Abstract
In this study, we investigated the role of elevated sarcoplasmic reticulum (SR) Ca2+ leak through ryanodine receptors (RyR2s) in heart failure (HF)-related abnormalities of intracellular Ca2+ handling, using a canine model of chronic HF. The cytosolic Ca2+ transients were reduced in amplitude and slowed in duration in HF myocytes compared with control, changes paralleled by a dramatic reduction in the total SR Ca2+ content. Direct measurements of [Ca2+]SR in both intact and permeabilized cardiac myocytes demonstrated that SR luminal [Ca2+] is markedly lowered in HF, suggesting that alterations in Ca2+ transport rather than fractional SR volume reduction accounts for the diminished Ca2+ release capacity of SR in HF. SR Ca2+ ATPase (SERCA2)-mediated SR Ca2+ uptake rate was not significantly altered, and Na+/Ca2+ exchange activity was accelerated in HF myocytes. At the same time, SR Ca2+ leak, measured directly as a loss of [Ca2+]SR after inhibition of SERCA2 by thapsigargin, was markedly enhanced in HF myocytes. Moreover, the reduced [Ca2+]SR in HF myocytes could be nearly completely restored by the RyR2 channel blocker ruthenium red. The effects of HF on cytosolic and SR luminal Ca2+ signals could be reasonably well mimicked by the RyR2 channel agonist caffeine. Taken together, these results suggest that RyR2-mediated SR Ca2+ leak is a major factor in the abnormal intracellular Ca2+ handling that critically contributes to the reduced SR Ca2+ content of failing cardiomyocytes.
INTRODUCTION
In cardiac muscle, contraction and relaxation are controlled by the sarcoplasmic reticulum (SR), a Ca2+ storage and release organelle equipped with specialized molecules to pump Ca2+ into and release Ca2+ out of the SR lumen, namely the SR Ca2+ ATPase (SERCA2) and the ryanodine receptor (RyR2) channel, respectively (1). Contraction is triggered when a small amount of Ca2+ enters the myocyte via voltage-dependent Ca2+ channels and is amplified by Ca2+-induced Ca2+ release (CICR) (2) from the SR through RyR2s, causing activation of the myofilaments. After CICR, Ca2+ release robustly terminates and enters a refractory state, processes that are governed by the decline in intra-SR [Ca2+] that accompanies SR Ca2+ release (3–5). Once the RyR2 channels close, Ca2+ can be effectively pumped back to the SR by SERCA2, contributing to cardiac relaxation. Although most of the Ca2+ constituting the cytosolic Ca2+ transient is taken up by the SR, some Ca2+ is extruded from the cell by the Na/Ca2+ exchanger (NCX) to balance that which entered via the Ca2+ channels in the plasmalemma. Of note, Ca2+ release channels do not remain completely quiescent in diastole but instead mediate a measurable SR Ca2+ leak that increases as a function of [Ca2+]SR, and limits the SR Ca2+ content (6,7).
Systolic heart failure (HF) is a disease state associated with weakening of myocardial contractility that ultimately results in catastrophic deterioration of ventricular pump function. Although there are many etiologies of HF, reduced amplitude and prolonged duration of the systolic Ca2+ transient are characteristic features of myocytes in HF (8–10). In most studies, including those in human myocytes, these HF-associated changes in Ca2+ transients are accompanied by a decrease in the SR Ca2+ content (11–14). Although it is clear that reduced SR Ca2+ content could lead to reduced systolic Ca2+ transients and weakened contractility, the specific mechanisms responsible for the reduced SR Ca2+ content in HF remain to be defined. In a number of studies, evidence from protein expression and SERCA2 activity measurements using homogenized tissue preparations suggests that SERCA2 function is inhibited in HF (15–17), although not all studies agree (18,19). At the same time, NCX function/expression is often up-regulated during HF (12,13,20). Both of these changes are expected to result in underfilled SR Ca2+ stores by facilitating Ca2+ removal from the myocyte at the expense of its uptake into the SR. Additionally, decreased SERCA2 activity could contribute to the slowed decay kinetics of the Ca2+ transient in HF, potentially affecting diastolic function. An increase in the SR Ca2+ leak could also contribute to the reduced SR Ca2+ content and abnormal Ca2+ transients in HF. Although there is considerable disagreement regarding the specific mechanisms governing SR Ca2+ leak during HF (21–23), mounting evidence suggests that the RyR2s become functionally hyperactive, rendering the SR membrane much leakier for Ca2+ compared with controls (24,25). Strikingly, mutations in RyR2 (or its auxillary proteins) that result in increased SR Ca2+ leak are commonly associated with catecholaminergic polymorphic ventricular tachycardia rather than HF (26,27). Therefore, the specific role of the increased SR Ca2+ leak in abnormal Ca2+ handling, and in particular to the reduction of [Ca2+]SR in HF, has yet to be experimentally defined.
Using a canine chronic model of HF, we recently showed that the RyR2 channel becomes excessively active, resulting in leaky SR Ca2+ stores in HF (25). The increase in RyR2 activity is caused by defective modulation of the channel by intra-SR luminal Ca2+, a mechanism that normally operates to terminate SR Ca2+ release and keep the RyR2 channels closed (i.e., refractory) during diastole. The goal of this study was to determine the contribution of elevated SR Ca2+ leak in HF-related alterations in Ca2+ handling relative to the contributions of potential changes in SERCA2 and NCX activities. To this end, we examined intracellular Ca2+ cycling by monitoring Ca2+ changes in both the extra- and intra-SR compartments in intact and permeabilized myocytes from normal and failing hearts as well as in normal cells exposed to various concentrations of the RyR2 agonist caffeine. Our studies show that enhanced SR Ca2+ leak via RyR2s is a primary factor in abnormal intracellular Ca2+ handling during heart failure and a critical mediator of reduced SR Ca2+ content in failing cardiomyocytes.
MATERIALS AND METHODS
All animal procedures were approved by The Ohio State University Institutional Animal Care and Use Committee. Mongrel dogs had HF induced by RV tachypacing for ≥4.5 months, using a previously described protocol (28). Cardiac structure and function of control and HF dogs were analyzed using electrocardiography and echocardiography. Left ventricular fractional shortening was 41.0 ± 1.6% for 8 control dogs and 14.3 ± 1.0% for 11 HF dogs. Myocytes were isolated using previously described techniques (25) from the midmyocardium of the left ventricular lateral free wall.
Whole-cell patch clamp recordings of transmembrane ionic currents were performed with an Axopatch 200B amplifier (Axon Instruments, MDS, Sunnyvale, CA). The external solution contained (in mM): 140 NaCl, 5.4 CsCl, 2.0 CaCl2, 0.5 MgCl2, 10 Hepes, and 5.6 glucose (pH 7.4). Patch pipettes were filled with a solution that contained (in mM): 123.4 CsCl, 20 TEACl, 5 MgATP, 5 NaCl, 1 MgCl2, 0.1 Tris GTP, 10 Hepes, and 0.1 Rhod-2 or Fluo-3 K-salt (pH 7.2). Electrical field stimulation experiments were performed using the following external solution (in mM): 140 NaCl, 5.4 KCl, 5.0 CaCl2, 0.5 MgCl2, 10 Hepes, and 5.6 glucose (pH 7.4).
Intracellular Ca2+ imaging was performed using Olympus Fluoview 1000 confocal microscope in line-scan or XY mode. To monitor the intra-SR Ca2+ levels, myocytes were loaded with 10 μM Fluo-5N AM for 3–6 h at 37°C. Cytosolic Ca2+ measurements were performed using either Rhod-2 or Fluo-3 Ca2+ indicators. When measured simultaneously, Fluo-5N and Rhod-2 were excited by 488- and 543-nm laser lines, and fluorescence was acquired at wavelengths of 500–530 and >590 nm, respectively. Fluo-3 was excited by the 488-nm line of an argon-ion laser, and the fluorescence was acquired at wavelengths >510 nm. To avoid movement artifacts, contraction was suppressed by either 40 μM cytochalasin D (for the experiments shown in Fig. 1) or 10 mM 2,3-butanedione monoxime (BDM) for the experiments shown in Fig. 6.
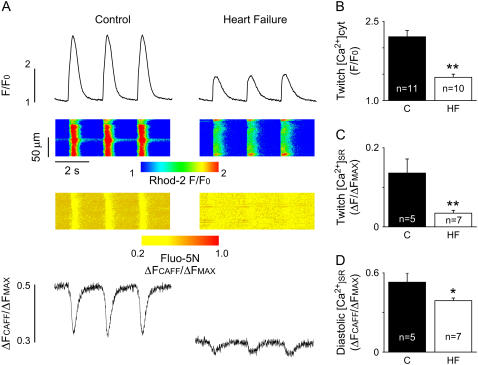
FIGURE 1.
Simultaneous measurements of cytosolic and intra-SR [Ca2+] transients. (A) Representative line-scan images of Rhod-2 (upper) and Fluo-5N (lower) fluorescence in control (C) and heart failure (HF) myocytes. Ca2+ transients were evoked by 0.5-Hz electrical field stimulation. (B) Average amplitudes (F/F0) of cytosolic Ca2+ transients were 2.21 ± 0.12 in control (C) and 1.44 ± 0.06 in HF myocytes. (C) Average amplitudes (ΔF/ΔFMAX) of SR Ca2+ depletion were 0.14 ± 0.04 in control (C) and 0.04 ± 0.007 in HF myocytes. (D) The average levels of free diastolic SR Ca2+ (ΔFCAF/ΔFMAX) in control (C) and HF myocytes were 0.53 ± 0.07 and 0.39 ± 0.02, respectively (*p < 0.05; **p < 0.01 versus controls).
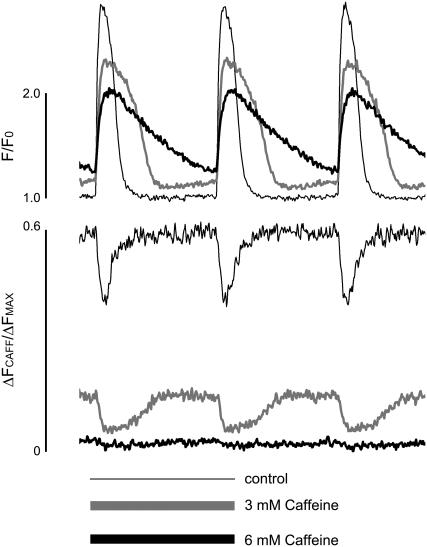
FIGURE 6.
Caffeine mimics the properties of global Ca2+ release observed in HF. Representative simultaneous recordings of cytosolic (measured with Rhod-2) and intra-SR [Ca2+] (measured with Fluo-5N) signals in voltage-clamped myocytes before and after application of the specified concentrations of caffeine. Caffeine produced significant deceleration of relaxation and decreased amplitudes of the cytosolic and intra-SR [Ca2+] transients. Ca2+ transients were evoked by 300-ms depolarizing steps from a holding potential of −50 mV to 0 mV every 2 s.
Intra-SR Ca2+ dynamics in Fluo-5N-loaded saponin-permeabilized myocytes were studied using an intracellular solution that contained (mM): 120 potassium aspartate, 20 KCl, 3 MgATP, 10 phosphocreatine, 5 U ml−1 creatine phosphokinase, 0.5 EGTA (pCa 7), and 20 HEPES (pH 7.2).
For quantitative studies, the temporal dynamics in fluorescence was expressed as ΔFCAFF/ΔFMAX = (F – FCAFF)/(FMAX − FCAFF), where F represents fluorescence at time t, FCAFF represents the fluorescence level of the cells after the application of 10 mM caffeine, and FMAX represents Fluo-5N fluorescence in the presence of 10 mM [Ca2+]. In permeabilized myocytes FMAX was determined by application of 10 mM [Ca2+] in the presence of 10 mM BDM and 1 μM ionomycin. In intact cells, FMAX was measured by application of 10 mM [Ca2+] in the presence of 10 mM BDM, 1 μM ionomycin, and 0.1% saponin. The amplitude of SR Ca2+ depletion during electrical field stimulation was expressed as ΔF/ΔFMAX = −(F – F0)/(FMAX − FCAFF), where F represents nadir fluorescence and F0 stands for baseline fluorescence. We previously determined that Fluo-5N fluorescence in the presence of 100 μM Ca2+ was quenched by <10% by 10 mM caffeine (29). Therefore, in this study Fluo-5N fluorescence was not corrected for caffeine quenching.
Results are mean ± SE, with n representing the number of different cells used. Statistical significance was evaluated either by Student's t-test or by ANOVA and t-test with Bonferroni correction, respectively. A value of p < 0.05 was considered significant.
RESULTS
Cytosolic and intra-SR [Ca2+]
We monitored intracellular Ca2+ cycling in intact control and HF myocytes by simultaneous imaging of [Ca2+] changes in the cytosolic and SR compartments using the Ca2+ indicators Rhod-2 and Fluo-5N, respectively. Representative recordings of cytosolic and intra-SR [Ca2+] changes in control and HF myocytes during field stimulation at 0.5 Hz are shown in panel A of Fig. 1, whereas panels B–D show averaged data on the amplitude characteristics of the cytosolic and luminal Ca2+ signals. Electrical stimulation elicited cytosolic Ca2+ transients associated with reciprocal dips in [Ca2+]SR. The peak amplitude of cytosolic Ca2+ transients was significantly reduced in HF myocytes compared with controls (Fig. 1 B), consistent with previous studies (13,15,25,30). At the same time, our [Ca2+]SR measurements demonstrated, for the first time, that both the baseline (i.e., diastolic) [Ca2+]SR and the extent of luminal [Ca2+] depletion during systolic Ca2+ release are dramatically reduced in HF myocytes (Fig. 1, C and D). These results are consistent with, and significantly extend, previous studies reporting changes in the total SR Ca2+ content as assessed by caffeine-induced Ca2+ release in HF (11–14).
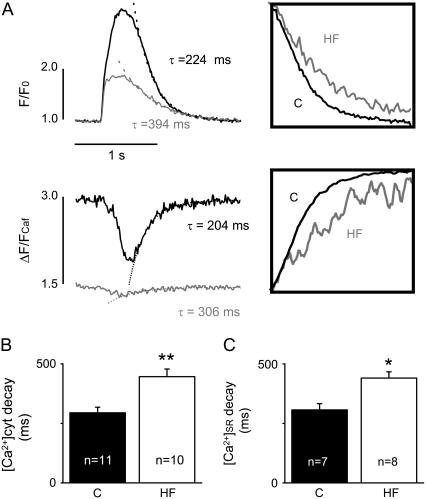
To learn more about the state of SR Ca2+ uptake/retention capacity in HF versus control myocytes, we examined the kinetics of both cytosolic and intra-SR Ca2+ transients. The time course of decline of the cytosolic Ca2+ transients was characteristically slowed in HF myocytes with respect to controls (Fig. 2, A and B). On average, the rate of Ca2+ transient decay was slowed by 36% in HF myocytes (Table 1). In addition to a reduction in Ca2+ sequestration, this alteration could be attributable to potential changes in Ca2+ transport across the sarcolemma such as slowed Ca2+ extrusion or increased Ca2+ entry on either the forward- or reverse-mode NCX, respectively. The time course of the [Ca2+]SR signal after SR Ca2+ release provides a more immediate measure of SR Ca2+ resequestration than the decay of the cytosolic Ca2+ transient. As demonstrated in Fig. 2 (A, lower panel, and C), the rate of [Ca2+]SR recovery after release was slower by 32% in HF myocytes than in controls (p < 0.05) (see also Table 1). These results suggest that the ability of SR to resequester (i.e., take up and retain) Ca2+ is impaired in HF myocytes.
FIGURE 2.
HF slows the kinetics of cytosolic and SR Ca2+ transients. (A) Representative time-dependent profiles of cytosolic (upper panel) and intra-SR (lower panel) Ca2+ transients induced by 0.5-Hz electrical field stimulation in control (C) and HF myocytes. The decay of the transients was fit by a single exponential function with specified time constants. Insets show Ca2+ transients with normalized amplitudes. (B) Average time constants of cytosolic Ca2+ transient decays were 295 ± 24 ms in control (C) and 447 ± 32 ms in HF myocytes. (C) Average time constants of the SR Ca2+ transient decays were 307 ± 26 ms in control (C) and 441 ± 26 ms in HF myocytes (*p < 0.05; **p < 0.01).
TABLE 1.
Rate constants of Ca2+ transients in control (C) and heart failure (HF) myocytes
| Rates | Control | n | Heart failure | n | HF/C |
|---|---|---|---|---|---|
| Rate twitch, s−1 | 3.6 ± 0.3 | 11 | 2.3 ± 0.2 | 10 | 64% |
| Rate NCX, s−1 | 0.39 ± 0.03 | 9 | 0.51 ± 0.04 | 9 | 131% |
| Rate SR*, s−1 | 3.4 ± 0.3 | 7 | 2.3 ± 0.1 | 8 | 68% |
Defined from the kinetics of [Ca2+]SR depletion signal; n = number of cells studied.
NCX activity
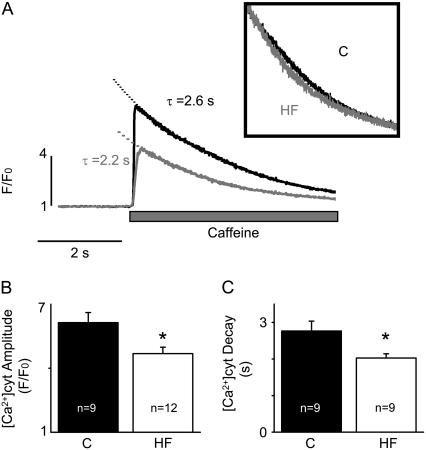
We obtained an estimate of intrinsic NCX-mediated Ca2+ extrusion activity in control versus HF myocytes by measuring the rate of cytosolic Ca2+ decline during Ca2+ transients induced by caffeine. Fig. 3 provides representative traces of Ca2+ transients elicited by 10 mM caffeine (panel A) and pooled data for the amplitude and decay time constants of caffeine-induced Ca2+ transients in control and HF myocytes (panels B and C, respectively). The amplitude of the caffeine-induced Ca2+ transients was reduced, consistent with the measurements of [Ca2+]SR above. The rate of decay of caffeine-induced Ca2+ transients showed 31% acceleration in HF myocytes, suggesting an increase in NCX Ca2+ extrusion activity (Table 1). Enhanced Ca2+ extrusion via NCX could, to some extent, contribute to the reduced [Ca2+]SR in HF myocytes. However, this influence is likely to be minor considering that the rate of Ca2+ extrusion via NCX is severalfold slower than the rate of Ca2+ resequestration into SR (Table 1).
FIGURE 3.
HF reduces SR Ca2+ content and moderately increases the rate of cytosolic Ca2+ removal by NCX. (A) Representative recordings of Ca2+ transients induced by rapid application of 10 mM caffeine in control (C) and HF myocytes. The inset shows caffeine-induced Ca2+ transients with normalized amplitude. (B) Average amplitudes (F/F0) of caffeine-induced Ca2+ transients were 6.14 ± 0.47 in control and 4.69 ± 0.30 in HF myocytes, respectively. (C) The decay time constants of the caffeine-induced Ca2+ transients in control and HF myocytes were 2.8 ± 0.3 and 2.0 ± 0.1 s, respectively (*p < 0.05).
SERCA2-mediated Ca2+ uptake
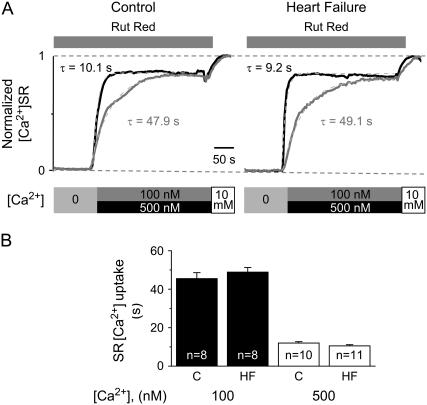
The ability of the SR to take up and retain Ca2+ is determined by two competing processes: SERCA2-mediated transport of Ca2+ to the SR lumen and Ca2+ leak through RyR2s. To assess the relative roles of these mechanisms in the HF-related decrease of SR net Ca2+ sequestration capacity, we performed direct measurements of SERCA2-mediated Ca2+ uptake in saponin-permeabilized myocytes in the presence the RyR2 antagonist ruthenium red (Rut Red). In these experiments, the SR was first depleted in a Ca2+-free solution using caffeine (10 mM). Then SR Ca2+ uptake was initiated by addition of either 100 or 500 nM Ca2+ to the bathing solution and tracked by measuring the elevation of [Ca2+]SR with Fluo-5N. Representative traces of SR Ca2+ uptake along with the experimental protocol are shown in Fig. 4 A, whereas Fig. 4 B presents pooled data on the time constants of SR Ca2+ uptake in control and HF myocytes. In HF myocytes, the time constants of SR Ca2+ uptake were not significantly different (p > 0.5) from those measured in control myocytes. Thus, the intrinsic Ca2+ uptake activity of SERCA2 appears to be unchanged in HF myocytes.
FIGURE 4.
SERCA2-mediated SR Ca2+ uptake is not significantly altered in HF myocytes. (A) Time course of SR Ca2+ uptake in control and HF permeabilized myocytes, measured with Fluo-5N-loaded SR, in the presence of 10 μM ruthenium red (Rut Red). The SR [Ca2+] was depleted with 10 mM caffeine in Ca2+-free solution, and SR Ca2+ uptake was initiated by the addition of either 100 or 500 nM of Ca2+. (B) Average time constants (from exponential fit) of SR Ca2+ uptake were 45.5 ± 3.1 s in control (C) and 48.9 ± 2.4 s in HF myocytes, when measured in the presence of 100 nM Ca2+, and 12.0 ± 0.7 s in control and 10.5 ± 0.6 s in HF myocytes, when measured in the presence of 500 nM Ca2+.
SR Ca2+ leak
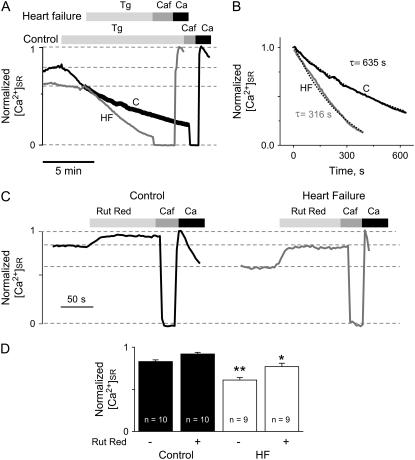
We directly measured the rate of SR Ca2+ leak by monitoring the time-dependent decline of [Ca2+]SR in permeabilized myocytes, after inhibition of SERCA2 by thapsigargin (Fig. 5 A). At steady state, [Ca2+]SR is determined by the balance between SERCA2-mediated Ca2+ uptake and Ca2+ leak through RyR2s. Similar to the results in intact cells (Fig. 1 D), the baseline [Ca2+]SR was dramatically lowered in permeabilized myoyctes in the HF group compared with controls. Inhibition of SERCA2 resulted in a decline of [Ca2+]SR in both control and HF myocytes with slopes proportional to Ca2+ leak rates. Consistent with a predominant role of Ca2+ leak in HF-related reduction of [Ca2+]SR, the SR Ca2+ leak rate was markedly enhanced in HF myocytes compared with controls (Fig. 5, A and B, and see Fig. 7 B). To ensure that the increased Ca2+ leak observed in HF myocytes was not caused by nonlinear properties of Fluo-5N, the kinetics of [Ca2+]SR decline in the presence of thapsigargin was analyzed over the same range of Fluo-5N signal in both control and HF myocytes (Fig. 5 B).
FIGURE 5.
HF increases SR Ca2+ leak. (A) Time-dependent profiles of intra-SR Ca2+ signals. Application of 10 μM of thapsigargin (Tg) evokes a steady loss of intra-SR Ca2+, visualized by the decline of the Fluo-5N signal, which was significantly faster in permeabilized myocytes from failing hearts. Then 10 mM caffeine (Caf) was applied to determine the Fluo-5N signal with depleted SR. Maximal Fluo-5N signal was determined by simultaneous application of 1 μM ionomycin with 10 mM Ca2+ and 10 mM BDM. (B) Time course of normalized Fluo-5N signals in control and HF myocytes measured in the presence of Tg, as demonstrated in panel A. Kinetic analysis was performed in both control and HF over the same range of Fluo-5N signal, as indicated in panel A by the wide black line. Dotted lines represent exponential fit to the data. (C) Time-dependent profiles of intra-SR Fluo-5N signals before and after application of 10 μM ruthenium red (Rut Red) in control and HF cells. (D) In control myocytes, normalized [Ca2+]SR levels were 0.83 ± 0.02 in the absence of, and 0.92 ± 0.02 in the presence of, Rut Red, respectively. In HF myocytes, normalized [Ca2+]SR levels were 0.61 ± 0.03 in the absence of, and 0.77 ± 0.04 in the presence of, Rut Red, respectively (*p < 0.05 versus control; **p < 0.01 versus control).
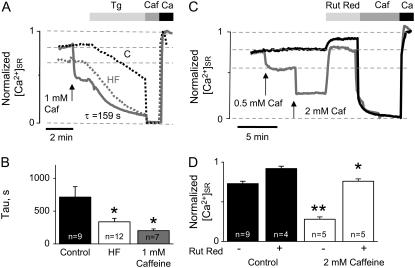
FIGURE 7.
Caffeine application to control myocytes mimics the properties of the SR Ca2+ leak observed in HF myocytes. (A) Time-dependent profiles of intra-SR Ca2+ signals. Application of 10 μM thapsigargin (Tg) evokes a steady loss of intra-SR Ca2+, visualized by the decline of Fluo-5N signal. Caffeine (1 mM) produces SR Ca2+ leak acceleration that is qualitatively similar to that occurring in HF. Dotted lines represent time-dependent profiles of intra-SR Ca2+ signals recorded in control (black) and HF (gray) myocytes. (B) Decline of Fluo-5N signal in the presence of thapsigargin was fit by an exponential function. The average time constants were 713 ± 160, 337 ± 53, and 201 ± 27 s in controls, HF myocytes, and control myocytes in the presence of 1 mM caffeine, respectively. (C) Time-dependent profiles of intra-SR Fluo-5N signals in control cells before and after application of 10 μM ruthenium red (Rut Red) in the absence and in the presence of specified concentrations of caffeine. Caffeine (Caf, 10 mM) was applied to determine Fluo-5N signal with Ca2+-depleted SR. The maximal Fluo-5N signal was determined by simultaneous application of ionomycin with 10 mM Ca2+ and 10 mM BDM. In the absence of caffeine, normalized [Ca2+]SR levels were 0.73 ± 0.03 before and 0.92 ± 0.03 during application of Rut Red, respectively. In the presence of 2 mM caffeine, normalized [Ca2+]SR levels were 0.28 ± 0.03 before and 0.76 ± 0.03 during the application of Rut Red, respectively (*p < 0.05 versus control; **p < 0.01 versus control).
To further assess the role of enhanced RyR2-mediated Ca2+ leak in HF-related [Ca2+]SR reduction, we investigated the relative effects of Rut Red on [Ca2+]SR in permeabilized control and HF myocytes (Fig. 5 C). Application of 10 μM Rut Red produced a modest increase in [Ca2+]SR in control myocytes, indicative of a basal SR Ca2+ leak occurring via RYR2s. In HF myocytes the effect of Rut Red on [Ca2+]SR was substantially greater, suggesting that RyR2-mediated SR Ca2+ leak plays a significant role in mediating the decrease in [Ca2+]SR in HF. However, it is to be noted that Rut Red did not raise [Ca2+]SR completely to the level of [Ca2+]SR seen in control myocytes (Fig. 5, C and D). This could indicate either a lower efficacy of Rut Red as a RyR2 inhibitor in HF myocytes exhibiting a greater RyR2-mediated SR Ca2+ leak or the existence of a Ca2+ leak component mediated by a pathway(s) other then the RyR2s such as inositol trisphosphate receptors (IP3Rs) the expression of which is increased in HF (31).
Comparison with the effects of caffeine
To further examine whether, and to what extent, HF-related changes in Ca2+ cycling could be attributed to a Ca2+ leak via RyR2s, we investigated changes in cellular Ca2+ cycling caused by the RyR2 agonist caffeine in normal myocytes. Fig. 6 shows representative traces of cytosolic and SR luminal Ca2+ concentrations in a voltage-clamped myocyte stimulated at 0.5 Hz before and after exposure to two different concentrations of caffeine (3 and 6 mM). Caffeine decreased the amplitude and prolonged the duration of Ca2+ transients. At the same time, caffeine led to a decrease in both basal and systolic [Ca2+]SR. These effects of caffeine were concentration dependent with higher caffeine doses causing more profound changes. The HF-related changes in cytosolic and luminal Ca2+ signals could be approximately emulated by 3 mM caffeine. Similar results were obtained in four other myocytes.
The application of caffeine reproduced the leaky SR phenotype in permeabilized control myocytes. As shown in Fig. 7, A and C, application of low doses of caffeine to a normal myocyte induced a reduction in [Ca2+]SR similar to that observed in HF myocytes, and the application of thapsigargin produced a Ca2+ leak with a rate comparable, albeit somewhat faster than, that in HF myocytes. Pooled data on the rate of SR Ca2+ leak recorded in the presence of 1 mM caffeine in comparison with basal leak rates measured in control and HF myocyes are presented in Fig. 7 B. The effects of two incremental additions of caffeine (0.5 and 2 mM) on basal [Ca2+]SR are documented in Fig. 7 C. Again, application of 0.5 mM of caffeine lowered [Ca2+]SR to a level corresponding to that in HF, and the higher dose induced a larger although still incomplete depletion of [Ca2+]SR as revealed by addition of 10 mM at the end of the experiment. Importantly, Rut Red added in the presence of 1 mM caffeine failed to elevate [Ca2+]SR to the level attained with the same dose of Rut Red in the absence of caffeine (Fig. 7, C and D). Reminiscent of the experiments in HF myocytes, these results suggest that inhibition of RyR2s by Rut Red is incomplete at the concentration used (10 μM) or that the inhibition can be overcome by caffeine. Collectively, these results suggest that the HF-related changes in cellular Ca2+ handling can be reproduced reasonably well in control myocytes with caffeine at concentrations of 0.5–3 mM, depending on experimental settings.
DISCUSSION
In this study, we investigated the role of elevated SR Ca2+ leak through RyR2s in HF-related abnormalities of intracellular Ca2+ handling, using a canine model of chronic HF. Our study shows for the first time that the increase in the RyR2-mediated Ca2+ leak is a major factor in the reduced SR Ca2+ content and the decreased amplitude and slowed time course of cytosolic Ca2+ transients in cardiac myocytes from failing hearts.
Role of enhanced SR Ca2+ leak in abnormal Ca2+ handling in HF
Consistent with previous studies (13,15,25,30), the cytosolic Ca2+ transients were markedly reduced in amplitude and slowed in duration in HF myocytes compared with control myocytes, changes paralleled by a significant reduction in the total SR Ca2+ content. These alterations in Ca2+ handling are considered to be hallmarks of HF (8–10). The HF-induced reduction of the size of the functional Ca2+ store could be caused either by a potential decrease in SR volume (per-myocyte volume) or by a reduction of [Ca2+] in the lumen of SR in HF myocytes (32). Our direct measurements of [Ca2+]SR in both intact and permeabilized cardiac cells demonstrated that SR luminal [Ca2+] is manifestly lowered in HF myocytes, suggesting that alterations in Ca2+ transport rather than fractional SR volume contraction accounts for the diminished Ca2+ release capacity of SR in HF. Potential contributors to the decreased [Ca2+]SR in HF include reduced SR Ca2+-ATPase function, enhanced NCX Ca2+ extrusion activity, and/or increased SR Ca2+ leak. Based on our experiments, SR Ca2+ uptake rate was not significantly altered in HF myocytes. This lack of functional changes is consistent with the unchanged levels of SERCA2a expressed in failing myocytes using this particular model of HF (25). Although NCX activity was accelerated in HF myocytes, these changes in NCX function were relatively modest (∼30%) and could not account for the altered Ca2+ transients in HF myocytes. Indeed, we found that HF myocytes exhibited a similar reduction in [Ca2+]SR regardless of whether they were intact or permeabilized (Fig. 1, A and D, and Fig. 5, C and D), suggesting that NCX is not a major contributor to the reduced [Ca2+]SR in HF under our experimental conditions. At the same time, SR Ca2+ leak, measured directly as a reduction in [Ca2+]SR after inhibition of SERCA2 by thapsigargin, was markedly enhanced in HF myocytes. Moreover, the reduced [Ca2+]SR in HF myocytes could be nearly completely restored by the RyR2 channel blocker Rut Red. Thus, based on our experiments, enhanced SR Ca2+ leak is a critical factor determining the reduced [Ca2+]SR in HF with altered SERCA2- and NCX-mediated Ca2+ transport playing no major role in modulating [Ca2+]SR.
Importantly, the increased SR Ca2+ leak not only contributed to the reduced SR Ca2+ content but also influenced the dynamics of resequestration of Ca2+ into the SR and was also likely to be responsible for the slowed kinetics of the cytosolic Ca2+ transients in HF myocytes. Indeed, the kinetics of recovery of both [Ca2+]SR and cytosolic Ca2+ signals after each release was slowed in HF myocytes despite the fact that SERCA2 and NCX activities exhibited either no change or were increased, respectively, in HF myocytes. Our findings are consistent with those of Litwin et al. (33), which showed that sustained SR Ca2+ release rather than inhibition of SR Ca2+ uptake accounts for the slowed kinetics of the cytosolic Ca2+ transients in myocytes from infarcted rabbits. These results along with those obtained with caffeine (see below) suggest that caution must be exercised when using kinetics of Ca2+ transient decay as a measure of SERCA2 Ca2+ transport activity, especially in disease states where SR Ca2+ leak can profoundly affect the Ca2+ transient time course.
Effects of caffeine
The effects of HF on cytosolic and SR luminal Ca2+ signals could be reasonably well mimicked by the RyR2 channel agonist caffeine, further supporting the notion that Ca2+ leak via RyR2s is a major contributor to the HF-related changes in myocyte Ca2+ handing. Similar to the HF phenotype, caffeine at intermediate to high concentrations reduced the amplitude and slowed the time course of cytosolic Ca2+ transients and increased baseline (diastolic) [Ca2+]cyt in control cells. The inhibitory effects of intermediate and high concentrations of caffeine on the amplitude and rate of decay of the cytosolic Ca2+ transient are consistent with previous studies (34). Caffeine also reduced both the diastolic and systolic [Ca2+]SR and slowed the recovery rate of [Ca2+]SR after Ca2+ release. The HF-induced changes in the parameters of cytosolic and luminal Ca2+ signals could be approximately matched with the addition of 0.5–3 mM caffeine, permitting us to “calibrate” the impact of HF on SR Ca2+ leak in terms of effects of a well-studied RyR opener.
Considerations of Ca2+ homeostasis
Based on studies using low concentrations of the RyR2 channel modulators caffeine and tetracaine, it has been shown (35) that the SR Ca2+ release mechanism in cardiac myocytes possesses a capacity for self-regulation, which allows myocytes to maintain Ca2+ transients of constant amplitude in the face of interventions that alter RyR2 activity. Thus, a reduction in Ca2+ transient amplitude by such an intervention would result in both reduced Ca2+ extrusion via NCX and reduced Ca2+-dependent inactivation of ICa and, hence, increased retention of Ca2+ in the myocyte, which would contribute to the Ca2+ transient during the next Ca2+ release cycle. Therefore, one might expect that the RyR2-mediated leak in HF would lead only to a transient change in the amplitude of cytosolic Ca2+ transients without producing steady-state inhibitory (or potentiatory) effects on cytosolic Ca2+ transients. However, as we demonstrated by the application of moderate to high doses of caffeine, the ability of the SR to preserve normal Ca2+ release amplitude depends on the extent to which the RyR2 function is altered. When the RyR2 becomes excessively active, the SR loses its capacity to retain Ca2+ during diastole, resulting in a partial or even complete elimination of the SR Ca2+ store. Because the majority of Ca2+ contributing to the Ca2+ transient comes from the SR, this unavoidably leads to reduced Ca2+ transients as is manifested in HF myocytes.
Mechanisms for enhanced RyR2 activity
The specific biochemical defect(s) causing RyR2 to become leaky in HF is not known. It has been proposed that RyR2 phosphorylation either by PKA or CAMKII may contribute to the SR leak in HF by rendering RyR2s hyperactive (21,36–39). Alternatively, enhanced RyR2 activity could be caused by modification of the channel protein by reactive oxygen or reactive nitrogen species generated in HF (40,41). Recently, using the same canine model, we demonstrated that the enhanced RyR2 activity in HF is caused by sensitization of the RyR2 to activation by luminal Ca2+ (25). This finding explains why SR Ca2+ leak is high despite the reduced SR luminal Ca2+ levels in HF myocytes, considering the well-known positive relationship between SR Ca2+ load and RyR2 activity (42). Consistent with this mechanism, our direct measurements of [Ca2+]SR showed that Ca2+ release terminates at much lower [Ca2+]SR in HF myocytes compared with normal myocytes, indicative of an extended range of Ca2+ concentrations enhancing RyR2 channel activity in HF. It remains to be seen whether these changes in RyR2 function are also modulated by changes in RyR2 phosphorylation or redox modulation. Because regulation of RyR2s by luminal Ca2+ may involve RyR2's luminal binding partners, including calsequestrin, triadin, and junctin (43,44), it is also conceivable that alterations in interactions of RyR2 with these proteins contribute to abnormal RyR2 channel function in HF. Although the levels of these proteins are not changed, RyR2 expression is markedly reduced in this model of HF (25). Because both triadin and junctin appear to increase RyR2 activity (44), an increased ratio of triadin and junctin to RyR2 could contribute to increased RyR2 function in HF. Additionally, CASQ2 glycosylation is dramatically altered in HF, indicative of defective junctional SR trafficking and Ca2+ release complex assembly (45).
Comparison with previous studies
Our results corroborate previous studies that demonstrated that an elevated SR Ca2+ leak exists in myocytes from failing hearts using rabbit and dog HF models (24,25). Moreover, our data show that enhanced SR Ca2+ leak can be a primary determinant of altered Ca2+ handling in HF myocytes. At this time it is unclear whether the prevailing role of SR Ca2+ leak and the apparent lack of effects on SR Ca2+ uptake are specific features of this particular disease model or are more general properties of HF. The contribution of SR Ca2+ leak to HF-related alterations in SR function may vary among different models and stages of HF and is difficult to quantify because of the presence of competing Ca2+ fluxes, including those mediated by SERCA2 and NCX. Application of approaches developed in this study to other models and stages of HF should certainly clarify this issue.
Using a shorter-term (3–6 weeks) canine model of tachypacing-induced HF, Hobai and O'Rourke (13) showed that reduced SR Ca2+ content is responsible for depressed SR Ca2+ release in HF. Although potential changes in SERCA2 function and SR Ca2+ leak were not estimated in that study, the marked prolongation of the Ca2+ transient decay indicates that either the uptake or retention of Ca2+ by the SR, or both, were affected through potential inhibition of SERCA2 and/or stimulation of SR Ca2+ leak. At the same time, NCX Ca2+ extrusion activity was enhanced by 50% and was also likely to be contributing to the reduced SR Ca2+ content. A study using a rabbit model of HF found a substantial SR Ca2+ leak (24), but its significance was deemed secondary to enhanced NCX-mediated Ca2+ extrusion in view of the marked up-regulation of NCX activity in this model. In human HF, NCX was shown to change little, and alterations in Ca2+ handling were ascribed to the reduced Ca2+ uptake capacity of the SR, manifested as slowed Ca2+ transient decay (14). Because the slowed kinetics of the Ca2+ transient could be attributable to both slowed SERCA2-mediated Ca2+ uptake and increased SR Ca2+ leak, it is possible that in human HF, increased SR Ca2+ leak is an important factor in acquisition of the HF-related Ca2+ transient phenotype, as we show in our chronic canine model of HF. Although it demonstrates the importance of a SR Ca2+ leak, our study does not rule out other potential mechanisms, including failure of SR Ca2+ release activation caused by structural changes (46) or alterations in myocyte electrical excitability (47), contributing to defective Ca2+ signaling in the failing heart.
CONCLUSION
In conclusion, this study used a large animal model of chronic heart failure, closely relevant to human disease, to demonstrate that elevated Ca2+ leak from the SR via RyR2s can be a major factor in determining the altered properties of cytosolic Ca2+ transients in HF. Our findings support the notion that targeting the RyR2 might be a logical therapeutic approach to treating HF (48). However, implementing a successful therapy based on RyR2 targeting could be challenging, considering the complex nature of store-mediated Ca2+ signaling. Indeed, inhibition of RyR2s may fix the leak but at the same time impair systolic function. Establishing the precise molecular mechanism(s) responsible for HF-related abnormalities in RyR2s should facilitate the development of a rational strategy to normalize Ca2+ cycling and myocardial performance in HF.
Acknowledgments
Authors thank Arun Sridhar for his help in myocyte isolation.
This work was supported by the American Heart Association (A.B., D.T., C.A.C.), National Institutes of Health Grants HL074045 and HL063043 (S.G.), and by the Slovak Research and Development Agency under contract No. LPP-0099-06. Pacemakers were generously provided by Medtronic, Inc., Minneapolis, MN.
Andriy Belevych, Zuzana Kubalova, and Dmitry Terentyev contributed equally to this work.
Editor: David A. Eisner.
References
- 1.Bers, D. M. 2001. Excitation-contraction coupling and cardiac contractile force. Dordrecht, Boston: Kluwer Academic Publishers. xxiv.
- 2.Fabiato, A. 1985. Time and calcium dependence of activation and inactivation of calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J. Gen. Physiol. 85:247–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Terentyev, D., S. Viatchenko-Karpinski, H. H. Valdivia, A. L. Escobar, and S. Gyorke. 2002. Luminal Ca2+ controls termination and refractory behavior of Ca2+-induced Ca2+ release in cardiac myocytes. Circ. Res. 91:414–420. [DOI] [PubMed] [Google Scholar]
- 4.Shannon, T. R., T. Guo, and D. M. Bers. 2003. Ca2+ scraps: local depletions of free [Ca2+] in cardiac sarcoplasmic reticulum during contractions leave substantial Ca2+ reserve. Circ. Res. 93:40–45. [DOI] [PubMed] [Google Scholar]
- 5.Sobie, E. A., K. W. Dilly, J. dos Santos Cruz, W. J. Lederer, and M. S. Jafri. 2002. Termination of cardiac Ca2+ sparks: an investigative mathematical model of calcium-induced calcium release. Biophys. J. 83:59–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lukyanenko, V., S. Viatchenko-Karpinski, A. Smirnov, T. F. Wiesner, and S. Gyorke. 2001. Dynamic regulation of sarcoplasmic reticulum Ca2+ content and release by luminal Ca2+-sensitive leak in rat ventricular myocytes. Biophys. J. 81:785–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shannon, T. R., K. S. Ginsburg, and D. M. Bers. 2002. Quantitative assessment of the SR Ca2+ leak-load relationship. Circ. Res. 91:594–600. [DOI] [PubMed] [Google Scholar]
- 8.Hasenfuss, G., and B. Pieske. 2002. Calcium cycling in congestive heart failure. J. Mol. Cell. Cardiol. 34:951–969. [DOI] [PubMed] [Google Scholar]
- 9.Houser, S. R., and K. B. Margulies. 2003. Is depressed myocyte contractility centrally involved in heart failure? Circ. Res. 92:350–358. [DOI] [PubMed] [Google Scholar]
- 10.Sipido, K. R., and D. Eisner. 2005. Something old, something new: changing views on the cellular mechanisms of heart failure. Cardiovasc. Res. 68:167–174. [DOI] [PubMed] [Google Scholar]
- 11.Lindner, M., E. Erdmann, and D. J. Beuckelmann. 1998. Calcium content of the sarcoplasmic reticulum in isolated ventricular myocytes from patients with terminal heart failure. J. Mol. Cell. Cardiol. 30:743–749. [DOI] [PubMed] [Google Scholar]
- 12.Pogwizd, S. M., M. Qi, W. Yuan, A. M. Samarel, and D. M. Bers. 1999. Upregulation of Na+/Ca2+ exchanger expression and function in an arrhythmogenic rabbit model of heart failure. Circ. Res. 85:1009–1019. [DOI] [PubMed] [Google Scholar]
- 13.Hobai, I. A., and B. O'Rourke. 2001. Decreased sarcoplasmic reticulum calcium content is responsible for defective excitation-contraction coupling in canine heart failure. Circulation. 103:1577–1584. [DOI] [PubMed] [Google Scholar]
- 14.Piacentino, V., 3rd, C. R. Weber, X. Chen, J. Weisser-Thomas, K. B. Margulies, D. M. Bers, and S. R. Houser. 2003. Cellular basis of abnormal calcium transients of failing human ventricular myocytes. Circ. Res. 92:651–658. [DOI] [PubMed] [Google Scholar]
- 15.Pieske, B., B. Kretschmann, M. Meyer, C. Holubarsch, J. Weirich, H. Posival, K. Minami, H. Just, and G. Hasenfuss. 1995. Alterations in intracellular calcium handling associated with the inverse force-frequency relation in human dilated cardiomyopathy. Circulation. 92:1169–1178. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt, U., R. J. Hajjar, P. A. Helm, C. S. Kim, A. A. Doye, and J. K. Gwathmey. 1998. Contribution of abnormal sarcoplasmic reticulum ATPase activity to systolic and diastolic dysfunction in human heart failure. J. Mol. Cell. Cardiol. 30:1929–1937. [DOI] [PubMed] [Google Scholar]
- 17.Jiang, M. T., A. J. Lokuta, E. F. Farrell, M. R. Wolff, R. A. Haworth, and H. H. Valdivia. 2002. Abnormal Ca2+ release, but normal ryanodine receptors, in canine and human heart failure. Circ. Res. 91:1015–1022. [DOI] [PubMed] [Google Scholar]
- 18.Movsesian, M. A., M. Karimi, K. Green, and L. R. Jones. 1994. Ca2+-transporting ATPase, phospholamban, and calsequestrin levels in nonfailing and failing human myocardium. Circulation. 90:653–657. [DOI] [PubMed] [Google Scholar]
- 19.Schwinger, R. H., M. Bohm, U. Schmidt, P. Karczewski, U. Bavendiek, M. Flesch, E. G. Krause, and E. Erdmann. 1995. Unchanged protein levels of SERCA II and phospholamban but reduced Ca2+ uptake and Ca2+-ATPase activity of cardiac sarcoplasmic reticulum from dilated cardiomyopathy patients compared with patients with nonfailing hearts. Circulation. 92:3220–3228. [DOI] [PubMed] [Google Scholar]
- 20.Pieske, B., L. S. Maier, D. M. Bers, and G. Hasenfuss. 1999. Ca2+ handling and sarcoplasmic reticulum Ca2+ content in isolated failing and nonfailing human myocardium. Circ. Res. 85:38–46. [DOI] [PubMed] [Google Scholar]
- 21.Marx, S. O., S. Reiken, Y. Hisamatsu, T. Jayaraman, D. Burkhoff, N. Rosemblit, and A. R. Marks. 2000. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 101:365–376. [DOI] [PubMed] [Google Scholar]
- 22.Marks, A. R. 2003. A guide for the perplexed: towards an understanding of the molecular basis of heart failure. Circulation. 107:1456–1459. [DOI] [PubMed] [Google Scholar]
- 23.Bers, D. M., D. A. Eisner, and H. H. Valdivia. 2003. Sarcoplasmic reticulum Ca2+ and heart failure: roles of diastolic leak and Ca2+ transport. Circ. Res. 93:487–490. [DOI] [PubMed] [Google Scholar]
- 24.Shannon, T. R., S. M. Pogwizd, and D. M. Bers. 2003. Elevated sarcoplasmic reticulum Ca2+ leak in intact ventricular myocytes from rabbits in heart failure. Circ. Res. 93:592–594. [DOI] [PubMed] [Google Scholar]
- 25.Kubalova, Z., D. Terentyev, S. Viatchenko-Karpinski, Y. Nishijima, I. Gyorke, R. Terentyeva, D. N. da Cunha, A. Sridhar, D. S. Feldman, R. L. Hamlin, C. A. Carnes, and S. Györke. 2005. Abnormal intrastore calcium signaling in chronic heart failure. Proc. Natl. Acad. Sci. USA. 102:14104–14109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Priori, S. G., C. Napolitano, N. Tiso, M. Memmi, G. Vignati, R. Bloise, V. Sorrentino, and G. A. Danieli. 2001. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation. 103:196–200. [DOI] [PubMed] [Google Scholar]
- 27.di Barletta, M. R., S. Viatchenko-Karpinski, A. Nori, M. Memmi, D. Terentyev, F. Turcato, G. Valle, N. Rizzi, C. Napolitano, S. Gyorke, P. Volpe, and S. G. Priori. 2006. Clinical phenotype and functional characterization of CASQ2 mutations associated with catecholaminergic polymorphic ventricular tachycardia. Circulation. 114:1012–1019. [DOI] [PubMed] [Google Scholar]
- 28.Nishijima, Y., D. S. Feldman, J. D. Bonagura, Y. Ozkanlar, P. J. Jenkins, V. A. Lacombe, W. T. Abraham, R. L. Hamlin, and C. A. Carnes. 2005. Canine nonischemic left ventricular dysfunction: a model of chronic human cardiomyopathy. J. Card. Fail. 11:638–644. [DOI] [PubMed] [Google Scholar]
- 29.Kubalova, Z., I. Gyorke, R. Terentyeva, S. Viatchenko-Karpinski, D. Terentyev, S. C. Williams, and S. Gyorke. 2004. Modulation of cytosolic and intra-sarcoplasmic reticulum calcium waves by calsequestrin in rat cardiac myocytes. J. Physiol. 561:515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beuckelmann, D. J., M. Nabauer, and E. Erdmann. 1992. Intracellular calcium handling in isolated ventricular myocytes from patients with terminal heart failure. Circulation. 85:1046–1055. [DOI] [PubMed] [Google Scholar]
- 31.Go, L. O., M. C. Moschella, J. Watras, K. K. Handa, B. S. Fyfe, and A. R. Marks. 1995. Differential regulation of two types of intracellular calcium release channels during end-stage heart failure. J. Clin. Invest. 95:888–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Venetucci, L., A. W. Trafford, and D. A. Eisner. 2003. Illuminating sarcoplasmic reticulum calcium. Circ. Res. 93:4–5. [DOI] [PubMed] [Google Scholar]
- 33.Litwin, S. E., D. Zhang, and J. H. Bridge. 2000. Dyssynchronous Ca2+ sparks in myocytes from infarcted hearts. Circ. Res. 87:1040–1047. [DOI] [PubMed] [Google Scholar]
- 34.Negretti, N., S. C. O'Neill, and D. A. Eisner. 1993. The effects of inhibitors of sarcoplasmic reticulum function on the systolic Ca2+ transient in rat ventricular myocytes. J. Physiol. 468:35–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eisner, D. A., A. W. Trafford, M. E. Diaz, C. L. Overend, and S. C. O'Neill. 1998. The control of Ca release from the cardiac sarcoplasmic reticulum: regulation versus autoregulation. Cardiovasc. Res. 38:589–604. [DOI] [PubMed] [Google Scholar]
- 36.Wehrens, X. H., S. E. Lehnart, and A. R. Marks. 2005. Intracellular calcium release and cardiac disease. Annu. Rev. Physiol. 67:69–98. [DOI] [PubMed] [Google Scholar]
- 37.Ai, X., J. W. Curran, T. R. Shannon, D. M. Bers, and S. M. Pogwizd. 2005. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ. Res. 97:1314–1322. [DOI] [PubMed] [Google Scholar]
- 38.Maier, L. S., and D. M. Bers. 2007. Role of Ca2+/calmodulin-dependent protein kinase (CaMK) in excitation-contraction coupling in the heart. Cardiovasc. Res. 73:631–640. [DOI] [PubMed] [Google Scholar]
- 39.Litwin, S. E. 2006. “Ryanogate”: who leaked the calcium? Circ. Res. 98:165–168. [DOI] [PubMed] [Google Scholar]
- 40.Zima, A. V., and L. A. Blatter. 2006. Redox regulation of cardiac calcium channels and transporters. Cardiovasc. Res. 71:310–321. [DOI] [PubMed] [Google Scholar]
- 41.Tocchetti, C. G., W. Wang, J. P. Froehlich, S. Huke, M. A. Aon, G. M. Wilson, G. Di Benedetto, B. O'Rourke, W. D. Gao, D. A. Wink, J. P. Toscano, M. Zaccolo, D. M. Bers, H. H. Valdivia, H. Cheng, D. A. Kass, and N. Paolocci. 2007. Nitroxyl improves cellular heart function by directly enhancing cardiac sarcoplasmic reticulum Ca2+ cycling. Circ. Res. 100:96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gyorke, S., I. Gyorke, V. Lukyanenko, D. Terentyev, S. Viatchenko-Karpinski, and T. F. Wiesner. 2002. Regulation of sarcoplasmic reticulum calcium release by luminal calcium in cardiac muscle. Front. Biosci. 7:d1454–d1463. [DOI] [PubMed] [Google Scholar]
- 43.Zhang, L., J. Kelley, G. Schmeisser, Y. M. Kobayashi, and L. R. Jones. 1997. Complex formation between junctin, triadin, calsequestrin, and the ryanodine receptor. Proteins of the cardiac junctional sarcoplasmic reticulum membrane. J. Biol. Chem. 272:23389–23397. [DOI] [PubMed] [Google Scholar]
- 44.Gyorke, I., N. Hester, L. R. Jones, and S. Gyorke. 2004. The role of calsequestrin, triadin, and junctin in conferring cardiac ryanodine receptor responsiveness to luminal calcium. Biophys. J. 86:2121–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kiarash, A., C. E. Kelly, B. S. Phinney, H. H. Valdivia, J. Abrams, and S. E. Cala. 2004. Defective glycosylation of calsequestrin in heart failure. Cardiovasc. Res. 63:264–272. [DOI] [PubMed] [Google Scholar]
- 46.Song, L. S., E. A. Sobie, S. McCulle, W. J. Lederer, C. W. Balke, and H. Cheng. 2006. Orphaned ryanodine receptors in the failing heart. Proc. Natl. Acad. Sci. USA. 103:4305–4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harris, D. M., G. D. Mills, X. Chen, H. Kubo, R. M. Berretta, V. S. Votaw, L. F. Santana, and S. R. Houser. 2005. Alterations in early action potential repolarization causes localized failure of sarcoplasmic reticulum Ca2+ release. Circ. Res. 96:543–550. [DOI] [PubMed] [Google Scholar]
- 48.Wehrens, X. H., and A. R. Marks. 2004. Novel therapeutic approaches for heart failure by normalizing calcium cycling. Nat. Rev. Drug Discov. 3:565–573. [DOI] [PubMed] [Google Scholar]