Abstract
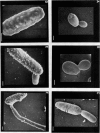
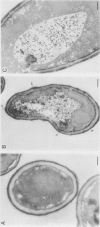
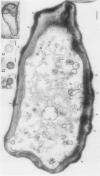
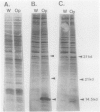
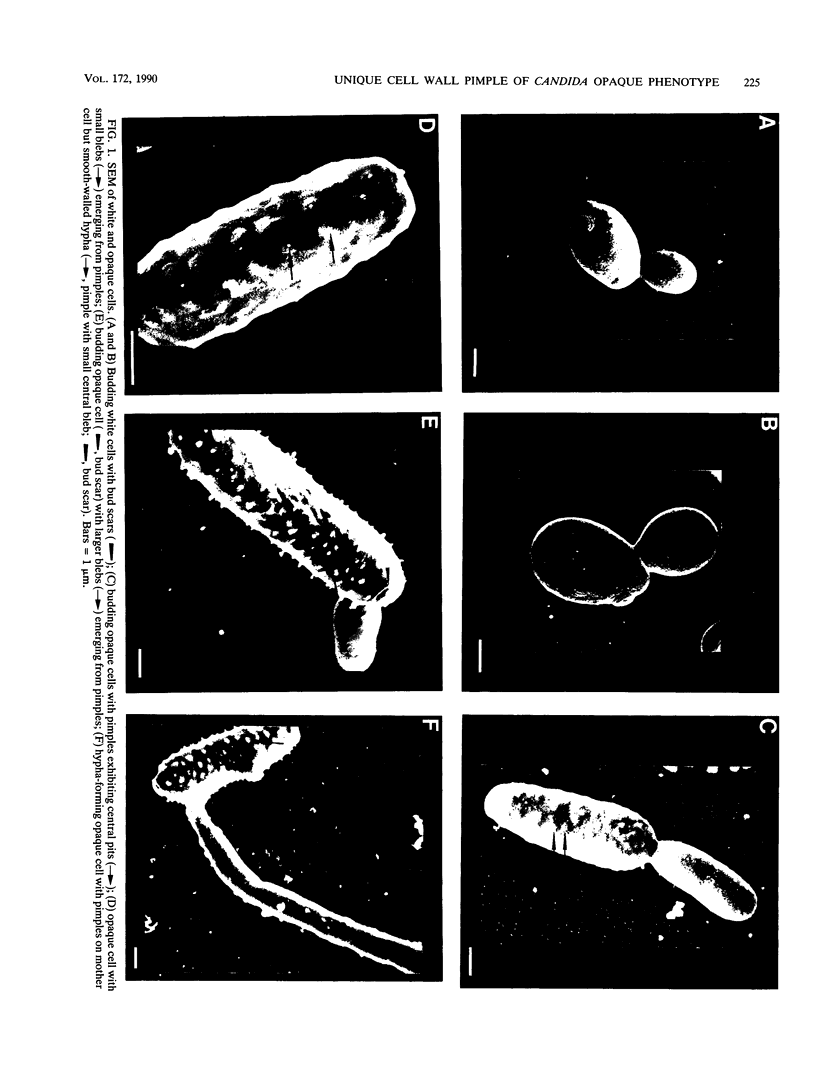
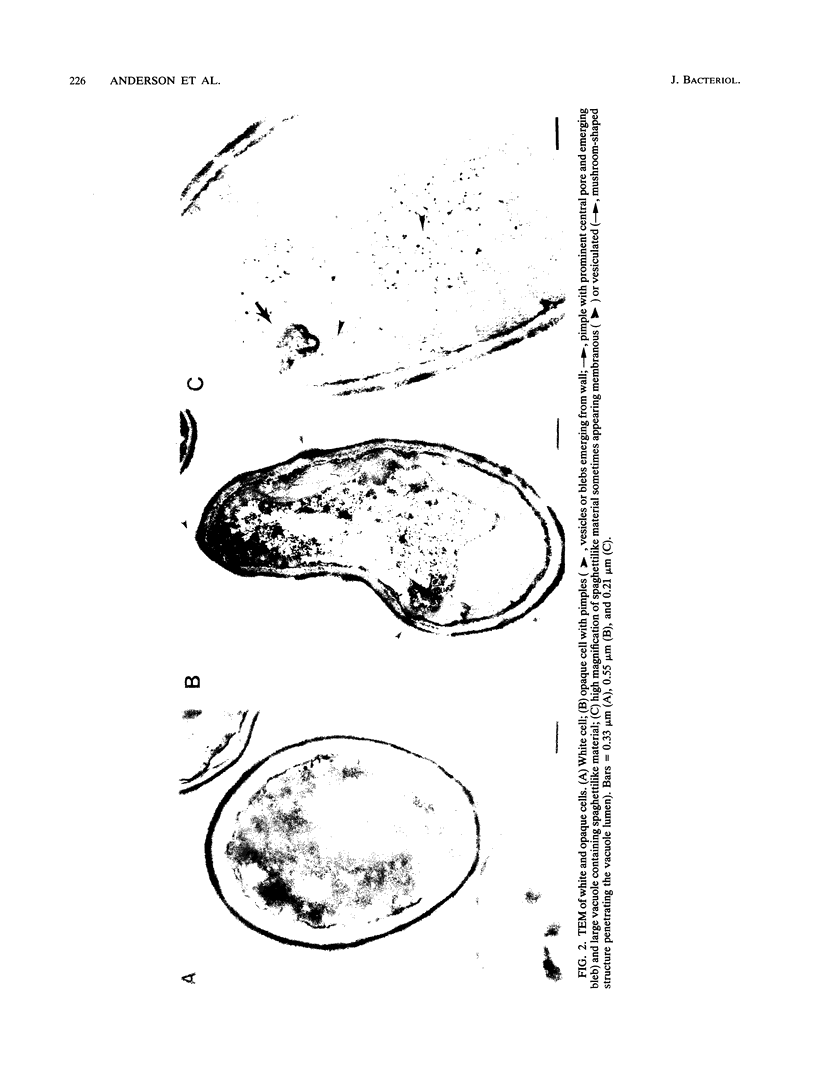
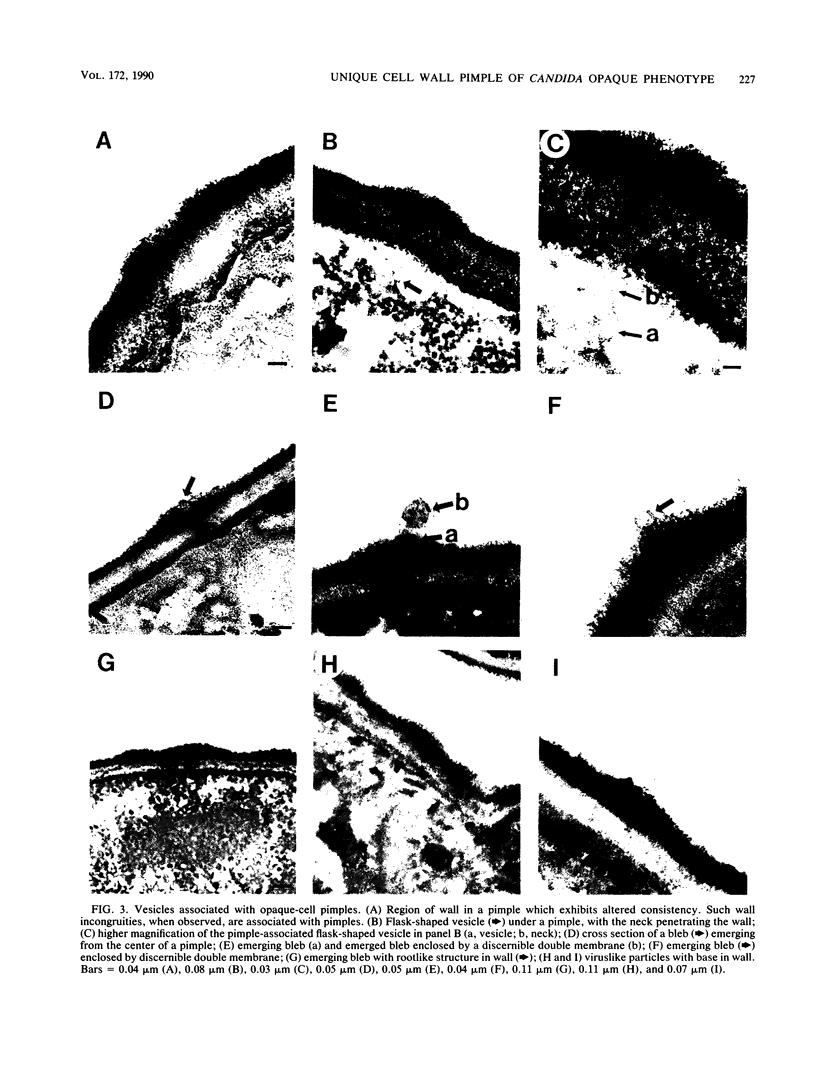
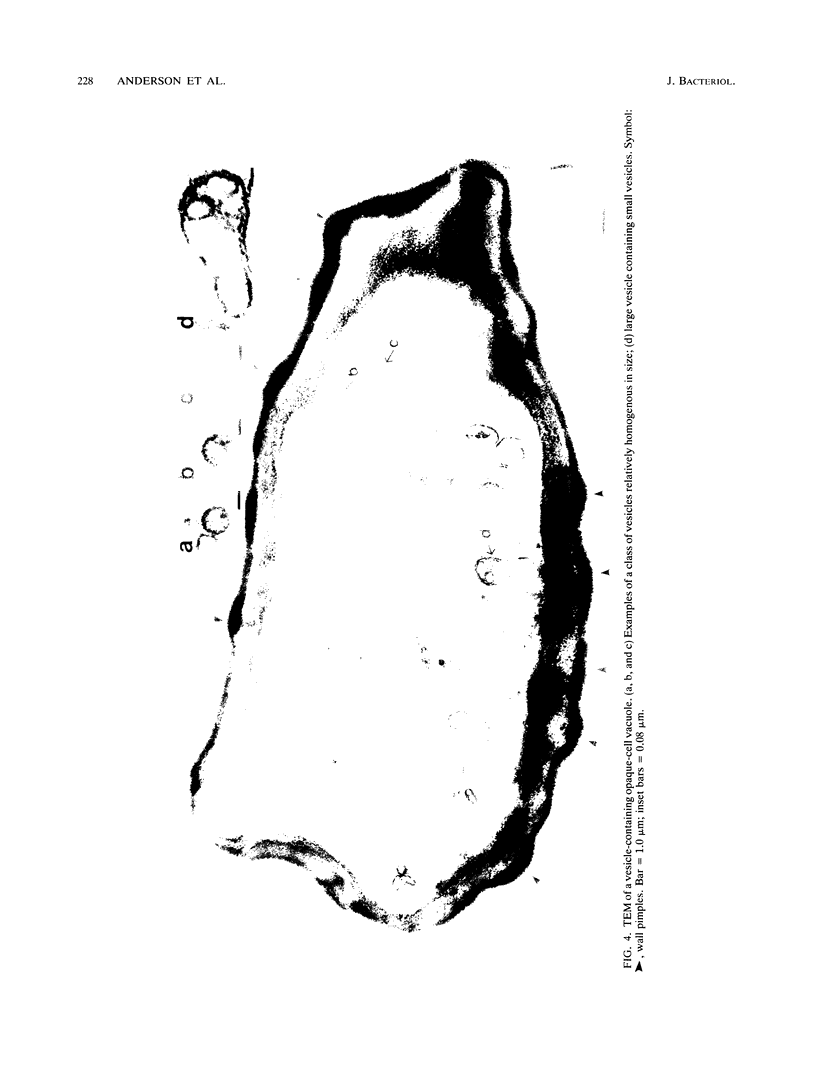
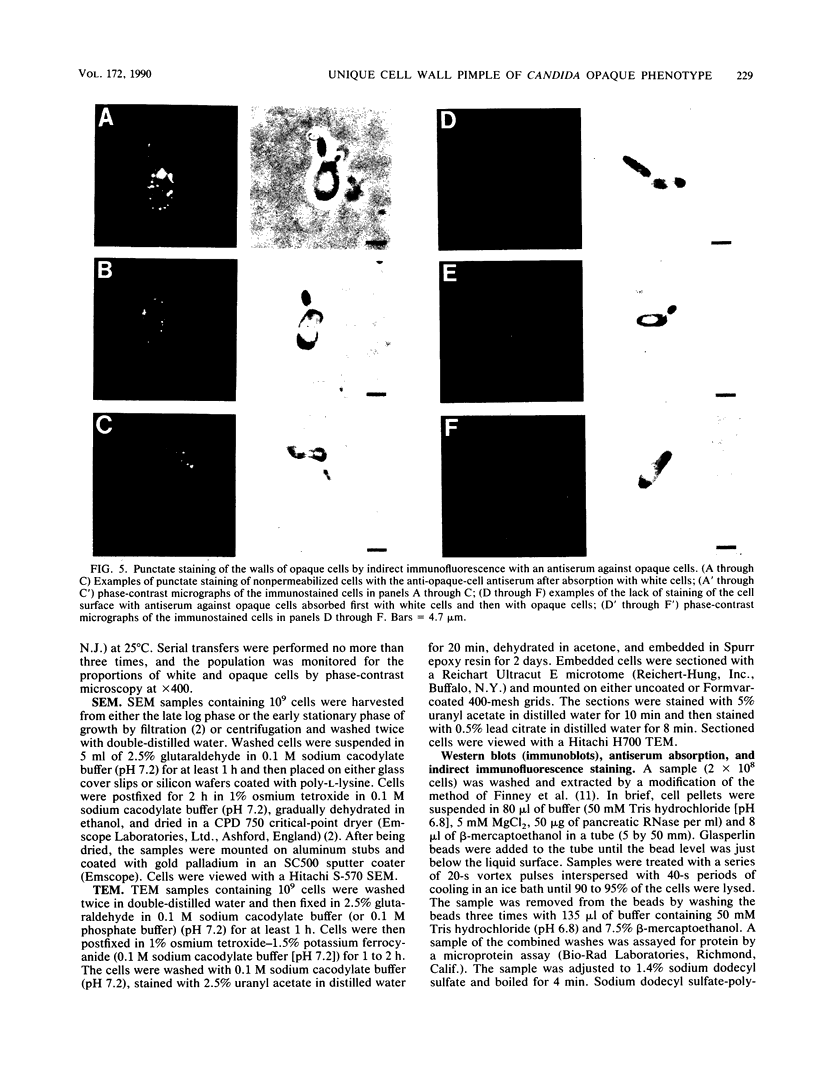
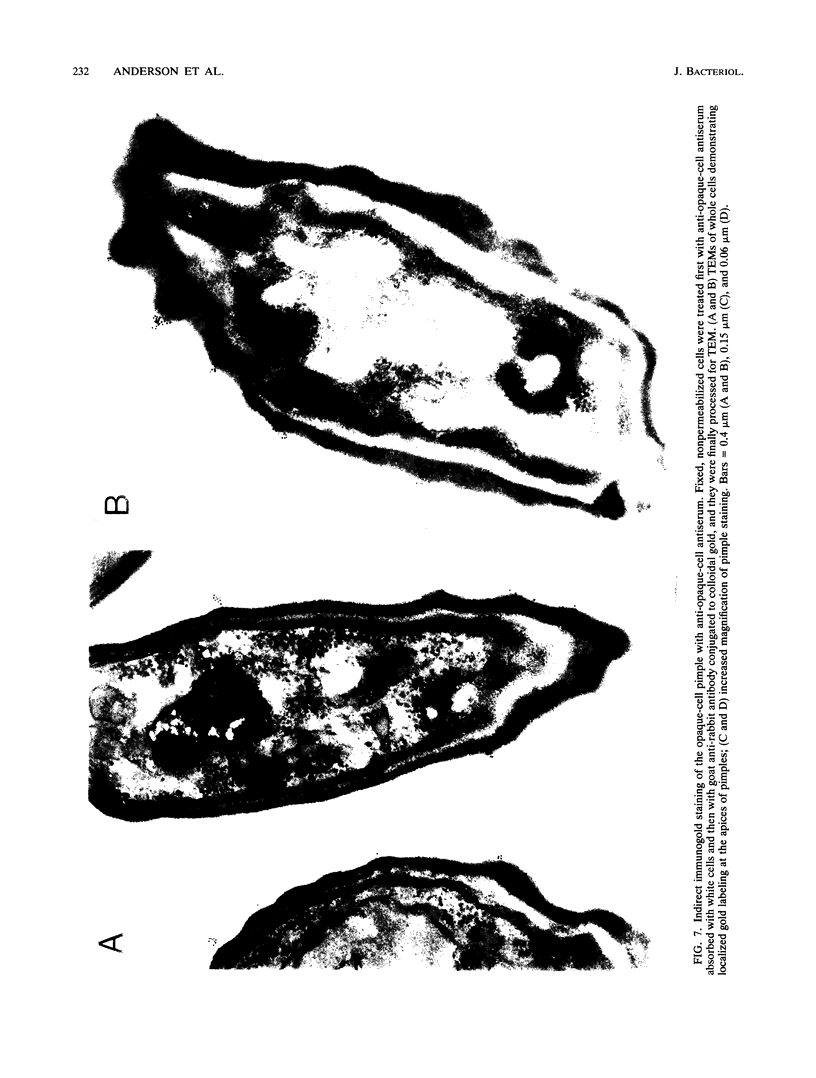
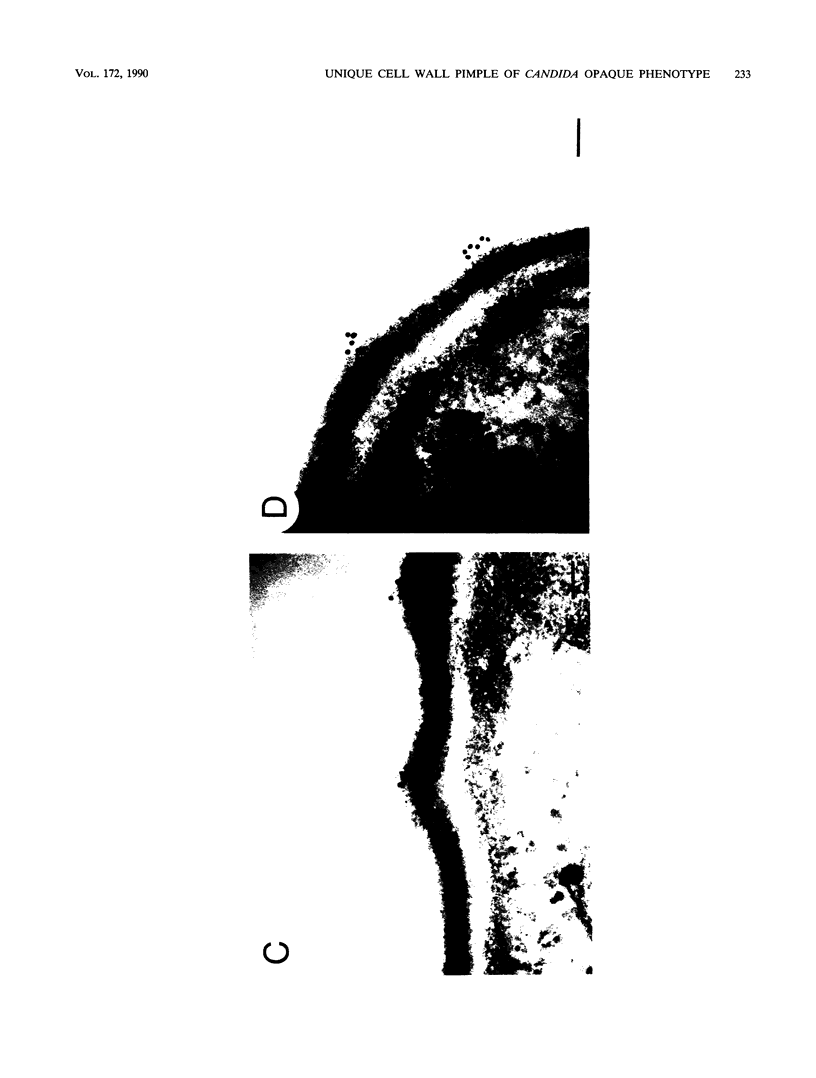
Cells of Candida albicans WO-1 switch frequently and reversibly between two colony-forming phenotypes, white and opaque. In the white form, budding cells appear similar to those of most other strains of C. albicans, but in the opaque form, budding cells are larger, are bean shaped, and possess pimples on the wall. These pimples exhibit a unique and complex morphology. With scanning electron microscopy, a central pit can be discerned, and in many cases, a bleb can be observed emerging from the pimple center. With transmission electron microscopy, channels are evident in some pimples and vesicles are apparent under the pimple in the cytoplasm, in the actual wall of the pimple, or emerging from the tip of the pimple. A large vacuole predominates in the opaque-cell cytoplasm. This vacuole is usually filled with spaghettilike membranous material and in a minority of cases is filled with vesicles, many of which exhibit a relatively uniform size. An antiserum to opaque cells recognizes three opaque-cell-specific antigens with molecular masses of approximately 14.5, 21, and 31 kilodaltons (kDa). Absorption with nonpermeabilized opaque cells demonstrated that only the 14.5-kDa antigen is on the cell surface; indirect immunogold labeling demonstrated that it is localized in or on the pimple. The possibility is suggested that the vacuole of opaque cells is the origin of membrane-bound vesicles which traverse the wall through specialized pimple structures and emerge from the pimple with an intact outer double membrane, a unique phenomenon in yeast cells. The opaque-cell-specific 14.5-kDa antigen either is in the pimple channel or is a component of the emerging vesicle. The functions of the unique opaque-cell pimple and emerging vesicle are not known.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. M., Soll D. R. Unique phenotype of opaque cells in the white-opaque transition of Candida albicans. J Bacteriol. 1987 Dec;169(12):5579–5588. doi: 10.1128/jb.169.12.5579-5588.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J., Cundiff L., Schnars B., Gao M. X., Mackenzie I., Soll D. R. Hypha formation in the white-opaque transition of Candida albicans. Infect Immun. 1989 Feb;57(2):458–467. doi: 10.1128/iai.57.2.458-467.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedell G. W., Soll D. R. Effects of low concentrations of zinc on the growth and dimorphism of Candida albicans: evidence for zinc-resistant and -sensitive pathways for mycelium formation. Infect Immun. 1979 Oct;26(1):348–354. doi: 10.1128/iai.26.1.348-354.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeke J. D., Garfinkel D. J., Styles C. A., Fink G. R. Ty elements transpose through an RNA intermediate. Cell. 1985 Mar;40(3):491–500. doi: 10.1016/0092-8674(85)90197-7. [DOI] [PubMed] [Google Scholar]
- Borst P., Greaves D. R. Programmed gene rearrangements altering gene expression. Science. 1987 Feb 6;235(4789):658–667. doi: 10.1126/science.3544215. [DOI] [PubMed] [Google Scholar]
- Calderone R. A., Cihlar R. L., Lee D. D., Hoberg K., Scheld W. M. Yeast adhesion in the pathogenesis of endocarditis due to Candida albicans: studies with adherence-negative mutants. J Infect Dis. 1985 Oct;152(4):710–715. doi: 10.1093/infdis/152.4.710. [DOI] [PubMed] [Google Scholar]
- Fine D., Schochetman G. Type D primate retroviruses: a review. Cancer Res. 1978 Oct;38(10):3123–3139. [PubMed] [Google Scholar]
- Finney R., Langtimm C. J., Soll D. R. The programs of protein synthesis accompanying the establishment of alternative phenotypes in Candida albicans. Mycopathologia. 1985 Jul;91(1):3–15. doi: 10.1007/BF00437280. [DOI] [PubMed] [Google Scholar]
- Kennedy M. J., Rogers A. L., Hanselmen L. R., Soll D. R., Yancey R. J., Jr Variation in adhesion and cell surface hydrophobicity in Candida albicans white and opaque phenotypes. Mycopathologia. 1988 Jun;102(3):149–156. doi: 10.1007/BF00437397. [DOI] [PubMed] [Google Scholar]
- Kwon-Chung K. J., Lehman D., Good C., Magee P. T. Genetic evidence for role of extracellular proteinase in virulence of Candida albicans. Infect Immun. 1985 Sep;49(3):571–575. doi: 10.1128/iai.49.3.571-575.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee K. L., Buckley H. R., Campbell C. C. An amino acid liquid synthetic medium for the development of mycelial and yeast forms of Candida Albicans. Sabouraudia. 1975 Jul;13(2):148–153. doi: 10.1080/00362177585190271. [DOI] [PubMed] [Google Scholar]
- Pomés R., Gil C., Nombela C. Genetic analysis of Candida albicans morphological mutants. J Gen Microbiol. 1985 Aug;131(8):2107–2113. doi: 10.1099/00221287-131-8-2107. [DOI] [PubMed] [Google Scholar]
- Rikkerink E. H., Magee B. B., Magee P. T. Opaque-white phenotype transition: a programmed morphological transition in Candida albicans. J Bacteriol. 1988 Feb;170(2):895–899. doi: 10.1128/jb.170.2.895-899.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slutsky B., Buffo J., Soll D. R. High-frequency switching of colony morphology in Candida albicans. Science. 1985 Nov 8;230(4726):666–669. doi: 10.1126/science.3901258. [DOI] [PubMed] [Google Scholar]
- Slutsky B., Staebell M., Anderson J., Risen L., Pfaller M., Soll D. R. "White-opaque transition": a second high-frequency switching system in Candida albicans. J Bacteriol. 1987 Jan;169(1):189–197. doi: 10.1128/jb.169.1.189-197.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll D. R., Kraft B. A comparison of high frequency switching in the yeast Candida albicans and the slime mold Dictyostelium discoideum. Dev Genet. 1988;9(4-5):615–628. doi: 10.1002/dvg.1020090438. [DOI] [PubMed] [Google Scholar]
- Soll D. R., Langtimm C. J., McDowell J., Hicks J., Galask R. High-frequency switching in Candida strains isolated from vaginitis patients. J Clin Microbiol. 1987 Sep;25(9):1611–1622. doi: 10.1128/jcm.25.9.1611-1622.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll D. R., Staebell M., Langtimm C., Pfaller M., Hicks J., Rao T. V. Multiple Candida strains in the course of a single systemic infection. J Clin Microbiol. 1988 Aug;26(8):1448–1459. doi: 10.1128/jcm.26.8.1448-1459.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll D. R. The regulation of cellular differentiation in the dimorphic yeast Candida albicans. Bioessays. 1986 Jul;5(1):5–11. doi: 10.1002/bies.950050103. [DOI] [PubMed] [Google Scholar]
- Watson K., Arthur H. Cell surface topography of Candida and Leucosporidium yeasts as revealed by scanning electron microscopy. J Bacteriol. 1977 Apr;130(1):312–317. doi: 10.1128/jb.130.1.312-317.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]