Abstract
Physiological cardiac hypertrophy is associated with mitochondrial adaptations that are characterized by activation of PGC-1α and increased fatty acid oxidative (FAO) capacity. It is widely accepted that phosphoinositide-3 Kinase (PI3K) signaling to Akt1 is required for physiological cardiac growth. However, the signaling pathways that coordinate physiological hypertrophy and metabolic remodeling are incompletely understood. We now show that activation of PI3K is sufficient to increase myocardial FAO capacity and that inhibition of PI3K signaling prevents mitochondrial adaptations in response to physiological hypertrophic stimuli despite increased expression of PGC1-α. We also show that activation of the downstream kinase Akt is not required for the mitochondrial adaptations that are secondary to PI3K activation. Thus in physiological cardiac growth, PI3K is an integrator of cellular growth and metabolic remodeling. Although PI3K signaling to Akt1 is required for cellular growth, Akt-independent pathways mediate the accompanying mitochondrial adaptations.
Introduction
Cardiac hypertrophy has classically been categorized as “physiological” as occurs during development or in response to exercise training and “pathological” as occurs following pressure overload. Physiological hypertrophy is a compensated state with preserved or enhanced function over time whereas pathological hypertrophy often progresses to heart failure. The functional consequence of cardiac hypertrophy may be due to differences in signaling pathways that contribute to pathological vs. physiological cardiac growth (Dorn and Force, 2005).
Cardiac hypertrophy can dramatically alter myocardial substrate metabolism depending on the stimulus for hypertrophy. The metabolic flexibility of the heart allows it to maintain energy needs in response to various signals for growth, and disruption of this flexibility can impair function (Ritchie and Delbridge, 2006). Physiological hypertrophy (during maturation from birth to adulthood or following exercise training) is associated with mitochondrial biogenesis and an increased capacity to oxidize fatty acids (FA) and glucose (Burelle et al., 2004; Lehman et al., 2000). Pathological hypertrophy, which often decompensates and progresses to heart failure, is associated with reduced FA metabolism and increased dependence on glucose utilization (Allard et al., 1994; Arany et al., 2006; Massie et al., 1995).
Isoforms of peroxisome proliferator activated receptors (PPAR) and PPAR gamma coactivator-1 (PGC-1) are key transcriptional regulators of cardiac metabolism (Huss and Kelly, 2005). PPARα is a ligand activated nuclear receptor involved in transcription of many FA oxidation genes. The transcriptional coactivators PGC-1α and PGC-1β are important regulators of mitochondrial remodeling in the heart, by coactivating PPARs and nuclear respiratory factors 1/2 (NRF1/2) to coordinately increase mitochondrial biogenesis and oxidative capacity (Huss et al., 2002; Lehman et al., 2000; Russell et al., 2004; Vega et al., 2000). However, the signaling pathways that coordinate the mitochondrial adaptations in the context of cardiac hypertrophy are not fully understood.
It is widely accepted that PI3K signaling to Akt is an important regulator of cellular and organ growth. Class Ia PI3Ks consist of a p110α catalytic subunit and a p85/p55 regulatory subunit, which can be activated by growth factors to phosphorylate phosphoinositide-4,5-P2 at the 3 position to produce phosphoinositide-3,4,5-P3 (PIP3). PIP3 initiates activation of downstream targets, such as phosphoinositide dependent kinase 1 (PDK1) and Akt, that modulate a wide variety of cellular processes including metabolism, cell growth, protein synthesis, and gene transcription. In the heart, activation of p110α induces compensated hypertrophy, whereas inhibition of PI3K results in a reduction in cardiac size (Luo et al., 2005; Shioi et al., 2000). p110α signaling is necessary for physiological cardiac growth in response to exercise training, but is dispensable for pathological hypertrophy (Luo et al., 2005; McMullen et al., 2003).
Akt1 and Akt2 are the most abundant Akt isoforms in the heart. Akt1 is required for physiological hypertrophy in response to exercise training and IGF1 stimulation (DeBosch et al., 2006). Transgenic overexpression of Akt isoforms in the heart results in a greater degree of cardiac hypertrophy with a broad spectrum of functional consequences from increased contractility to decreased ejection fraction and heart failure, which may depend in part on the degree of Akt overexpression (Condorelli et al., 2002; Matsui et al., 2001; Shioi et al., 2002). It is currently unknown if activation of PI3K and/or activation of Akt signaling plays any role in the metabolic remodeling that accompanies physiological cardiac growth.
The aim of the current study was to determine the role of myocardial PI3K/Akt signaling in the metabolic and mitochondrial adaptations that occur in the context of physiological cardiac hypertrophy. By examining various mouse mutants with activation or inhibition of PI3K and Akt signaling respectively we show that PI3K signaling coordinately regulates physiological cardiac growth and the associated mitochondrial adaptations. In contrast to most other metabolic effects of PI3K signaling, these changes are independent of Akt activation.
Results
Constitutive Activation of PI3K Increases Mitochondrial Fatty Acid Oxidative Capacity in the Heart
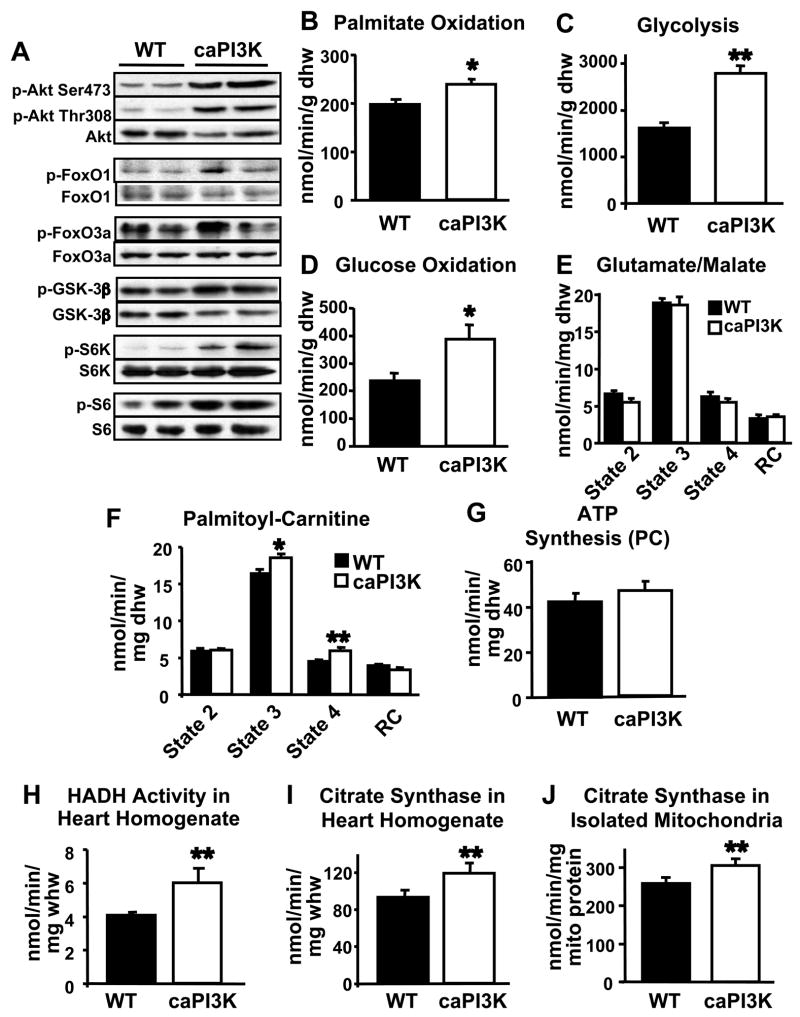
To determine if activation of phosphoinositide 3-kinase (PI3K) in the heart increases mitochondrial oxidative capacity, we measured substrate metabolism and mitochondrial function in hearts from mice with cardiac-restricted constitutive activation of PI3K (caPI3K). These mice have cardiac hypertrophy (~20%) with preserved function (Shioi et al., 2000). Relative to controls, phosphorylation of Akt and its downstream targets are increased in caPI3K hearts (Figure 1A). Palmitate oxidation, glycolysis, and glucose oxidation in perfused working hearts from 5–7 week-old caPI3K mice was increased (Figure 1B–1D) and indices of cardiac function in isolated working hearts, including left ventricular developed pressure and cardiac output, were modestly increased in caPI3K mice relative to controls (Supplementary Table 1). Mitochondrial respiration and ATP production in permeabilized cardiac fibers from caPI3K mice were unchanged when using either glutamate or pyruvate as a substrate (Figure 1E and data not shown) but the maximal rate of mitochondrial respiration (State 3) using palmitoyl-carnitine as a substrate was increased in caPI3K cardiac fibers compared to controls (Figure 1F). Despite a significant increase in state 4 of mitochondrial respiration (in the presence of oligomycin), ATP synthesis rates and ATP/O ratio (the amount of ATP produced per molecule of oxygen consumed) were unchanged relative to controls, suggesting that mitochondrial oxygen consumption was tightly coupled to ATP production in caPI3K hearts (Figure 1G and data not shown). Activities of 3′hydroxyacyl-CoA dehydrogenase (HADH), a key enzyme in β-oxidation, and citrate synthase (CS), a rate limiting step in the citric acid cycle, were increased in caPI3K cardiac tissue (Figures 1H and 1I). Increased mitochondrial enzymatic activities, particularly CS activity, could suggest changes in mitochondrial number or morphology. Electron microscopic studies indicated that cardiac structure, mitochondrial number, and mitochondrial morphology were unchanged in caPI3K hearts compared to controls (Supp. Figure 1). Additionally, mitochondrial DNA content was also unchanged in caPI3K hearts. When normalized to mitochondrial protein, citrate synthase activity measured in isolated mitochondria from caPI3K hearts was increased (Figure 1J), indicating that the increased mitochondrial enzymatic activities are likely due to increased enzymatic activity per mitochondria, rather than changes in mitochondrial number.
Figure 1.
Activation of PI3K increases mitochondrial fatty acid (FA) metabolism in the heart. Phosphorylation of PI3K and Akt targets in caPI3K hearts (A). Palmitate oxidation (B), glycolysis (C), and glucose oxidation (D) in isolated working hearts from 5–7 week-old caPI3K and control mice. Mitochondrial respiration rates in saponin-permeabilized cardiac fibers exposed to 5 mM glutamate/2 mM malate (E) or 20 μM palmitoyl-carnitine/5 mM malate (PC) (F) as substrate. ATP synthesis rates (G) with PC in cardiac fibers. 3′Hydroxyacyl-CoA dehydrogenase (HADH) (H) and citrate synthase (CS) (I) enzymatic activity rates in whole heart homogenates. CS activity in isolated mitochondria (J) from caPI3K and control mice (n=4–8 per group; * p<0.05, ** p<0.01 vs. WT).
Dominant Inhibition of PI3 Kinase Reduces Fatty Acid Oxidative Capacity in the Heart
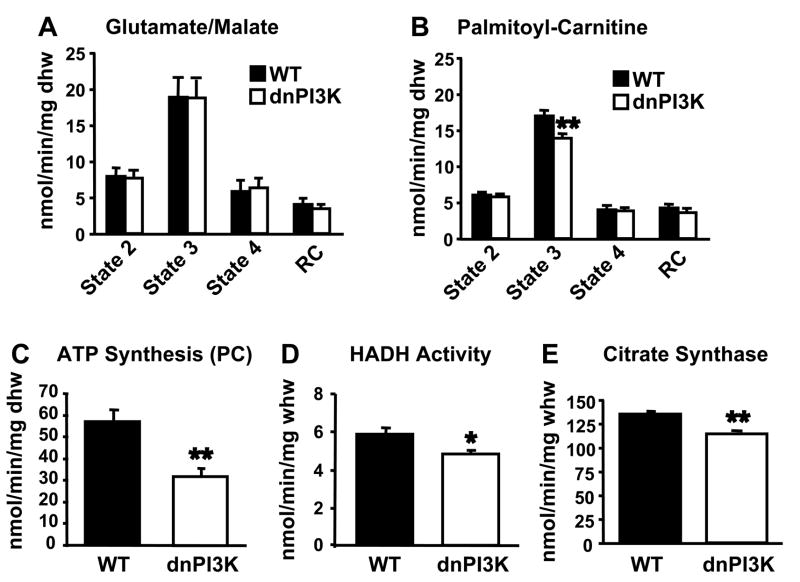
We next determined the effect of inhibition of PI3K signaling on mitochondrial oxidative capacity using mice with cardiac restricted expression of a dominant negative p110α (dnPI3K). Hearts from dnPI3K mice are significantly smaller than control hearts, but maintain function in vivo (Shioi et al., 2000). Mitochondrial respiration and ATP production in saponin-permeabilized cardiac fibers from 5 to 7-week-old dnPI3K hearts were unchanged relative to controls using either glutamate or pyruvate as substrate (Figure 2A and data not shown). However, state 3 of respiration and ATP production were significantly reduced in dnPI3K fibers treated with palmitoyl-carnitine as substrate (Figures 2B and 2C). Reduced palmitoyl-carnitine respiration was correlated with decreased palmitate oxidation, cardiac power, and cardiac output in isolated working hearts from older dnPI3K mice (Supp. Figure 2). Enzymatic activities of HADH and CS were significantly reduced in heart homogenates from dnPI3K mice (Figure 2D and 2E). However, electron microscopy of cardiac tissue revealed no changes in cardiac structure, mitochondrial number, or mitochondrial morphology (Supp. Figure 3).
Figure 2.
Inhibition of PI3K decreases mitochondrial FA metabolism in the heart. Mitochondrial respiration rates in saponin-permeabilized cardiac fibers from 5–7 week-old dnPI3K and control mice exposed to glutamate/malate (A) or palmitoyl-carnitine (PC) (B) as substrate. ATP synthesis rates (C) with PC in cardiac fibers. HADH (D) and CS (E) activity rates in whole heart homogenates (n=5–8 per group; * p<0.05, ** p<0.01 vs. WT).
Inhibition of PI3K signaling Prevents the Increase in Myocardial Fatty Acid Oxidative Capacity in Response to Exercise Training
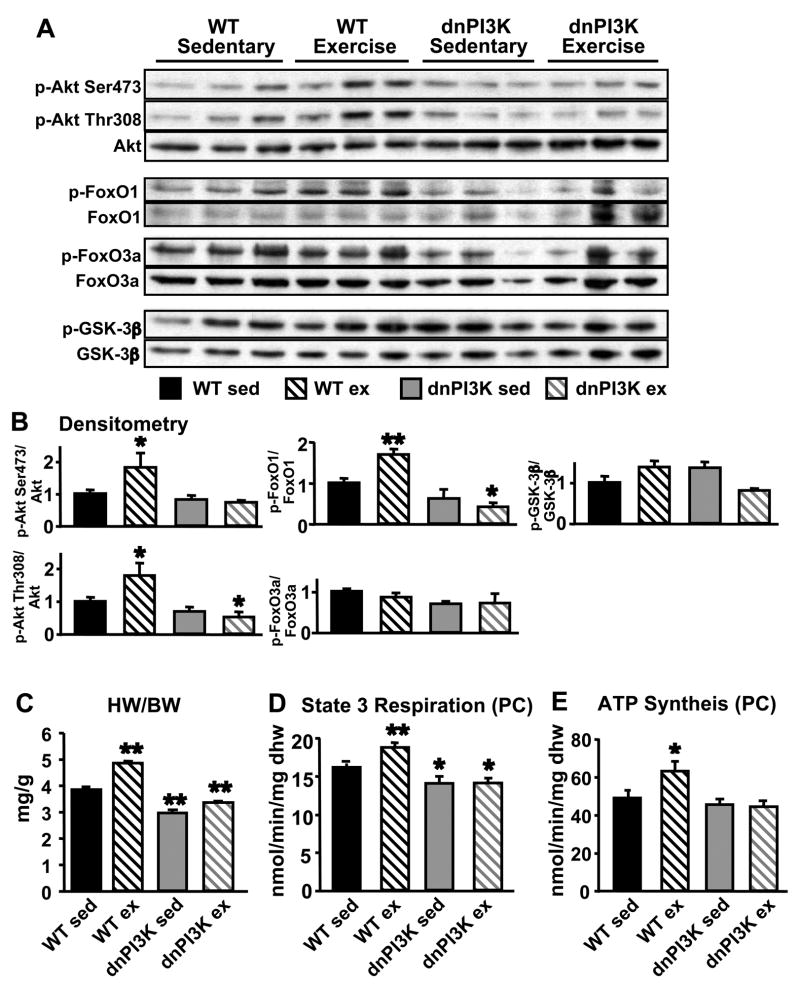
To determine if PI3K signaling is required for the cardiac mitochondrial adaptations to physiological hypertrophy, we exposed 8–10 week-old dnPI3K and control mice to 3 weeks of exercise swimming training. In previously published studies, inhibition of PI3K signaling in dnPI3K mice prevented exercise-induced cardiac hypertrophy but had no effect on cardiac function measured by echocardiography (McMullen et al., 2003). We measured phosphorylation levels of Akt and its downstream targets in hearts from exercise-trained and sedentary mice (Figure 3A). Densitometric analysis showed that phosphorylation ratios of Akt and FoxO1 were significantly attenuated in response to exercise training in dnPI3K hearts compared to controls (Figure 3B). Swim-trained WT mice exhibited robust cardiac hypertrophy, but hypertrophy in dnPI3K mice was prevented (Figure 3C), despite similar degrees of exercise training as evidenced by equivalent increases in citrate synthase activity in skeletal muscle of exercise-trained dnPI3K and WT mice (Supplementary Figure 4). Swim-trained WT mice show enhanced mitochondrial respiration and ATP production with palmitoyl-carnitine substrate compared to sedentary controls (Figure 3D and 3E). Consistent with observations in younger animals, 11–13 week-old dnPI3K sedentary mice show decreased state 3 of respiration compared to WT sedentary controls. Swimming training was unable to increase mitochondrial respiration or ATP synthesis rates in dnPI3K hearts (Figure 3D and 3E).
Figure 3.
Inhibition of PI3K prevents cardiac hypertrophy and mitochondrial adaptations in response to exercise training. Western blot analysis (A) and densitometric ratios (B) of phosphorylated targets of PI3K and Akt in hearts from wildtype sedentary (WT sed), wildtype exercise-trained (WT ex), dnPI3K sedentary (dnPI3K sed) and dnPI3K-exercise trained (dnPI3K ex) mice. Heart weight to body weight (HW/BW) ratio in exercise-trained dnPI3K and control mice (C). Mitochondrial respiration (D) and ATP synthesis (E) with palmitoyl-carnitine in cardiac fibers from wildtype and dnPI3K sedentary and exercise-trained mice. (n=4–6 per group; * p<0.05, ** p<0.01 vs. WT sed).
Fatty Acid Oxidation (FAO) Enzyme and Oxidative Phosphorylation Complex (OXPHOS) Transcript Levels in caPI3K and dnPI3K Hearts
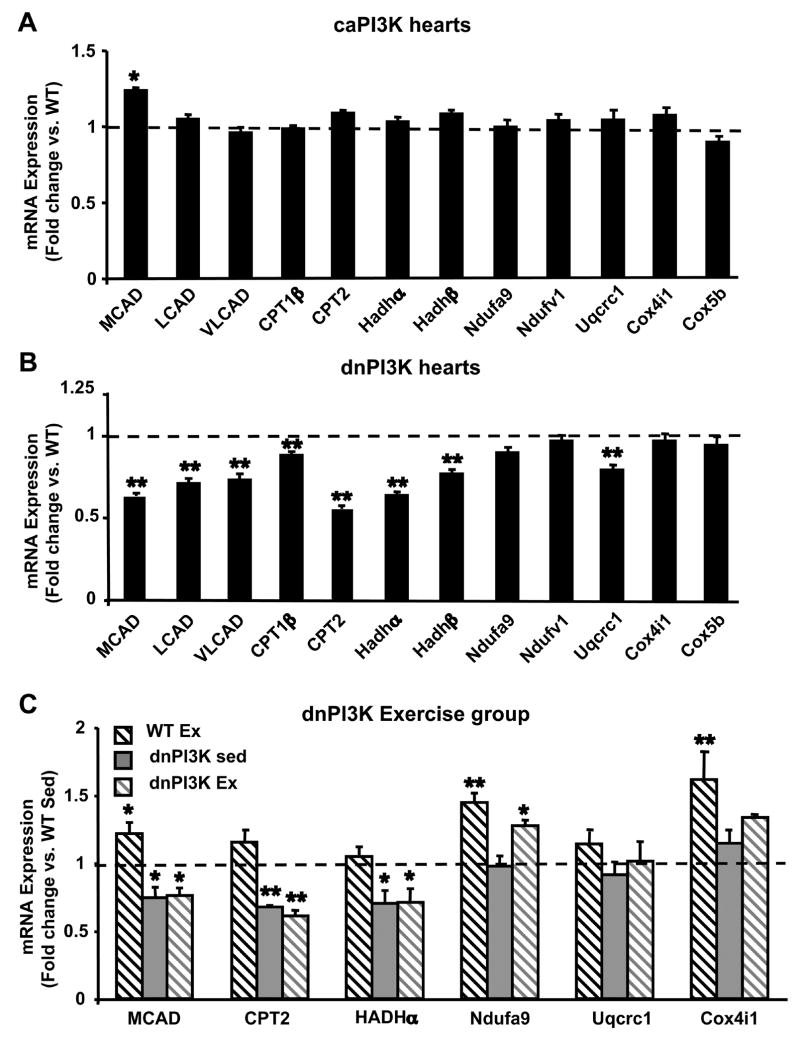
To investigate whether PI3K-mediated changes in mitochondrial FAO capacity reflect transcriptional changes, we determined mRNA levels of FAO enzymes and OXPHOS subunits by quantitative real-time PCR in caPI3K and dnPI3K hearts. Although medium chain acyl-CoA dehydrogenase (MCAD) transcript levels were increased in caPI3K hearts, FAO enzyme and OXPHOS subunit mRNA levels remained largely unchanged (Figure 4A). In contrast, mRNA levels of MCAD, long- (LCAD), and very long- (VLCAD) chain acyl-CoA dehydrogenases, muscle carnitine palmitoyl transferase 1 (CPT1β), carnitine palmitoyl transferase 2 (CPT2), 3′hydroxyacyl-CoA dehydrogenase α-(Hadhα) and β-subunits (Hadhβ), and a subunit of complex III (Uqcrc1) were reduced in dnPI3K hearts from 5–7 week old female mice compared to controls (Figure 4B). In hearts from 11–13-week-old male wildtype mice, swimming exercise-training induced increases in mRNA levels of MCAD, a subunit of complex I (Ndufa9), and a subunit of complex IV (Cox4i1) compared to sedentary controls (Figure 4C). Hearts from exercise trained dnPI3K mice failed to increase mRNA levels of MCAD compared to sedentary controls, and attenuated the exercise-induced increase in Cox4i1 mRNA levels (Figure 4C).
Figure 4.
mRNA quantification of FA oxidation enzymes and oxidative phosphorylation (OXPHOS) complex subunits in hearts from caPI3K and dnPI3K mice (expressed as fold change vs. WT). (A) mRNA levels in hearts from caPI3K mice, (n=8 per group). (B) mRNA levels in hearts from dnPI3K mice, (n=12 per group). (C) mRNA levels (fold change vs. sedentary wildtype (WT Sed)) in hearts from 12-week-old exercise trained wildtype (WT Ex), sedentary dnPI3K (dnPI3K Sed), and exercise-trained dnPI3K (dnPI3K Ex) mice, (n=4–6 per group). MCAD - medium chain acyl-CoA dehydrogenase (AD); LCAD - long chain AD; VLCAD - very long chain AD; CPT1-β - carnitine palmitoyl transferase 1, muscle isoform; CPT2 - carnitine palmitoyl transferase 2; Hadh α/β - 3′hydroxyacyl-CoA dehydrogenase α or β subunit; Ndufa9 - NADH dehydrogenase (ubiquinone) 1 alpha subcomplex 9; Ndufv1 – NADH dehydrogenase (ubiquinone) flavoprotein 1; Uqcrc1 - ubiquinol-cytochrome c reductase core protein 1; Cox4i1 – cytochrome c oxidase, subunit IV isoform 1; Cox5b - cytochrome c oxidase, subunit Vb. (*p<0.05, ** p<0.01 vs. WT or WT Sed)
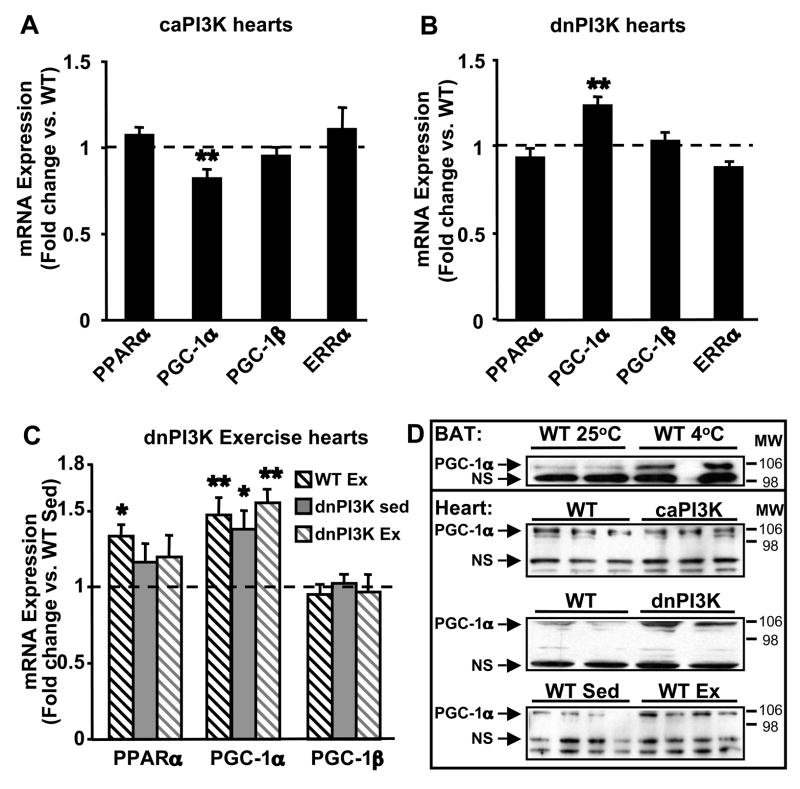
We also determined mRNA expression of PPARα, PGC-1 isoforms, and estrogen-related receptor alpha (ERRα) which are transcriptional regulators of FAO and OXPHOS genes (Huss and Kelly, 2004). No changes in mRNA levels of PPARα, PGC-1β, or ERRα were observed in caPI3K hearts, but mRNA levels of PGC-1α were decreased compared to controls (Figure 5A). No changes in mRNA levels of PPARα, PGC-1β, or ERRα were observed in dnPI3K hearts (Figure 5B). Despite down-regulation of FAO and OXPHOS genes in dnPI3K hearts, mRNA levels of PGC-1α were increased 1.23 fold compared to controls (Figure 5B). Although exercise training induced PPARα mRNA levels in WT hearts compared to WT sedentary controls, mRNA levels of PPARα were unchanged in exercise-trained dnPI3K hearts relative to WT exercise or dnPI3K sedentary controls (Figure 5C). PGC-1α mRNA levels were induced by exercise training in WT mice. Despite reduced mitochondrial respiration, hearts from sedentary dnPI3K and exercise-trained dnPI3K mice showed increased PGC-1α mRNA expression to levels similar to those observed in exercise-trained wildtype mice (Figure 5C). No changes were observed in PGC-1β mRNA levels in hearts from exercise-trained and sedentary, wildtype or dnPI3K mice. Western analysis of nuclear enriched fractions of cardiac tissue confirmed that protein expression of PGC-1α was significantly decreased in caPI3K hearts relative to controls (Densitometric ratio of PGC-1α to the non-specific band: WT = 1.21 ± 0.04 vs. caPI3K = 0.83 ± 0.10; p<0.05) (Figure 5D). Conversely, PGC-1α protein expression was increased in both exercise-trained WT hearts and sedentary dnPI3K hearts, relative to sedentary controls (Figure 5D).
Figure 5.
Expression of transcriptional regulators of FA oxidation enzymes and OXPHOS complex subunits in hearts from caPI3K and dnPI3K mice. (A) mRNA levels in hearts from caPI3K mice vs. WT expression. (n=8 per group). (B) mRNA levels in hearts from dnPI3K mice vs. WT. (n=12 per group). (C) mRNA levels in hearts from exercise-trained wildtype (WT Ex), sedentary dnPI3K (dnPI3K Sed), and exercise-trained dnPI3K (dnPI3K Ex) mice vs. sedentary wildtype (WT Sed) (n=4–6 per group). (D) Western analysis for PGC-1α in nuclear enriched fractions from caPI3K, dnPI3K, WT Ex, and control hearts. PGC-1α expression in brown adipose tissue (BAT) from cold exposed WT mice was used as an antibody control (NS = non-specific band for loading control). PPARα - peroxisome proliferator-activated receptor α; PGC-1α and -1β - PPARγ coactivator 1α and 1β; ERRα – estrogen related receptor α. (*p<0.05, ** p<0.01 vs. WT or WT Sed).
Constitutive Activation of Akt Reduces Mitochondrial Fatty Acid Metabolism in the Heart
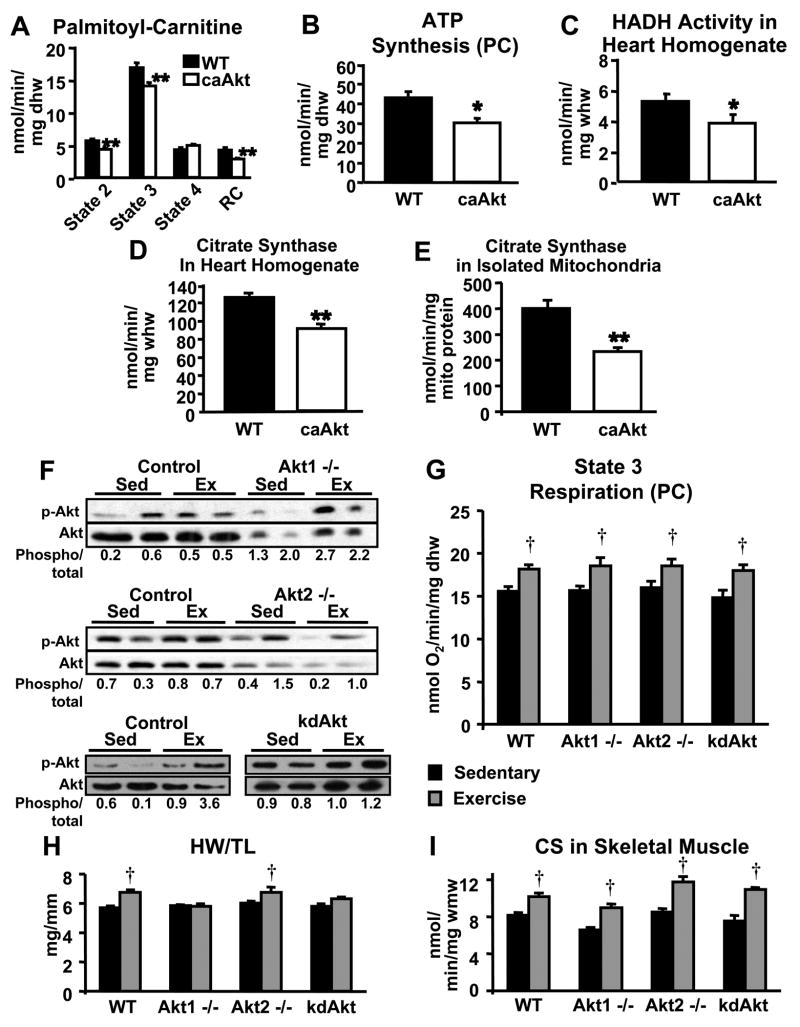
To determine if Akt mediates the effects of PI3K on myocardial mitochondrial fatty acid oxidative capacity, we determined mitochondrial function in hearts of mice with cardiac restricted expression of a constitutively active Akt1 (T308D/S473D) (caAkt). Hearts from 2-week-old caAkt mice exhibit robust hypertrophy with preserved cardiac function that progressively declines by 14-weeks of age, and heart failure ensues by 20-weeks of age (Shioi et al., 2002). To avoid the confounding effects of heart failure on the mitochondrial phenotype of caAkt hearts, we studied mice between 5 and 7 weeks of age. States 2 and 3 of mitochondrial respiration with palmitoyl-carnitine as substrate were decreased in caAkt cardiac fibers compared to controls (Figure 6A). Rates of ATP synthesis were also reduced in caAkt fibers (Figure 6B), to a degree similar to that of dnPI3K hearts at the same age. Enzymatic activities of HADH and CS were markedly reduced in heart homogenates from caAkt mice relative to controls (Figure 6C and 6D). When normalized to mitochondrial protein, CS activity in mitochondria isolated from caAkt hearts was also reduced suggesting diminished enzymatic activity per mitochondria (Figure 6E).
Figure 6.
Activation of Akt in the heart reduces mitochondrial function, and isoform specific deletion of Akt1 or Akt2 or cardiac specific overexpression of a kinase-dead Akt1 (kdAkt) does not prevent exercise-induced increases in mitochondrial FAO capacity in the heart. Mitochondrial respiration (A) and ATP production (B) with palmitoyl-carnitine (PC) in cardiac fibers from 5–7 week-old caAkt and control mice. HADH and CS activities (C) and (D) in whole heart homogenates. CS activity in isolated mitochondria (E). Western analysis for phosphorylation of Akt (Ser473) in hearts from sedentary (Sed) and exercise-trained (Ex) Akt1 −/−, Akt2 −/−, kdAkt, and control mice (F). Densitometric ratio of phospho-Akt to total Akt is shown under each sample (Phospho/total). Mitochondrial respiration with PC in cardiac fibers (G), heart weight to tibia length (HW/TL) ratio (H), and CS activity in skeletal muscle (I) from sedentary and exercise-trained Akt1 −/−, Akt2 −/−, kdAkt, and control mice. (n=4–6 per group; * p<0.05, ** p<0.01 vs. WT; † p<0.05 vs. sedentary control).
Germline Deletion of Akt1 or Akt2 or Overexpression of a Kinase-Dead Akt1 Isoform Does Not Prevent Exercise-Induced Increases in Mitochondrial Respiration
We next determined mitochondrial function in sedentary or exercise-trained mice with either germline deletion of Akt1 or Akt2, or cardiac restricted expression of a kinase deficient Akt1 (K197M) (kdAkt) (Shioi et al., 2002) to evaluate if mitochondrial adaptations to exercise-induced cardiac hypertrophy require the activity of a specific Akt isoform. Akt1 −/− mice are characterized by decreased body size and normal glucose tolerance, whereas Akt2 −/− mice exhibit impaired glucose tolerance and hepatic insulin resistance (Chen et al., 2001; Cho et al., 2001a; Cho et al., 2001b; Garofalo et al., 2003). Cardiac function and dimensions, as measured by echocardiography, were not significantly different between Akt1 −/−, Akt2 −/−, and control mice, and were unchanged between sedentary and exercise-trained mice (Supplementary Table 2). Exercise increased phosphorylation of Akt in hearts from control animals for each group (Figure 6F). Phosphorylation of residual Akt isoforms increased with exercise training in Akt1 −/− hearts, whereas phosphorylation levels of the residual Akt isoforms in Akt2 −/− hearts was variable and did not globally increase with exercise training (Figure 6F). Hearts from kdAkt mice overexpress an Akt isoform that can still be phosphorylated and we show that exercise training increases its phosphorylation (Figure 6F). State 3 of respiration with palmitoyl-carnitine as substrate was unchanged in cardiac fibers from sedentary Akt1 −/−, Akt2 −/−, and kdAkt relative to WT controls (Figure 6G). Additionally, mitochondrial respiration with PC was increased in cardiac fibers from exercise-trained Akt1 −/−, Akt2 −/−, kdAkt, and control mice, compared to sedentary mice of the same genotype (Figure 6G). Cardiac hypertrophy, as measured by heart weight to tibia length was increased in exercise-trained Akt2 −/− and control mice, but was prevented in exercise-trained Akt1 −/− mice and was not significantly increased in exercise-trained kdAkt mice (Figure 6H). The same degree of exercise training was achieved in Akt1 −/−, Akt2 −/−, kdAkt, and control mice as evidenced by increased citrate synthase activity in mixed gastrocnemius muscle from exercise-trained mice, relative to sedentary mice of the same genotype (Figure 6I). Thus, whereas Akt1 is required for physiological cardiac hypertrophy, it does not mediate the mitochondrial adaptations that accompany exercise training. Moreover, the studies in Akt2 −/− hearts, in which mitochondrial respirations increased despite variable phosphorylation of residual Akt isoforms with exercise and a preserved mitochondrial response to exercise in kdAkt hearts, provide additional evidence for the absence of a role for Akt in mediating the metabolic adaptations to exercise-induced cardiac hypertrophy.
Inhibition of Akt Signaling in caPI3K Hearts Attenuates Hypertrophy but Does Not Prevent Mitochondrial Metabolic Remodeling
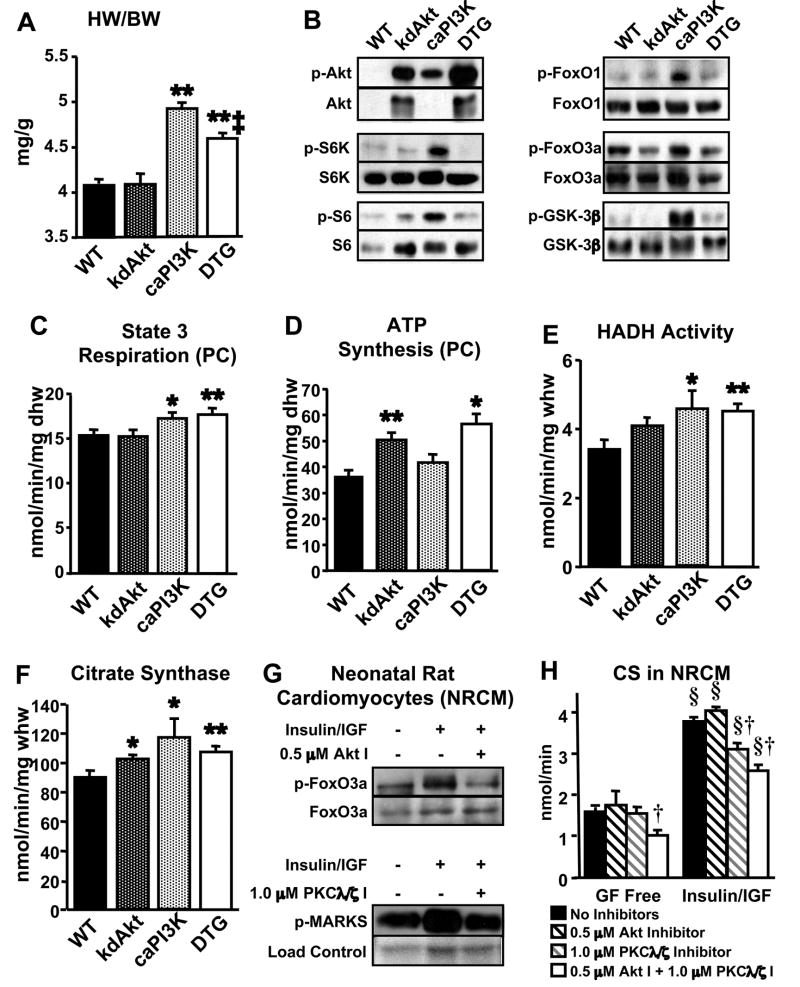
Given the deleterious effects of Akt overexpression on mitochondrial oxidative capacity and the absence of any impact of germline Akt deletion on mitochondrial function, we hypothesized that PI3K regulates fatty acid oxidative capacity in the heart independently of Akt signaling. To address this question, we performed an epistatic experiment by crossing caPI3K mice to kdAkt mice to produce double transgenic mice (DTG). No differences were observed in body weights of DTG mice compared to single transgenic or wildtype controls. Heart weight to body weight (HW/BW) ratios were not different between kdAkt and wildtype mice (Figure 7A). HW/BW ratio was increased in caPI3K mice (1.21 ± 0.01 fold) and this hypertrophy was significantly attenuated in DTG mice (1.12 ± 0.01 fold, p<0.01 vs. caPI3K) (Figure 7A), recapitulating previously published observations (Shioi et al., 2002). Western analysis of heart homogenates revealed that phosphorylation of multiple downstream targets of Akt was significantly increased in caPI3K hearts (Figure 7B). The increases in phosphorylation of Akt targets were blocked in hearts from DTG mice, indicating that signaling downstream of Akt is significantly attenuated in DTG hearts (Figure 7B).
Figure 7.
Expression of a kinase-dead Akt (kdAkt) does not impair PI3K-mediated mitochondrial adaptations despite attenuation of cardiac hypertrophy and inhibition of downstream Akt signaling. (A) Heart weight to body weight (HW/BW) ratio of double transgenic (DTG) and control mice (n=12–20 per group). (B) Phosphorylation of PI3K and Akt targets. State 3 of mitochondrial respiration (C) and ATP production (D) with palmitoyl-carnitine (PC) in cardiac fibers (n=5–9 per group). HADH and CS activities (E) and (F) in whole heart homogenates (n=5–9 per group). (G) Phosphorylation of Akt and PKCλ/ζ targets in neonatal rat cardiomyocytes (NRCM) in the presence or absence of insulin (100nM)/IGF-1 (10nM) and Akt inhibitor (Akt I) or PKCλ/ζ inhibitor (PKCλ/ζ I). Loading control for p-MARKS is Ponceau stained membrane. (H) Total CS activity in NRCM treated with Growth Factor Free (GF Free) media or insulin/IGF-1 media plus Akt or PKCλ/ζ inhibitors. (*p<0.05, ** p<0.01 vs. WT; ‡ p<0.05 vs. caPI3K; § p<0.01 vs. GF Free control; † p<0.05 vs. all other groups (GF Free) or vs. no inhibitor or Akt I following Insulin/IGF-1).
State 3 of mitochondrial respiration with palmitoyl-carnitine (PC) as substrate was unchanged in cardiac fibers from kdAkt compared to wildtype controls (Figure 7C). As previously observed, caPI3K cardiac fibers showed enhanced state 3 of respiration (1.13 ± 0.04 fold over WT) with PC substrate and this enhancement was not attenuated in DTG mice (1.15 ± 0.05 fold over WT) (Figure 7C). Interestingly, ATP synthesis rates with PC were increased in cardiac fibers from kdAkt mice compared to wildtype controls (Figure 7D). Again, ATP production was unchanged in caPI3K cardiac fibers with PC compared to WT, but synthesis rates remained increased in DTG fibers (Figure 7D).
HADH activity was significantly increased in DTG hearts compared to WT to a similar degree as caPI3K (Figures 7E). Citrate synthase activity was also significantly increased in kdAkt, caPI3K, and DTG hearts (Figure 7F).
Inhibition of Insulin- and IGF-Mediated PKCλ/ζ Signaling, but not Akt Signaling, Reduces Citrate Synthase Activity in Neonatal Rat Cardiomyocytes
To begin to elucidate possible alternative signaling pathways that contribute to PI3K-mediated mitochondrial remodeling, we treated neonatal rat cardiomyocytes (NRCM) with insulin and IGF-1 in the presence of Akt or PKCλ/ζ inhibitors and assessed citrate synthase activity. Insulin and IGF-1 treatment for 72 hours was sufficient to increase activity of Akt and PKCλ/ζ signaling as evidenced by increased phosphorylation of their downstream targets FoxO3a and MARKS, respectively (Figure 7G). We performed a dose titration of both PKCλ/ζ and Akt inhibitors and found that 1.0 μM PKCλ/ζ inhibitor (PKCλ/ζ I) or 0.5 μM Akt inhibitor (Akt I) in the presence of insulin/IGF reduced phosphorylation of downstream targets to the level of cells not exposed to insulin/IGF (Figure 7G), while higher levels of inhibitors tended to decrease cell viability. We found a strong correlation between total citrate synthase activity and cell number in NRCM preparations (Supplementary figure 5A), and at the concentrations used, Akt inhibitors did not reduce IGF-1 mediated activation of PKCλ/ζ signaling nor did the PKCλ/ζ I attenuate IGF-1 mediated activation of Akt targets (Supplementary figure 5B). Total citrate synthase (CS) activity was unchanged between NRCM in serum free media treated with or without 1.0 μM PKCζ I or 0.5 μM Akt I, but the combination of both inhibitors reduced CS activity (Figure 7H). Insulin/IGF-1 treatment of NRCM increased absolute levels of CS activity and inhibition of Akt signaling did not prevent this increase in CS activity (Figure 7H). Inhibition of PKCλ/ζ in the presence of insulin/IGF-1 stimulation significantly reduced total CS activity compared to insulin/IGF-1 alone, and the combination of PKCλ/ζ and Akt inhibitors did not reduce CS below that of PKCλ/ζ inhibition alone (Figure 7H).
Discussion
In this study, we show that phosphoinositide-3 Kinase (PI3K) is a critical modulator of mitochondrial fatty acid (FA) metabolism in the heart and that this metabolic regulation is independent of Akt. Constitutive activation of PI3K in the heart is sufficient to increase FA utilization and selectively up-regulate mitochondrial oxidative capacity for FA substrates. Inhibition of PI3K prevents the increase in FAO capacity that occurs in response to physiological cardiac hypertrophy, despite increased PGC-1α mRNA and protein levels. Downstream of PI3K, disruption of Akt signaling did not prevent the mitochondrial adaptations to exercise or PI3K activation. Inhibition of cardiac Akt signaling in the context of exercise training or PI3K activation attenuates hypertrophy, but does not diminish mitochondrial respiration or FAO enzyme activity. Conversely, constitutive activation of cardiac Akt results in cardiac hypertrophy but impairs mitochondrial function, thereby dissociating a conserved signal for cellular growth from mitochondrial adaptations. Thus, the current study identifies a necessary role for PI3K in coordinating myocardial fatty acid oxidative capacity with physiological cardiac hypertrophy, independently of PGC-1α expression. We also demonstrate that the long-term metabolic and mitochondrial effects of PI3K can be dissociated from its downstream target Akt, thereby representing a novel paradigm in long-term regulation of metabolism by PI3K signaling.
Previous studies have shown that inhibition of PI3K blocks the heart’s ability to hypertrophy in response to exercise training (McMullen et al., 2003). We currently show that PI3K inhibition in hearts from exercise-trained mice also prevents the increase in mitochondrial FA oxidative capacity, despite a significant increase in PGC-1α expression. The requirement of p110α in the regulation of cardiac growth and the associated metabolic adaptation implies a tight link between physiological hypertrophy and enhanced mitochondrial FA oxidative capacity. Activation of PI3K therefore leads to a balanced increase in oxidative energy production and cardiac growth that may protect against the progression to heart failure seen in pathological hypertrophy. This protective effect may be specific to the growth factor and exercise training responsive Class Ia PI3-kinases. Indeed, previous studies have shown that whereas class Ia PI3Ks regulate hypertrophy, class Ib PI3Ks modulate contractility in the heart (Crackower et al., 2002).
Acute activation of PI3K in the heart, as occurs with insulin stimulation, alters substrate utilization by increasing glucose utilization and reducing FA oxidation (Belke et al., 2002). PI3K is necessary for insulin-mediated glucose uptake and thereby plays a primary role in acutely increasing glucose utilization and reciprocally decreasing FA oxidation in response to insulin (Pessin and Saltiel, 2000). Decreased FA utilization induced by insulin stimulation seems at odds with our observation that increased PI3K activation and increased FA oxidation are associated with exercise-induced or physiological cardiac hypertrophy (Burelle et al., 2004; Luo et al., 2005; McMullen et al., 2003). The current study distinguishes between acute and chronic PI3K signaling and confirms the role of chronic PI3K signaling in increasing mitochondrial FA oxidative capacity during physiological cardiac hypertrophy. Thus, while acute activation of PI3K by insulin stimulation increases glucose utilization and suppresses FA oxidation in the heart, chronic activation of PI3K in the heart enhances FA oxidation by increasing mitochondrial oxidative capacity for FA substrates.
Quantification of mRNA levels of FA oxidation enzymes revealed divergent downstream effects of PI3K signaling in the heart. Thus, activation of PI3K enhances FA oxidative capacity in the absence of an increase in transcript levels of FAO enzymes, with the exception of MCAD, thereby suggesting that activation of PI3K in response to physiologic stimuli may enhance mitochondrial function via post-transcriptional mechanisms. In contrast, inhibition of PI3K is associated with decreased mRNA levels of FAO enzymes and prevents their increase in response to exercise training. This suggested that PI3K signaling might modulate expression or activity of the transcriptional regulators of FAO gene expression. However, dnPI3K hearts showed no changes in PPARα, PGC-1β, or ERRα mRNA levels, which are transcriptional regulators of enzymes and subunits in FAO and OXPHOS pathways in the heart (Huss and Kelly, 2004). Indeed, despite diminished mitochondrial FAO capacity in hearts from exercise-trained and sedentary dnPI3K mice, we observed increased PGC-1α mRNA and protein levels, to a similar degree as observed in exercise-trained wildtype hearts. Thus down-regulation of FAO gene expression in dnPI3K hearts cannot be accounted for by reduced expression of the transcriptional regulators of FAO enzymes. However, the possibility of changes in the activity of PPAR transcription factors or PGC-1 transcriptional co-activators cannot be ruled out. While diminished activity of transcriptional co-activators may account for the down-regulation of FAO transcript levels in the heart when PI3K is inhibited, the lack of a significant increase in mitochondrial FAO genes in caPI3K hearts would argue against a change in the activity of transcriptional regulators of mitochondrial FA oxidative capacity when PI3K is chronically activated in the heart.
Our results show that exercise training synergistically increases mitochondrial FAO, PGC-1α expression, and expression of mitochondrial genes, yet chronic activation of PI3K in the heart does not recapitulate this transcriptional profile. The differences in theses two models may be a consequence of the duration of PI3K activation. Intermittent activation of PI3K, as occurs in exercise training, may be more beneficial for mitochondrial function, and is a potential reason why exercise-trained hearts and hearts with constitutive activation of PI3K differ in the expression of mitochondrial enzymes and their transcriptional regulators. Indeed, we have evidence that models of chronic Akt activation in the heart show diminished mitochondrial function and decreased expression of PGC-1α and its target genes (manuscript in preparation). Thus it appears that intermittent activation of PI3K during exercise training is necessary for coordinated increases in mitochondrial enzyme gene expression and FAO capacity, but constitutive activation of PI3K signaling leads to sustained Akt activation, which can suppress the increased expression of PGC-1α and its target mitochondrial FAO enzymes in the heart. Nevertheless our data strongly suggests that sustained PI3K activation might be sufficient to promote increased mitochondrial FAO independently of a coordinate increase in PGC-1α-mediated transcriptional upregulation.
Our initial efforts to determine if Akt mediates the mitochondrial adaptations in response to PI3K signaling revealed that constitutive activation of Akt in the heart leads to diminished mitochondrial FAO capacity. This result might not be surprising in light of data from many groups showing that chronic Akt activation in the heart is detrimental to cardiac function (Nagoshi et al., 2005; O’Neill and Abel, 2005; Shiojima et al., 2005). In addition, hearts from mice with germline deletion of Akt1 and Akt2 and hearts with reduced Akt signaling via overexpression of a kinase dead Akt1 isoform respond normally by increasing mitochondrial function in response to exercise training. These observations led us to hypothesize that the modulation of mitochondrial function by PI3K is independent of Akt signaling. We confirmed the findings previously reported by Shioi and colleagues that cardiac-restricted expression of a kinase dead Akt1 diminishes signaling downstream of Akt in the heart and attenuates cardiac hypertrophy in caPI3K mice (Shioi et al., 2002). We further show that inhibition of Akt signaling does not prevent the PI3K-mediated increase in mitochondrial FA oxidative capacity. Additionally, in contrast to dnPI3K hearts in which ATP synthesis was reduced with palmitoyl-carnitine, attenuation of Akt signaling in kdAkt (Shioi et al., 2002) and DTG hearts increased ATP production, further supporting the hypothesis that mitochondrial remodeling in response to PI3K signaling is distinct from the mitochondrial effects of Akt signaling in the heart.
Our experiments in neonatal rat cardiomyocytes identify a potential Akt-independent target that may mediate the PI3K-dependent metabolic effects on cardiomyocytes. PKCλ/ζ was an attractive target from a metabolic standpoint as atypical PKC isoforms have been shown to play a role in GLUT4 translocation in muscle (Bandyopadhyay et al., 2000; Etgen et al., 1999) and have distinct effects from Akt on lipid metabolism in the liver (Matsumoto et al., 2003; Taniguchi et al., 2006). In our neonatal rat cardiomyocyte experiments we found a strong correlation between citrate synthase (CS) activity and cell number. These results indicate that in neonatal myocytes, growth factor signaling promotes a proliferative response that occurs in concert with a coordinate increase in mitochondrial content, whereas in the intact heart a coordinated increase in mitochondrial function accompanies myocyte hypertrophy. We defined conditions in which growth factor stimulation coordinately increased CS activity and cellular hyperplasia despite inhibition of Akt activation. By contrast, inhibition of growth-factor activation of PKCλ/ζ signaling led to a significant decrease in mitochondrial CS activity in the absence of any change in cellular viability. At the concentrations used, these inhibitors were relatively specific for their respective substrates although we cannot completely rule out off target effects. Nevertheless, these observations raise the possibility that PKCλ/ζ signaling may be one potential PIP3/PDK1 downstream signal that is required for PI3K dependent mitochondrial remodeling, and provides preliminary evidence that alternative PI3K targets can influence mitochondrial function in response to growth factor stimulation. These data do not rule out possible roles for other PIP3 targets, such as serum- and glucocorticoid-regulated kinase (SGK), p90 ribosomal S6 kinase (RSK), p21-activated kinase 1 (PAK1), Rac which can modulate actin remodeling (Han et al., 1998) or PLCγ which activates pathways downstream of IP3 and DAG (Xie et al., 2005). PI3K significantly affects a broad range of cellular signaling pathways in cardiac muscle, and more work will be needed to establish all of the Akt-independent but PIP3-dependent signaling molecules that are required for regulation of mitochondrial remodeling in response to PI3K activation.
In summary, the current study identifies a central role for PI3K in coordinating physiological cardiac growth and metabolic capacity in the heart. Inhibition of PI3K is sufficient to prevent the mitochondrial adaptations to exercise-induced hypertrophy, despite increases in PGC-1α mRNA and protein levels, indicating that up-regulation of PGC-1α expression and PI3K activation may work in parallel to increase myocardial mitochondrial oxidative capacity during physiological cardiac growth. Additionally, whereas previous studies have identified an obligate requirement of PI3K activation of Akt1 as a key regulator of physiological cardiac cellular growth (DeBosch et al., 2006; McMullen et al., 2003), here we show that PI3K likely activates Akt-independent signaling pathways to mediate the metabolic and mitochondrial adaptation that accompanies physiological cardiac hypertrophy.
Materials and Methods
Generation of Animals
Mice with cardiac restricted expression of a dominant negative p110α (dnPI3K), the inter-SH2 fusion of p110α which maintains PI3K in a constitutively active state (caPI3K), a kinase deficient (K197M) mutant Akt1 (kdAkt), or a constitutively active Akt1 (T308D/S473D) mutant (caAkt) were generated in the laboratory of Dr. Seigo Izumo and have been previously described (Shioi et al., 2000; Shioi et al., 2002). Germline Akt1 and Akt2 null mice were generated in the laboratory of Dr. Morris J. Birnbaum and have been previously described (Cho et al., 2001a; Cho et al., 2001b). The animals were fed a standard chow and housed in temperature-controlled facilities with a 12-h light and 12-h dark cycle (lights on at 6:00 A.M.). All animal experimentation was conducted in accordance with guidelines approved by the Institutional Animal Care and Use Committee of the University of Utah.
Swimming Exercise Training
Eight to 10-week-old dnPI3K and control mice were exposed to swimming exercise training as previously described (Wilkins et al., 2004). Briefly, each day consisted of two bouts of swimming, separated by 4 hours, beginning with a 10-minute duration on the first day. The two bouts of swimming were increased by 10 minutes each day until two bouts of 90 minutes were achieved. Mice were swum for 13 additional days (21 days total) and were sacrificed 18 hours after the last swim.
Substrate Metabolism in Isolated Working Hearts
Palmitate and glucose oxidation were measured in isolated working hearts as described (Mazumder et al., 2004). Hearts were perfused with 0.4 mM palmitate and 5 mM glucose without insulin.
Mitochondrial Respiration in Permeabilized Cardiac Fibers
Mitochondrial oxygen consumption and ATP production were measured in permeabilized cardiac fibers from 5 to 7-week-old mice of each model using techniques that have been previously described (Boudina et al., 2007).
Enzyme Activity Assays
Mitochondrial Isolation
Mitochondria were isolated from fresh heart tissue by differential centrifugation as described (Brand et al., 2005). Mitochondria were resuspended in STE buffer and protein was quantified using Micro BCA kit (Pierce) with BSA as a standard.
Citrate Synthase (CS)
CS activity was determined spectrophotometrically using whole heart or mixed gastrocnemius muscle homogenates as described (Boudina et al., 2005; Clark and Rodnick, 1998; Rodnick and Sidell, 1997). CS activity was also measured using 10 μg of isolated mitochondrial protein and 10 μL of neonatal rat cardiomyocyte extracts.
3′-Hydroxyacyl-CoA Dehydrogenase (HADH)
HADH was determined spectrophotometrically using whole heart homogenates as described (Boudina et al., 2005; Clark and Rodnick, 1998).
Mitochondrial DNA Content
Mitochondrial DNA content was quantified by real-time polymerase chain reaction (RT-PCR). Briefly, total DNA was extracted and purified from heart tissue with DNEasy Kit (Qiagen Inc., Valencia, CA). Four ng of DNA was used to quantify mitochondrial and nuclear DNA markers. RT-PCR was performed using an ABI Prism 7900HT instrument (Applied Biosystems, Foster City, CA) in 384-well plate format with SYBR Green I chemistry and ROX internal reference (Invitrogen). Analysis of results was automated using scripting with SDS 2.1 (Applied Biosystems), Microsoft Access, and Microsoft Excel. β-actin was used as a nuclear DNA marker.
Electron Microscopy
Electron microscopy (EM) samples were prepared and processed at the Core Research Microscopy Facility at the University of Utah. Briefly, small pieces of endocardial and sub-endocardial tissue from the left ventricle were fixed in 2.5% glutaraldehyde and 1% paraformaldehyde in 0.1 M sodium cacodylate, with 2.4% sucrose and 8 mM CaCl2 (pH 7.4) for at least a day. Samples were post fixed in 2% osmium tetroxide in 0.1 M sodium cacodylate, en bloc stained with aqueous uranyl acetate, and dehydrated through a graded series of ethanol washes (50% up to 100%). Fixed samples were then infiltrated with and embedded in Spurr’s plastic, and processed for EM. Mitochondrial morphology was assessed at 3500x, 18,000x, and 70,000x magnifications and mitochondrial number was determined in 16 pictures per group (n=4 hearts and 4 pictures per heart) at 18,000x magnification.
Gene expression
mRNA was quantified by real-time polymerase chain reaction using an ABI Prism 7900HT instrument in 384-well plate format as described (Boudina et al., 2007). Cyclophilin (CPHN) was used as a template normalizer in caPI3K studies. Because CPHN levels were significantly increased in dnPI3K samples, vascular endothelial growth factor A (VEGF), which was unchanged between groups, was used as a template normalizer for dnPI3K studies. Primer sequences are listed in Supplementary Table 3.
Western blot analysis
PI3K/Akt Signaling Targets
Total proteins were extracted from frozen hearts as described (Boudina et al., 2005). Proteins were resolved by SDS-PAGE and electro-transferred onto a PVDF membrane (Millipore Corp., Bedford, MA). The following antibodies were used: phospho-Akt (Thr308), phospho-Akt (Ser473), Akt, phospho-p70S6-Kinase (Thr389), p70S6-Kinase, phospho-S6, S6, phospho-FoxO1 (Thr24)/phospho-FoxO3a (Thr32), FoxO1, phospho-GSK-3β, p-MARKS (Cell Signaling Technology, Danvers, MA), FoxO3a (Upstate, Billerica, MA), and GSK-3β (Santa Cruz Biotechnology, Santa Cruz, CA). Protein detection was carried out with the appropriate horseradish peroxidase-conjugated secondary antibody and ECL or ECL Plus detection systems (Amersham Biosciences, Piscataway, N.J.).
PGC-1α Western Analysis
For PGC-1α, nuclear enriched fractions were isolated from cardiac tissue using NE-PER® nuclear and cytoplasmic extraction reagents (Pierce Biotechnology, Rockford, IL). The polyclonal antibody for PGC-1α was a gift from Dr. Morris Birnbaum.
Primary cell culture
Primary neonatal rat cardiomyocytes (NRCM) were harvested using a modified protocol (Chien et al., 1985; Sen et al., 1988). Following mechanical and enzymatic (collagenase/pancreatin) separation of 1–3 day old rat pup hearts, Percoll-gradient purified cardiocytes were plated on gelatin-coated plates at a density of 160,000/cm2 in 10% horse serum, 5% fetal bovine serum, and 100 nM BrdU containing DMEM/M199 media (Invitrogen). Following 24-hrs recovery, media was replaced with serum and growth factor free (GF Free) media and treatments were started. Cells were treated with or without 100 nM insulin and 10 nM IGF-1 (National Hormone & Peptide Program, Torrance, CA) and the indicated concentrations of Akt (Calbiochem, No.124008) and PKCλ/ζ (Biosource, Camarillo, CA) inhibitors for 72-hrs. Media and treatments were refreshed every 24-hrs. Following treatment, cells were harvested and counted for citrate synthase activity assay or Western blot analysis.
Statistical Analyses
Data are expressed as mean ± SE. Unpaired Student’s t test was used to analyze data sets with two groups. All other differences were analyzed by ANOVA, and significance was assessed by Fisher’s protected least significant difference test. For all analyses, P<0.05 was accepted as indicating a significant difference. Statistical calculations were performed using the Statview 5.0.1 software package (SAS Institute, Cary, NC).
Supplementary Material
Acknowledgments
This work was supported by grants RO1HL070070 and UO1HL70525 from the National Institutes of Health, and the Ben and Iris Margolis Foundation to E. Dale Abel who is an Established Investigator of the American Heart Association. B. T. O’Neill was supported by a physician scientist-training award from the American Diabetes Association; V. Zaha by a postdoctoral fellowship from the American Heart Association; A. Wende by NIH grant 5T32 HL007576 and S. Boudina by post-doctoral fellowships from the Juvenile Diabetes Research Foundation and the American Heart Association (Western Affiliates). Morris J. Birnbaum is supported by grant RO1DK056886 and the Cox Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allard MF, Schonekess BO, Henning SL, English DR, Lopaschuk GD. Contribution of oxidative metabolism and glycolysis to ATP production in hypertrophied hearts. Am J Physiol. 1994;267:H742–750. doi: 10.1152/ajpheart.1994.267.2.H742. [DOI] [PubMed] [Google Scholar]
- Arany Z, Novikov M, Chin S, Ma Y, Rosenzweig A, Spiegelman BM. Transverse aortic constriction leads to accelerated heart failure in mice lacking PPAR-gamma coactivator 1alpha. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:10086–10091. doi: 10.1073/pnas.0603615103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay G, Kanoh Y, Sajan MP, Standaert ML, Farese RV. Effects of adenoviral gene transfer of wild-type, constitutively active, and kinase-defective protein kinase C-lambda on insulin-stimulated glucose transport in L6 myotubes. Endocrinology. 2000;141:4120–4127. doi: 10.1210/endo.141.11.7766. [DOI] [PubMed] [Google Scholar]
- Belke DD, Betuing S, Tuttle MJ, Graveleau C, Young ME, Pham M, Zhang D, Cooksey RC, McClain DA, Litwin SE, et al. Insulin signaling coordinately regulates cardiac size, metabolism, and contractile protein isoform expression. J Clin Invest. 2002;109:629–639. doi: 10.1172/JCI13946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudina S, Sena S, O’Neill BT, Tathireddy P, Young ME, Abel ED. Reduced mitochondrial oxidative capacity and increased mitochondrial uncoupling impair myocardial energetics in obesity. Circulation. 2005;112:2686–2695. doi: 10.1161/CIRCULATIONAHA.105.554360. [DOI] [PubMed] [Google Scholar]
- Boudina S, Sena S, Theobald H, Sheng X, Wright JJ, Hu XX, Aziz S, Johnson JI, Bugger H, Zaha VG, et al. Mitochondrial energetics in the heart in obesity related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes. 2007 doi: 10.2337/db07-0481. [DOI] [PubMed] [Google Scholar]
- Brand MD, Pakay JL, Ocloo A, Kokoszka J, Wallace DC, Brookes PS, Cornwall EJ. The basal proton conductance of mitochondria depends on adenine nucleotide translocase content. Biochem J. 2005;392:353–362. doi: 10.1042/BJ20050890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burelle Y, Wambolt RB, Grist M, Parsons HL, Chow JC, Antler C, Bonen A, Keller A, Dunaway GA, Popov KM, et al. Regular exercise is associated with a protective metabolic phenotype in the rat heart. Am J Physiol Heart Circ Physiol. 2004;287:H1055–1063. doi: 10.1152/ajpheart.00925.2003. [DOI] [PubMed] [Google Scholar]
- Chen WS, Xu PZ, Gottlob K, Chen ML, Sokol K, Shiyanova T, Roninson I, Weng W, Suzuki R, Tobe K, et al. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 2001;15:2203–2208. doi: 10.1101/gad.913901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien KR, Sen A, Reynolds R, Chang A, Kim Y, Gunn MD, Buja LM, Willerson JT. Release of arachidonate from membrane phospholipids in cultured neonatal rat myocardial cells during adenosine triphosphate depletion. Correlation with the progression of cell injury. J Clin Invest. 1985;75:1770–1780. doi: 10.1172/JCI111889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB, 3rd, Kaestner KH, Bartolomei MS, Shulman GI, Birnbaum MJ. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta) Science. 2001a;292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. The Journal of biological chemistry. 2001b;276:38349–38352. doi: 10.1074/jbc.C100462200. [DOI] [PubMed] [Google Scholar]
- Clark RJ, Rodnick KJ. Morphometric and biochemical characteristics of ventricular hypertrophy in male rainbow trout (Oncorhynchus mykiss) J Exp Biol. 1998;201:1541–1552. doi: 10.1242/jeb.201.10.1541. [DOI] [PubMed] [Google Scholar]
- Condorelli G, Drusco A, Stassi G, Bellacosa A, Roncarati R, Iaccarino G, Russo MA, Gu Y, Dalton N, Chung C, et al. Akt induces enhanced myocardial contractility and cell size in vivo in transgenic mice. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:12333–12338. doi: 10.1073/pnas.172376399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crackower MA, Oudit GY, Kozieradzki I, Sarao R, Sun H, Sasaki T, Hirsch E, Suzuki A, Shioi T, Irie-Sasaki J, et al. Regulation of myocardial contractility and cell size by distinct PI3K-PTEN signaling pathways. Cell. 2002;110:737–749. doi: 10.1016/s0092-8674(02)00969-8. [DOI] [PubMed] [Google Scholar]
- DeBosch B, Treskov I, Lupu TS, Weinheimer C, Kovacs A, Courtois M, Muslin AJ. Akt1 is required for physiological cardiac growth. Circulation. 2006;113:2097–2104. doi: 10.1161/CIRCULATIONAHA.105.595231. [DOI] [PubMed] [Google Scholar]
- Dorn GW, 2nd, Force T. Protein kinase cascades in the regulation of cardiac hypertrophy. J Clin Invest. 2005;115:527–537. doi: 10.1172/JCI24178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etgen GJ, Valasek KM, Broderick CL, Miller AR. In vivo adenoviral delivery of recombinant human protein kinase C-zeta stimulates glucose transport activity in rat skeletal muscle. The Journal of biological chemistry. 1999;274:22139–22142. doi: 10.1074/jbc.274.32.22139. [DOI] [PubMed] [Google Scholar]
- Garofalo RS, Orena SJ, Rafidi K, Torchia AJ, Stock JL, Hildebrandt AL, Coskran T, Black SC, Brees DJ, Wicks JR, et al. Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKB beta. J Clin Invest. 2003;112:197–208. doi: 10.1172/JCI16885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Luby-Phelps K, Das B, Shu X, Xia Y, Mosteller RD, Krishna UM, Falck JR, White MA, Broek D. Role of substrates and products of PI 3-kinase in regulating activation of Rac-related guanosine triphosphatases by Vav. Science. 1998;279:558–560. doi: 10.1126/science.279.5350.558. [DOI] [PubMed] [Google Scholar]
- Huss JM, Kelly DP. Nuclear receptor signaling and cardiac energetics. Circulation research. 2004;95:568–578. doi: 10.1161/01.RES.0000141774.29937.e3. [DOI] [PubMed] [Google Scholar]
- Huss JM, Kelly DP. Mitochondrial energy metabolism in heart failure: a question of balance. J Clin Invest. 2005;115:547–555. doi: 10.1172/JCI200524405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huss JM, Kopp RP, Kelly DP. Peroxisome proliferator-activated receptor coactivator-1alpha (PGC-1alpha) coactivates the cardiac-enriched nuclear receptors estrogen-related receptor-alpha and -gamma. Identification of novel leucine-rich interaction motif within PGC-1alpha. The Journal of biological chemistry. 2002;277:40265–40274. doi: 10.1074/jbc.M206324200. [DOI] [PubMed] [Google Scholar]
- Lehman JJ, Barger PM, Kovacs A, Saffitz JE, Medeiros DM, Kelly DP. Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Invest. 2000;106:847–856. doi: 10.1172/JCI10268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, McMullen JR, Sobkiw CL, Zhang L, Dorfman AL, Sherwood MC, Logsdon MN, Horner JW, DePinho RA, Izumo S, et al. Class IA phosphoinositide 3-kinase regulates heart size and physiological cardiac hypertrophy. Mol Cell Biol. 2005;25:9491–9502. doi: 10.1128/MCB.25.21.9491-9502.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massie BM, Schaefer S, Garcia J, McKirnan MD, Schwartz GG, Wisneski JA, Weiner MW, White FC. Myocardial high-energy phosphate and substrate metabolism in swine with moderate left ventricular hypertrophy. Circulation. 1995;91:1814–823. doi: 10.1161/01.cir.91.6.1814. [DOI] [PubMed] [Google Scholar]
- Matsui T, Tao J, del Monte F, Lee KH, Li L, Picard M, Force TL, Franke TF, Hajjar RJ, Rosenzweig A. Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation. 2001;104:330–335. doi: 10.1161/01.cir.104.3.330. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Ogawa W, Akimoto K, Inoue H, Miyake K, Furukawa K, Hayashi Y, Iguchi H, Matsuki Y, Hiramatsu R, et al. PKClambda in liver mediates insulin-induced SREBP-1c expression and determines both hepatic lipid content and overall insulin sensitivity. J Clin Invest. 2003;112:935–944. doi: 10.1172/JCI18816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumder PK, O’Neill BT, Roberts MW, Buchanan J, Yun UJ, Cooksey RC, Boudina S, Abel ED. Impaired cardiac efficiency and increased fatty acid oxidation in insulin-resistant ob/ob mouse hearts. Diabetes. 2004;53:2366–2374. doi: 10.2337/diabetes.53.9.2366. [DOI] [PubMed] [Google Scholar]
- McMullen JR, Shioi T, Zhang L, Tarnavski O, Sherwood MC, Kang PM, Izumo S. Phosphoinositide 3-kinase(p110alpha) plays a critical role for the induction of physiological, but not pathological, cardiac hypertrophy. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:12355–12360. doi: 10.1073/pnas.1934654100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagoshi T, Matsui T, Aoyama T, Leri A, Anversa P, Li L, Ogawa W, del Monte F, Gwathmey JK, Grazette L, et al. PI3K rescues the detrimental effects of chronic Akt activation in the heart during ischemia/reperfusion injury. J Clin Invest. 2005;115:2128–2138. doi: 10.1172/JCI23073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill BT, Abel ED. Akt1 in the cardiovascular system: friend or foe? J Clin Invest. 2005;115:2059–2064. doi: 10.1172/JCI25900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessin JE, Saltiel AR. Signaling pathways in insulin action: molecular targets of insulin resistance. J Clin Invest. 2000;106:165–169. doi: 10.1172/JCI10582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie RH, Delbridge LM. Cardiac hypertrophy, substrate utilization and metabolic remodelling: cause or effect? Clin Exp Pharmacol Physiol. 2006;33:159–166. doi: 10.1111/j.1440-1681.2006.04342.x. [DOI] [PubMed] [Google Scholar]
- Rodnick KJ, Sidell BD. Structural and biochemical analyses of cardiac ventricular enlargement in cold-acclimated striped bass. Am J Physiol. 1997;273:R252–258. doi: 10.1152/ajpregu.1997.273.1.R252. [DOI] [PubMed] [Google Scholar]
- Russell LK, Mansfield CM, Lehman JJ, Kovacs A, Courtois M, Saffitz JE, Medeiros DM, Valencik ML, McDonald JA, Kelly DP. Cardiac-specific induction of the transcriptional coactivator peroxisome proliferator-activated receptor gamma coactivator-1alpha promotes mitochondrial biogenesis and reversible cardiomyopathy in a developmental stage-dependent manner. Circulation research. 2004;94:525–533. doi: 10.1161/01.RES.0000117088.36577.EB. [DOI] [PubMed] [Google Scholar]
- Sen A, Dunnmon P, Henderson SA, Gerard RD, Chien KR. Terminally differentiated neonatal rat myocardial cells proliferate and maintain specific differentiated functions following expression of SV40 large T antigen. The Journal of biological chemistry. 1988;263:19132–19136. [PubMed] [Google Scholar]
- Shioi T, Kang PM, Douglas PS, Hampe J, Yballe CM, Lawitts J, Cantley LC, Izumo S. The conserved phosphoinositide 3-kinase pathway determines heart size in mice. Embo J. 2000;19:2537–2548. doi: 10.1093/emboj/19.11.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioi T, McMullen JR, Kang PM, Douglas PS, Obata T, Franke TF, Cantley LC, Izumo S. Akt/protein kinase B promotes organ growth in transgenic mice. Mol Cell Biol. 2002;22:2799–2809. doi: 10.1128/MCB.22.8.2799-2809.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiojima I, Sato K, Izumiya Y, Schiekofer S, Ito M, Liao R, Colucci WS, Walsh K. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J Clin Invest. 2005;115:2108–2118. doi: 10.1172/JCI24682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi CM, Kondo T, Sajan M, Luo J, Bronson R, Asano T, Farese R, Cantley LC, Kahn CR. Divergent regulation of hepatic glucose and lipid metabolism by phosphoinositide 3-kinase via Akt and PKClambda/zeta. Cell Metab. 2006;3:343–353. doi: 10.1016/j.cmet.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Vega RB, Huss JM, Kelly DP. The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor alpha in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Mol Cell Biol. 2000;20:1868–1876. doi: 10.1128/mcb.20.5.1868-1876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins BJ, Dai YS, Bueno OF, Parsons SA, Xu J, Plank DM, Jones F, Kimball TR, Molkentin JD. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circulation research. 2004;94:110–118. doi: 10.1161/01.RES.0000109415.17511.18. [DOI] [PubMed] [Google Scholar]
- Xie Z, Singleton PA, Bourguignon LY, Bikle DD. Calcium-induced human keratinocyte differentiation requires src- and fyn-mediated phosphatidylinositol 3-kinase-dependent activation of phospholipase C-gamma1. Mol Biol Cell. 2005;16:3236–3246. doi: 10.1091/mbc.E05-02-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.