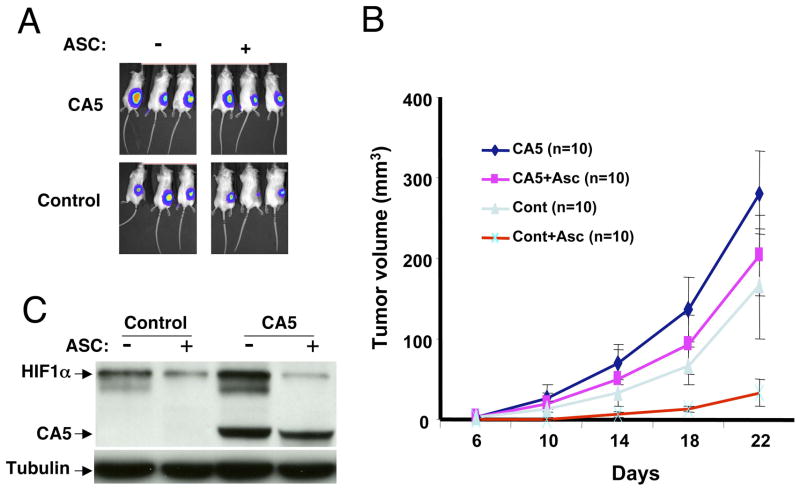
A. In vivo bioluminescent images at day 10 of representative CA5 overexpressing or control (Cont) P493 B tumors untreated (−) or treated (+) with NAC (40mM in drinking water). B. Volumes measured by caliper of CA5 overexpressing or control P493 B tumors untreated or treated with NAC (n = 10 for each group; mean ±SEM) are shown. C. Left panel. Immunoblot of HIF-1 demonstrates the hypoxic induction of endogenous HIF-1α in both control and CA5 overexpressing cells as compared with the constitutive expression of CA5. Right panel. Tumor cells in xenografts recovered from control (−) or NAC treated (+) mice display constitutive CA5 expression along with NAC-sensitive endogenous HIF-1α expression.
Figure 5.
A. Representative in vivo bioluminescent images of SCID mice xenografts of P493 cells overexpressing the stabilized HIF-1 CA5 mutant or control transduced P493 cells. Animals were untreated or treated with ascorbate (ASC) (5g/liter in drinking water). B. Time-dependent tumor volumes determined by caliper in 4 groups of mice injected with P493 cells overexpressing CA5 or control cells and either untreated or treated with ascorbate (ASC). Data are shown as mean ± SEM. C. Immunoblot of HIF-1α in control P493 cells or P493 cells expressing CA5 untreated or treated with ascorbate (ASC). Endogenous HIF-1α is shown with ectopic HIF CA5 mutant.
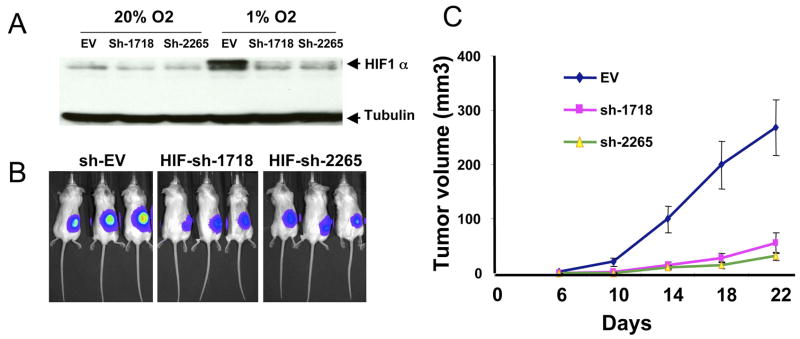
To determine the necessity of HIF-1α for P493 tumorigenesis, we used shRNA to reduce HIF-1α levels and found that tumorigenesis is profoundly inhibited with diminished HIF-1α (Figure 6). Thus, the reduction of HIF-1α expression, as occurs in response to NAC, is sufficient to inhibit MYC-mediated tumorigenesis. It is notable that while HIF-1α could negate and HIF-2α could stimulate endogenous Myc function, the stoichiometric over-expression of Myc in P493 cells (which do not express HIF-2α) can override the HIF-1 interaction network that attenuates endogenous Myc protein function (Gordan et al., 2007; Koshiji et al., 2004; Koshiji et al., 2005; Zhang et al., 2007). On the other hand, HIF-1α is not sufficient for tumorigenesis, since P493 xenografts expressing CA5 are completely suppressible by doxycycline (data not shown). Xenografts of the non-MYC-dependent human prostate cancer cell line, PC3, that expresses CA5, are also more resistant to NAC treatment as compared to control transduced PC3 cells (Figure 7). Collectively, these observations indicate that an important anti-tumorigenic activity of the anti-oxidants NAC and ascorbate is their ability to stimulate the degradation of HIF-1.
Figure 6. HIF-1α levels are necessary for Myc-induced P493 tumorigenesis.
A. Reduction of HIF-1α levels in P493 cells by stable shRNA expression as shown by immunoblotting. Control lysate (EV) is shown with two independent pools of P493 cells transduced with two different shRNAs (sh1718 and sh2265) targeting HIF-1α. B. In vivo bioluminescent images of tumors from control cells versus cells expressing HIF-1α shRNA. C. Tumor growth of control and HIF-1α expressing P493 cells as determined by caliper measurements (Mean + SEM; n = 5 for each group).
Figure 7. PC3 cells stably expressing the HIF-1α CA5 mutant are relatively resistant to NAC treatment.
A. Immunoblot of lysates from hypoxic (1% oxygen) cells showing the expression of CA5 that is resistant to NAC-induced degradation. Cells were treated with 10 mM NAC for 24 hours. B. Tumor growth of control PC3 cells or PC3 cells stably expressing the HIF-1α CA5 mutant in response to NAC treatment (40 mM in drinking water). Data are shown as mean + SEM with n = 5 for each group.
Our findings do not rule out minor HIF-independent anti-tumorigenic effects of antioxidants, but they challenge the current paradigm that anti-oxidants inhibit tumorigenesis predominantly by decreasing ROS-mediated DNA damage and mutations (Poirier, 2004; Sharma and Farmer, 2004). Although ROS can induce genomic instability and NAC can inhibit ROS, our results suggest that the anti-tumorigenic effect of NAC is derived predominantly from its effect on HIF-1. If genomic instability induced by ROS is the dominant factor in MYC-dependent P493 and MYC-independent PC3 cell tumorigenesis, then the HIF-1 mutant CA5 would not have overridden the effects of NAC. We provide experimental evidence indicating instead that CA5 could rescue tumorigenesis from the effects of NAC and that increased HIF-1α degradation represents a major molecular mechanism underlying the anti-tumorigenic effect of anti-oxidants. In particular, consistent with recent findings that inactivation of PHD2 by ROS produced by hypoxic cells increases HIF-1α levels (Kaelin, 2005b), we found that the anti-oxidants NAC and vitamin C decreased HIF-1α levels under conditions in which HIF-1α was hydroxylated by PHD2 and ubiquitinated by VHL. Hence, our findings not only uncover a basis for the anti-tumorigenic effect of anti-oxidants, but they also suggest the potential application of anti-oxidants for adjuvant therapy to prevent progression or recurrence of HIF-dependent tumors. We caution, however, that the doses of anti-oxidants used in our mouse studies exceed that currently used in the clinical setting, which may require a much higher dose.
EXPERIMENTAL PROCEDURES
Cell lines and hypoxic exposure
P493 human B cells and PC3-HRE-GFP cells (Raman et al., 2006) were maintained in RPMI 1640 with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin. VHL-null RCC4 cell line and a derivative cell line stably transfected with an expression vector encoding VHL, RCC4-VHL (gift of Celeste Simon, University of Pennsylvania, Philadelphia, PA) (Hu et al., 2003), were maintained in high glucose (4.5 mg/ml) DMEM with 10% FBS and 1% penicillin-streptomycin. P493-CA5 stable cells were generated by retroviral infection and selected with 800 μg/ml G418 as described previously (Krishnamachary et al., 2006). Polyclonal pools of cells were used for experiments. Non-hypoxic cells (20% O2) were maintained at 37°C in a 5% CO2, 95% air incubator. Hypoxic cells (1% O2) were maintained in a controlled atmosphere chamber (PLAS-LABS, Lansing, MI, USA) with a gas mixture containing 1% O2, 5% CO2, and 94% N2 at 37°C for indicated time. Cells were exposed to hypoxia for 24 hours before analysis unless otherwise indicated.
RNA interference experiments
siRNAs targeting human PHD1, PHD2 (siGENOME SMART POOL,) were purchased from Dharmacon Research Inc. Targeting sequences for PHD1 were pool of following 8 sequences: sense1: GAACCAGGCUGUCGAAGCAUU. antisense1: 5′-P-UGCUUCGACAGCCUGGUUCUU; sense2: AAUCAGAACUGGGACGUUAUU antisense2: 5′-UAACGUCCCAGUUCUGAUUUU; sense3: CAACAUCGAGCCACUCUUUUU; antisense3: 5′-AAAGAGUGGCUCGAUGUUGUU; sense4: ACAGAAAGGUGUCCAAGUAUU antisense4: 5′-UACUUGGACACCUUUCUGUUU. Targeting sequences for PHD2 were pool of following 8 sequences: sense1: CAAGGUAAGUGGAGGUAUAUU antisense1 5′-UAUACCUCCACUUACCUUGUU; sense2: GCGAUAAGAUCACCUGGAUUU antisense2: 5-AUCCAGGUGAUCUUAUCGCUU; sense3 GCUCAUCGCUGUUCCAGGAUU; antisense3 5′-UCCUGGAACAGCGAUGAGCUU; sense4: GAACAAGCACGGCAUCUGUUU; antisense4 5′-ACAGAUGCCGUGCUUGUUCUU. Transfection of siRNA was performed using Amaxa Nucleotransfection device according to manufacturer’s instruction. Briefly, 100nM PHD1, PHD2 siRNAs or Risc-free control siRNA (Dharmacon Research Inc.) were transfected into 2.5×106 P493 cells. After 48 to 72 hr, the cells were treated with or without N-acetylcysteine for 24 hr and harvested for indicated assays. P493-HIF-1α shRNA stably transduced cells (Polyclonal pools of P493-sh-1718 or P493-sh-2265) were generated by retrovirus infection and G418 selection (800 μg/ml) as described previously (Krishnamachary et al., 2006).
DNA Vectors and transfection
Expression vectors encoding wild-type FLAG-HIF-1α or mutant FLAG-HIF-1α (P402A/P564A) were described previously (Liu et al., 2007). Amaxa Nucleofector System was used for transient transfection of the vectors into P463 cells. (2 μg DNA, Kit V, 2.5 × 106 cells and program O-006 were used for transfection)
Western blot analysis
We obtained mouse antibody for HIF-1α from BD-BioSciences Pharmingen (Cat #610959), mouse antibody for HIF-2α from Santa Cruz Biotechnology (Cat # SC-28706), rabbit antibody for PHD1 from Bethyl Laboratories. Inc (Cat# A300-326A), rabbit antibody for PHD2 from Abcom (Ab26058), mouse antibody (9E10) for c-Myc from Zymed Inc., mouse antibody for phospho-Histone H2A-X from Upstate (Cat #05-636), mouse antibody for tubulin from CalBiochem (Cat# CP06), and performed western blot assay according to the manufacturer’s instructions.
mRNA quantitation (real-time PCR)
Total RNA was extracted using the RNeasy kit (QIAGEN) followed by DNAase (Ambion)-treatment according to the manufacturer’s instructions. Primers were designed using Beacon Designer software and cDNA was prepared using TaqMan Reverse Transcription Reagents (Roche, Applied Biosystems). Quantitative real-time PCR for HIF-1α, HIF-2α or c-Myc was performed using the ABI 7700 sequence detection system. A predeveloped probe and primers specific to 18S rRNA levels were used for normalization. All PCRs were performed in triplicate.
Animal studies
All mice were housed under pathogen-free conditions. The animal studies were performed according to the protocols approved by the Animal Care and Use Committee at Johns Hopkins University. For tumorigenesis study in a xenograft model, 30×106 P493-6 cells were injected subcutaneously into SCID mice. A group of mice was treated with 40 mM N-acetylcysteine (Sigma-Aldrich) or ascorbate (5g/L) in drinking water 7 days before tumor inoculation and throughout the study. The amount of NAC administered daily is estimated at 1 g/kg. The use of pharmacokinetics constant infusion calculation, assuming a half-life of 2 hours for NAC, yielded a steady state concentration of 9.2 mM or 1.5 g/L NAC for a 20g mouse with a blood volume of 1.6 mL (note that this concentration is underestimated, since plasma volume is only a fraction of blood volume).
The tumor volumes were measured using a caliber every 4 days and calculated using the formula: [length (mm) × width (mm) × width (mm) × 0.52]. Early development of tumor was also monitored by an in vivo Xenogen in vivo bioluminescent imaging system.
The conditional c-Myc-transgenic neonatal hepatocellular carcinoma model was as described (Beer et al., 2004). The animals were maintained on regular drinking water or water supplemented with 40 mM NAC or 0.01% doxycycline two weeks before mating of LAP-tTA (liver specific expression of the tetracycline suppressible transactivator) and TRE-Myc transgenic mice and throughout the study. Male mice (in which the TRE-Myc transgene integrated into the Y chromosome) with both transgenes were studied. The formation of tumors was monitored by daily inspection and confirmed by histological examination.
Genomic integrity study
We compared the genomic integrity of the P493 cells grown in vitro with those recovered from the xenografts of P493 cells. G-band analysis and SKY analysis of both cells were performed by Cytogenetics Core Facility at Johns Hopkins. The high density SNP analysis was provided by the Johns Hopkins SNP Center using Illumina’s BeadArray™ technology (http://snpcenter.grcf.jhmi.edu/). Array comparative genomic hybridization using Agilent microarrays and data analysis were performed by Microarray Core Facility, Johns Hopkins Cancer Center.
In vivo Imaging
Animals inoculated with P493 lymphoma cells, which were engineered with a luciferase report gene, were anesthetized and injected intraperitoneally with 3mg/200μl luminescent substrate D-LuciferinFirefly (XenoGEN Cat# XR-1001). The animals were then imaged and analyzed by using the Xenogen IVIS 200 Optical Imaging Device in the Johns Hopkins Molecular Imaging Center.
Flow cytometry
For GFP detection in PC3-HRE reporter cells, cells were cultured in a hypoxic chamber (1% Oxygen) for 24 hr in the presence or absence of 10 mM NAC. Cells were then washed and analyzed using a Becton Dickinson FACSCalibur flow cytometer. Cells cultured under normoxic conditions (20% O2) were used as control.
Immunoassay for human VEGF
VEGF concentration in cell culture supernatants was measured by using an immunoassay kit from R&D Systems (Quantikine Cat#DVE00) according to the manufacturer’s instruction. The data were normalized using the human VEGF standard curve provided by the immunoassay kit.
Supplementary Material
Acknowledgments
We thank L. Gardner, K. O’Donnell-Mendell, J. Kim and K. Zeller for comments and W. Kaelin for reagents. This work was supported in part by NIH grants P50CA103175, CA51497 and CA91596.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aitio ML. N-acetylcysteine -- passe-partout or much ado about nothing? Br J Clin Pharmacol. 2006;61:5–15. doi: 10.1111/j.1365-2125.2005.02523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer S, Zetterberg A, Ihrie RA, McTaggart RA, Yang Q, Bradon N, Arvanitis C, Attardi LD, Feng S, Ruebner B, et al. Developmental context determines latency of MYC-induced tumorigenesis. PLoS Biol. 2004;2:e332. doi: 10.1371/journal.pbio.0020332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berra E, Benizri E, Ginouves A, Volmat V, Roux D, Pouyssegur J. HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1alpha in normoxia. Embo J. 2003;22:4082–4090. doi: 10.1093/emboj/cdg392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahimi-Horn MC, Chiche J, Pouyssegur J. Hypoxia signalling controls metabolic demand. Curr Opin Cell Biol. 2007;19:223–229. doi: 10.1016/j.ceb.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Brunelle JK, Bell EL, Quesada NM, Vercauteren K, Tiranti V, Zeviani M, Scarpulla RC, Chandel NS. Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell Metab. 2005;1:409–414. doi: 10.1016/j.cmet.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Cameron E, Pauling L, Leibovitz B. Ascorbic acid and cancer: a review. Cancer Res. 1979;39:663–681. [PubMed] [Google Scholar]
- Christophorou MA, Ringshausen I, Finch AJ, Swigart LB, Evan GI. The pathological response to DNA damage does not contribute to p53-mediated tumour suppression. Nature. 2006;443:214–217. doi: 10.1038/nature05077. [DOI] [PubMed] [Google Scholar]
- Covello KL, Simon MC. HIFs, hypoxia, and vascular development. Curr Top Dev Biol. 2004;62:37–54. doi: 10.1016/S0070-2153(04)62002-3. [DOI] [PubMed] [Google Scholar]
- Giaccia A, Siim BG, Johnson RS. HIF-1 as a target for drug development. Nat Rev Drug Discov. 2003;2:803–811. doi: 10.1038/nrd1199. [DOI] [PubMed] [Google Scholar]
- Gordan JD, Bertout JA, Hu CJ, Diehl JA, Simon MC. HIF-2alpha promotes hypoxic cell proliferation by enhancing c-myc transcriptional activity. Cancer Cell. 2007;11:335–347. doi: 10.1016/j.ccr.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, Simon MC, Hammerling U, Schumacker PT. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 2005;1:401–408. doi: 10.1016/j.cmet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Hu CJ, Wang LY, Chodosh LA, Keith B, Simon MC. Differential roles of hypoxia-inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Mol Cell Biol. 2003;23:9361–9374. doi: 10.1128/MCB.23.24.9361-9374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel M, Bentink S, Berger H, Klapper W, Wessendorf S, Barth TF, Bernd HW, Cogliatti SB, Dierlamm J, Feller AC, et al. A biologic definition of Burkitt’s lymphoma from transcriptional and genomic profiling. N Engl J Med. 2006;354:2419–2430. doi: 10.1056/NEJMoa055351. [DOI] [PubMed] [Google Scholar]
- Kaelin WG. Proline hydroxylation and gene expression. Annu Rev Biochem. 2005a;74:115–128. doi: 10.1146/annurev.biochem.74.082803.133142. [DOI] [PubMed] [Google Scholar]
- Kaelin WG., Jr ROS: really involved in oxygen sensing. Cell Metab. 2005b;1:357–358. doi: 10.1016/j.cmet.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Karlsson A, Giuriato S, Tang F, Fung-Weier J, Levan G, Felsher DW. Genomically complex lymphomas undergo sustained tumor regression upon MYC inactivation unless they acquire novel chromosomal translocations. Blood. 2003;101:2797–2803. doi: 10.1182/blood-2002-10-3091. [DOI] [PubMed] [Google Scholar]
- Kelly BD, Hackett SF, Hirota K, Oshima Y, Cai Z, Berg-Dixon S, Rowan A, Yan Z, Campochiaro PA, Semenza GL. Cell type-specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1. Circ Res. 2003;93:1074–1081. doi: 10.1161/01.RES.0000102937.50486.1B. [DOI] [PubMed] [Google Scholar]
- Knowles HJ, Raval RR, Harris AL, Ratcliffe PJ. Effect of ascorbate on the activity of hypoxia-inducible factor in cancer cells. Cancer Res. 2003;63:1764–1768. [PubMed] [Google Scholar]
- Koshiji M, Kageyama Y, Pete EA, Horikawa I, Barrett JC, Huang LE. HIF-1alpha induces cell cycle arrest by functionally counteracting Myc. Embo J. 2004;23:1949–1956. doi: 10.1038/sj.emboj.7600196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiji M, To KK, Hammer S, Kumamoto K, Harris AL, Modrich P, Huang LE. HIF-1alpha induces genetic instability by transcriptionally downregulating MutSalpha expression. Mol Cell. 2005;17:793–803. doi: 10.1016/j.molcel.2005.02.015. [DOI] [PubMed] [Google Scholar]
- Krishnamachary B, Zagzag D, Nagasawa H, Rainey K, Okuyama H, Baek JH, Semenza GL. Hypoxia-inducible factor-1-dependent repression of E-cadherin in von Hippel-Lindau tumor suppressor-null renal cell carcinoma mediated by TCF3, ZFHX1A, and ZFHX1B. Cancer Res. 2006;66:2725–2731. doi: 10.1158/0008-5472.CAN-05-3719. [DOI] [PubMed] [Google Scholar]
- Limoli CL, Giedzinski E, Morgan WF, Swarts SG, Jones GD, Hyun W. Persistent oxidative stress in chromosomally unstable cells. Cancer Res. 2003;63:3107–3111. [PubMed] [Google Scholar]
- Liu YV, Baek JH, Zhang H, Diez R, Cole RN, Semenza GL. RACK1 competes with HSP90 for binding to HIF-1alpha and is required for O(2)-independent and HSP90 inhibitor-induced degradation of HIF-1alpha. Mol Cell. 2007;25:207–217. doi: 10.1016/j.molcel.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis SF, Vermolen BJ, Garini Y, Young IT, Guffei A, Lichtensztejn Z, Kuttler F, Chuang TC, Moshir S, Mougey V, et al. c-Myc induces chromosomal rearrangements through telomere and chromosome remodeling in the interphase nucleus. Proc Natl Acad Sci U S A. 2005;102:9613–9618. doi: 10.1073/pnas.0407512102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Dalgard CL, Mohyeldin A, McFate T, Tait AS, Verma A. Reversible inactivation of HIF-1 prolyl hydroxylases allows cell metabolism to control basal HIF-1. J Biol Chem. 2005;280:41928–41939. doi: 10.1074/jbc.M508718200. [DOI] [PubMed] [Google Scholar]
- Mantovani G, Maccio A, Madeddu C, Mura L, Gramignano G, Lusso MR, Massa E, Mocci M, Serpe R. Antioxidant agents are effective in inducing lymphocyte progression through cell cycle in advanced cancer patients: assessment of the most important laboratory indexes of cachexia and oxidative stress. J Mol Med. 2003;81:664–673. doi: 10.1007/s00109-003-0476-1. [DOI] [PubMed] [Google Scholar]
- Narayanan BA. Chemopreventive agents alters global gene expression pattern: predicting their mode of action and targets. Curr Cancer Drug Targets. 2006;6:711–727. doi: 10.2174/156800906779010218. [DOI] [PubMed] [Google Scholar]
- Pauling L, Nixon JC, Stitt F, Marcuson R, Dunham WB, Barth R, Bensch K, Herman ZS, Blaisdell BE, Tsao C, et al. Effect of dietary ascorbic acid on the incidence of spontaneous mammary tumors in RIII mice. Proc Natl Acad Sci U S A. 1985;82:5185–5189. doi: 10.1073/pnas.82.15.5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier MC. Chemical-induced DNA damage and human cancer risk. Nat Rev Cancer. 2004;4:630–637. doi: 10.1038/nrc1410. [DOI] [PubMed] [Google Scholar]
- Pouyssegur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441:437–443. doi: 10.1038/nature04871. [DOI] [PubMed] [Google Scholar]
- Raman V, Artemov D, Pathak AP, Winnard PT, Jr, McNutt S, Yudina A, Bogdanov A, Jr, Bhujwalla ZM. Characterizing vascular parameters in hypoxic regions: a combined magnetic resonance and optical imaging study of a human prostate cancer model. Cancer Res. 2006;66:9929–9936. doi: 10.1158/0008-5472.CAN-06-0886. [DOI] [PubMed] [Google Scholar]
- Reliene R, Fischer E, Schiestl RH. Effect of N-acetyl cysteine on oxidative DNA damage and the frequency of DNA deletions in atm-deficient mice. Cancer Res. 2004;64:5148–5153. doi: 10.1158/0008-5472.CAN-04-0442. [DOI] [PubMed] [Google Scholar]
- Reliene R, Schiestl RH. Antioxidant N-acetyl cysteine reduces incidence and multiplicity of lymphoma in Atm deficient mice. DNA Repair (Amst) 2006;5:852–859. doi: 10.1016/j.dnarep.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Sablina AA, Budanov AV, Ilyinskaya GV, Agapova LS, Kravchenko JE, Chumakov PM. The antioxidant function of the p53 tumor suppressor. Nat Med. 2005;11:1306–1313. doi: 10.1038/nm1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander S, Bullinger L, Karlsson A, Giuriato S, Hernandez-Boussard T, Felsher DW, Pollack JR. Comparative genomic hybridization on mouse cDNA microarrays and its application to a murine lymphoma model. Oncogene. 2005;24:6101–6107. doi: 10.1038/sj.onc.1208751. [DOI] [PubMed] [Google Scholar]
- Schuhmacher M, Staege MS, Pajic A, Polack A, Weidle UH, Bornkamm GW, Eick D, Kohlhuber F. Control of cell growth by c-Myc in the absence of cell division. Curr Biol. 1999;9:1255–1258. doi: 10.1016/s0960-9822(99)80507-7. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- Sharma RA, Farmer PB. Biological relevance of adduct detection to the chemoprevention of cancer. Clin Cancer Res. 2004;10:4901–4912. doi: 10.1158/1078-0432.CCR-04-0098. [DOI] [PubMed] [Google Scholar]
- Vafa O, Wade M, Kern S, Beeche M, Pandita TK, Hampton GM, Wahl GM. c-Myc can induce DNA damage, increase reactive oxygen species, and mitigate p53 function: a mechanism for oncogene-induced genetic instability. Mol Cell. 2002;9:1031–1044. doi: 10.1016/s1097-2765(02)00520-8. [DOI] [PubMed] [Google Scholar]
- Zhang H, Gao P, Fukuda R, Kumar G, Krishnamachary B, Zeller KI, Dang CV, Semenza GL. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell. 2007;11:407–420. doi: 10.1016/j.ccr.2007.04.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.