Abstract
The GABAA receptors (GABARs) are chloride-permeable ligand-gated ion channels responsible for fast inhibitory neurotransmission. These receptors are structurally heterogeneous, and in mammals can be formed from a combination of sixteen different subunit subtypes. Much of this variety comes from the six different α subunit subtypes. All neuronal GABARs contain an α subunit, and the identity of the α subtype affects the pharmacological properties of the receptors. The expression of each of the different α subtypes is regulated developmentally and regionally and changes with both normal physiological processes such development and synaptic plasticity, and pathological conditions such as epilepsy. In order to understand the functional significance of this structural heterogeneity, we examined the effect of the α subtype on the receptor’s response to GABA. Each of the six α subtypes was transiently co-expressed with the β3 and γ2L subunits in mammalian cells. The sensitivity to GABA was measured with whole-cell recordings. We also determined the activation, deactivation, desensitization, and recovery kinetics for the six isoforms using rapid-application recordings from excised macropatches. We found unique characteristics associated with each α subunit subtype. These properties would be expected to influence the post-synaptic response to GABA, creating functional diversity among neurons expressing different α subunits.
Keywords: GABA-A receptor, ion channel, kinetics, patch-clamp, recombinant, rapid application
1. INTRODUCTION
The functional and pharmacological properties of the GABAA receptors (GABARs) are determined in large part by their subunit composition (Korpi et al., 2002). To date, seven subunit families have been cloned, and many of these families have multiple subtypes. Six α subtypes, three β subtypes, three γ subtypes, and one member each in the δ, ε, π and θ subunit families have been found in mammalian species (Whiting et al., 1999). Formation of receptors with properties similar to those of native receptors appears to require a combination of at least an α, β, and a tertiary (γ, δ, ε, π or θ) subunit. The receptors are likely arranged with two α and two β subunits contributing to the pentameric structure (Baumann et al., 2002). The physiological significance of this multitude of subunits is only beginning to be understood. This study examined the effect of the α subtype on the macroscopic kinetic properties of the GABAR channel.
The α subunit family is the most diverse, with six different subtypes (α1-α6) found in mammalian species. Incorporation of an α subunit is required for production of GABA-activated channels in mammalian expression systems and an α subunit is almost certainly present in all native receptors (Fritschy et al., 1997). The α subtypes show relatively high structural homology (60-80%) but confer distinct functional and pharmacological properties (Mehta and Ticku, 1999; Korpi et al., 2002). The mRNA for each of the α subtypes exhibits a unique distribution pattern throughout the rat brain, and is differently regulated throughout development (Laurie et al., 1992a, 1992b; Wisden et al., 1992). GABARs produced by neurons in different brain regions and at different stages of development could therefore have very different characteristics due to variations in subunit composition. Recent studies indicate that, as suggested from their unique patterns of distribution, different α subtypes have different functional roles (Rudolph et al, 2001).
The production of the different α subtypes is also regulated by pharmacological and pathological conditions. In general terms, higher α1 expression is associated with conditions of lower excitability, while the α4 subunit, in particular, is associated with hyperexcitability. For example, expression of the α4 subunit is increased by withdrawal from neurosteroids, benzodiazepines, or alcohol; conditions also associated with an increase in anxiety and seizure susceptibility (Follesa et al., 2004). Onset of temporal lobe epilepsy in an animal model increased expression of both α3 and α4 subunits while decreasing α1 expression in hippocampal dentate granule cells (Brooks-Kayal et al., 1998) while genetically seizure-prone rats have an α subtype expression pattern similar to that seen in embryos, with lower expression of α1 and higher expression of α2, α3 and α5 (Poulter et al., 1999).
The kinetic properties of native GABARs vary with the type of neuron and its stage of development. Typically the IPSC decay rate is found to be slower in neurons early in development, changing to more rapid decay with adulthood (Takahashi, 2005). Different decay rates have also been reported for GABAR currents from different kinds of neurons (Xiang et al., 1998; Maric et al., 1999; Browne et al., 2001) and for extrasynaptic vs. synaptic receptors (Banks and Pearce, 2000). Some of these differences might be due to differences in the subunit composition of the receptors. Previous results from recombinant receptors suggest that the α subtype influences desensitization and deactivation kinetics, although these studies used a variety of experimental protocols and findings are often inconsistent among different laboratories (Gingrich et al., 1995; Tia et al., 1996a, 1996b; Lavoie et al., 1997; Burgard et al., 1999; McClellan and Twyman, 1999; Bianchi et al., 2002a; Lagrange et al., 2007). In order to directly compare the effect of the α subtype on channel properties, we examined the macroscopic kinetic properties of recombinant receptors containing each of the different α subtypes combined with the same β and γ subunit subtypes and under the same recording conditions.
2. RESULTS
The processes of channel activation, desensitization and deactivation are primary determinants of the shape and duration of the post-synaptic current. A complete characterization of the kinetic properties of recombinant receptors will clarify how changes in α subtype expression might influence the synaptic response of the receptors and will help predict the effect of these changes on GABAergic neurotransmission. We examined the properties of six different receptor isoforms containing different α subtypes, but the same β and γ subunits (β3 and γ2L). While the αxβ3γ2L combination is not necessarily the most common native isoform for each of the α subtypes (McKernan and Whiting, 1996), it was selected to provide a standard background for comparison. The γ2 subunit is the most widely expressed of the γ subtypes, and β2/3 subtypes are more common than the β1 in most regions (Laurie et al., 1992a; Wisden et al., 1992). The β2 and β3 subunits have high structural homology and confer similar pharmacological properties (Smith et al., 2004). The β3 is more highly expressed in the developing brain, while the β2 predominates in the adult brain (Laurie et al, 1992b). Many previous studies have used the β3 subunit, so we selected this subtype to allow a more direct comparison to earlier findings.
2.1. Whole-cell GABA sensitivity
To examine the effect of the GABAR α subtype on the receptor’s sensitivity to GABA, L929 fibroblasts were transiently transfected with β3 and γ2L subunits, and one of the six α subtypes. GABA was applied for 5 sec to cells voltage-clamped at -50 mV. GABA sensitivity varied widely among the different isoforms (Figure 1). The highest GABA sensitivity was observed with the α6 subunit, which was clearly separated from the other receptor isoforms. The average EC50 was 2.25 ± 0.53 μM (n=5), about four times more sensitive than the isoform with the next lowest EC50 (α5) and fifteen times more sensitive than the least sensitive isoform (α3). The α4 and α5 subunits conferred similar GABA sensitivities, with averages of 10.7 ± 1.8 μM (n=5) for α4β3γ2L and 9.4 ± 1.5 μM (n=5) for α5β3γ2L. Receptors containing α1 and α2 subunits were also similar to one another, with averages of 15.6 ± 2.0 μM (n=5, α1β3γ2L) and 25.0 ± 3.9 μM (n=5, α2β3γ2L). The lowest GABA sensitivity was associated with the α3 subunit, with an average EC50 of 35.8 ± 5.3 μM (n=5). Averaged hill numbers were not significantly different (p > 0.05) among the receptor isoforms and ranged from 1.04 ± 0.15 (α6) to 1.48 ± 0.12 (α1). The relative relationship of α6 < α4=α5 < α1=α2 < α3 is generally consistent with previously reported values using a variety of expression systems and subunit combinations (Dučićet al., 1995; Böhme et al., 2004).
Fig. 1. GABA sensitivity.
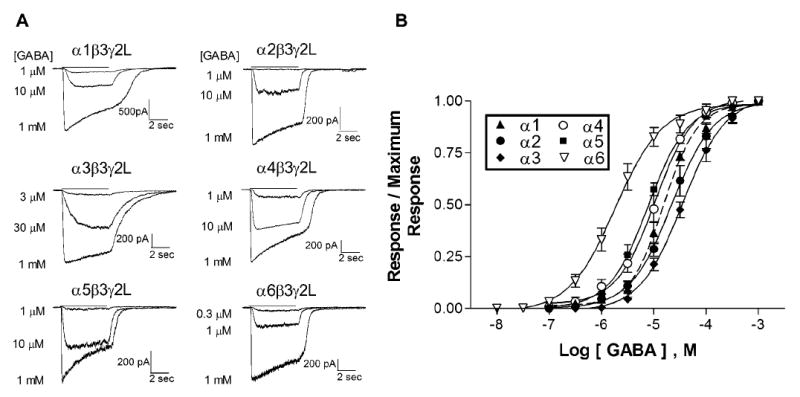
A. Mouse L929 fibroblasts were transiently transfected with one of the α subunit subtypes along with the β3 and γ2L subunits. GABA at a concentration ranging from 0.01 μM to 1 mM was applied for 5 sec (bar) to cells voltage clamped at -50 mV. Representative whole-cell currents obtained in response to the GABA concentration indicated are shown for each isoform.
B. GABA concentration-response relationships were determined for receptors containing each of the α subtypes. Points shown are averaged data from 5 cells from which complete concentration-response curves were obtained. EC50 values of the fits shown were 15.5 μM for α1β3γ2L (dashed line), 21.9 μM for α2β3γ2L, 35.3 μM for α3β3γ2L, 10.6 μM for α4β3γ2L, 8.9 μM for α5β3γ2L and 1.8 μM for α6β3γ2L. Hill numbers ranged from 0.97 (α6) to 1.35 (α1).
2.2. Deactivation Rate – 5 msec GABA applications
The post-synaptic current in neurons during phasic neurotransmission occurs in response to a brief exposure to a high concentration of GABA. Therefore, we used rapid application techniques to determine the effect of the α subtype on characteristics of the receptor likely to influence the properties of the synaptic response. These studies were performed using HEK-293T cells because of their higher transfection efficiency and protein expression level compared to the L929 cells, allowing a receptor density sufficient for recordings from most excised patches. We have observed no differences in the pharmacological or functional properties of GABAA receptors expressed in these two cell lines.
The rate of deactivation was determined with a 5 msec application of a maximally effective concentration of GABA. Because of its relatively low GABA EC50 in whole-cell studies, 3 mM GABA was used for rapid application studies of the α3-containing receptors, while 1 mM GABA was used for all the remaining isoforms. The decay of the current was fit with the sum of 2 exponential components (Figure 2, Table 1). The differences among the isoforms were largely in the duration of τslow and the relative contributions of the components (Table 1). Interestingly, the deactivation rates and GABA EC50’s were typically not correlated, even though both would be expected to be similarly influenced by any subunit-specific differences in the GABA binding steps. One of the slowest decay rates (weighted mean) was seen with the α6 subtype, consistent with its higher sensitivity to GABA. However, both the α4 and α5 subunits conferred very rapid deactivation rates, in apparent contrast to their relatively low GABA EC50’s. Additionally, the α3β3γ2L isoform, which had the lowest GABA sensitivity, also had one of the slowest rates of deactivation, comparable to that of the α6-containing receptors. Deactivation rates and measured EC50 values are influenced not just by agonist koff rates, but also by their activation rates and entry into long-lived open or closed states, which serve as high affinity agonist-bound states (Jones and Westbrook, 1995; Colquhoun, 1998). Therefore, an examination of the activation and desensitization kinetics of the isoforms may explain the general lack of correlation between GABA EC50 and deactivation rate. It is also possible that the relatively slow application rate associated with the whole-cell recording configuration could influence our ability to accurately capture the peak current, and therefore alter the measured EC50. In addition, the disruption of interactions of the receptor with cytoskeletal proteins through the process of patch excision could also alter channel properties and could result in differences between whole-cell and outside-out patch recordings (Chen and Olsen, 2007; Lagrange et al., 2007).
Fig. 2. Deactivation rate.
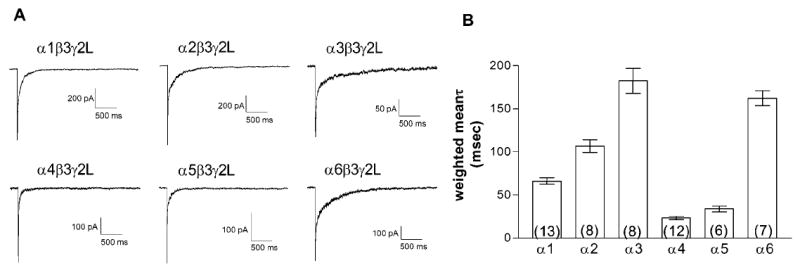
A. 1 or 3 mM GABA was applied for 5 msec to macropatches pulled from transiently transfected human HEK-293T cells. Representative currents are shown for patches held at -70 mV obtained from cells expressing each of the six isoforms, as indicated.
B. The current decay was fit with the sum of two exponential distributions. The weighted mean deactivation time was the sum of each time constant multiplied by its relative area. Bars represent the average ± SEM, and the number of patches is given by the number in parentheses.
Table 1.
Deactivation kinetics – 5 msec application
| Subtype | τfast (msec) | areafast | τslow (msec) |
|---|---|---|---|
| α1
(n= 13) |
14.61 ± 0.81 | 75.75 ± 2.00 % | 237.09 ± 16.40 |
| α2
(n= 8) |
18.38 ± 2.29 | 68.86 ± 2.82 % | 314.38 ± 25.61 |
| α3
(n=8) |
17.84 ± 2.62 | 69.45 ± 2.78 % | 577.35 ± 59.81 |
| α4
(n=12) |
9.91 ± 0.62 | 83.42 ± 2.18 % | 99.73 ± 8.25 |
| α5
(n=6) |
12.43 ± 1.41 | 74.06 ± 7.90% | 124.38 ± 23.45 |
| α6
(n=7) |
29.31 ± 4.42 | 59.66 ± 2.47% | 362.72 ± 18.05 |
2.3. Onset of Desensitization – 400 msec and 2 sec GABA applications
The effect of the α subtype on the time course of desensitization onset was examined using 400 msec and 2 sec applications of 1 or 3 mM GABA to outside-out patches (Figure 3). With a 400 msec application, the onset of desensitization was fit with the sum of two exponential components for all isoforms except for the α5-containing receptors (Figure 3B, Table 2). The decay of the α5β3γ2L receptors was fit with only a single component, with a time constant similar to the slower component seen with the other isoforms. Thus, it appears that this receptor does not substantially enter the fast desensitized state in response to GABA.
Fig. 3. Onset of Desensitization.
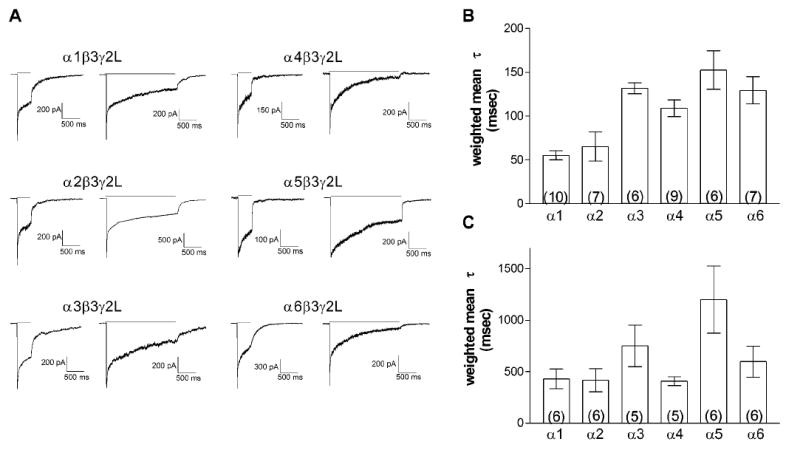
A. 1 or 3 mM GABA was applied for either 400 msec or 2 sec to excised, outside-out macropatches from HEK-293T cells transiently transfected with the subunit combination indicated. Bars indicate the duration of application. Representative currents are shown for patches held at -70 mV. Traces shown for the same isoform at each duration were not obtained from the same patch.
B. The onset of desensitization for the 400 msec application was fit with the sum of one (α5) or two exponential distributions. Bars represent the average weighted mean desensitization time constant ± SEM, and the number of patches is given by the number in parentheses.
C. The onset of desensitization for the 2 sec application was fit with the sum of two (α5) or three exponential distributions. The average weighted mean is shown as in B.
Table 2.
Desensitization properties – 400 msec application
| Subtype | 10-90% activation (msec) | τfast (msec) | areafast | τslow (msec) | residual current |
|---|---|---|---|---|---|
| α1
(n=14) |
0.603 ± 0.029 | 9.90 ± 0.84 | 68.4 ± 1.8% | 149.62 ± 10.86 | 34.2 ± 2.7% |
| α2
(n=7) |
0.735 ± 0.035 | 10.15 ± 1.33 | 60.5 ± 4.6% | 137.30 ± 23.25 | 38.4 ± 2.6% |
| α3
(n=6) |
1.788 ± 0.117 | 26.54 ± 4.98 | 43.5 ± 3.7% | 214.18 ± 11.33 | 52.3 ± 3.6% |
| α4
(n=9) |
0.951 ± 0.053 | 13.06 ± 1.77 | 52.91 ± 4.1% | 217.91 ± 16.76 | 34.1 ± 2.2% |
| α5
(n=6) |
1.247 ± 0.035 | N/A | N/A | 152.60 ± 21.87 | 61.6 ± 3.3 |
| α6
(n=7) |
1.052 ± 0.103 | 12.00 ± 2.49 | 51.7 ± 3.1% | 257.49 ± 30.33 | 31.7 ± 2.3% |
Receptors with α1 and α2 subunits were very similar in their desensitization properties while those with α4 and α6 subunits showed somewhat slower mean desensitization. The time constant for the fast component was comparable among all these isoform, and the difference in weighted mean was due predominantly to the slow component, which had a larger τ and greater relative contribution compared to the α1- and α2-containing receptors. In contrast, the α3β3γ2L isoform had relatively slower time constants for both components, with a substantial contribution from the slower component. The extent of desensitization for the 400 msec application was substantially less for the receptors with α3 and α5 subunits compared to all other isoforms.
In order to clarify contributions from the longer components of desensitization, 1 or 3 mM GABA was applied for 2 seconds to excised macropatches. The decay was fit with the sum of three exponential components except for the α5-containing receptors which, as observed with the 400 msec application, lacked the fastest component. The time constants of the three components are similar to those reported previously for 4 sec applications to α1β3γ2L receptors, suggesting that longer applications would not necessarily reveal additional components (Haas and Macdonald, 1999).
The general pattern among the different isoforms was comparable to the response to 400 msec applications. The fit of the decay for the α1- and α2-containing receptors revealed three components with similar time constants and relative areas. Again, the α3- and α5-containing receptors exhibited the slowest and least complete decay, with a substantial contribution from the longest component. Although they had weighted means similar to receptors with α1 or α2 subunits, the extent of desensitization of the α4- and α6-containing receptors was substantially greater than that of the other isoforms. A smaller residual current with α4β3γ2L compared to α1β3γ2L during 4 sec. application was also reported in a recent study using rapid application recordings of lifted whole cells (Lagrange et al,. 2007) and may reflect greater entry into or slower exit from the longer desensitized states.
2.4. Effect of α subtype on activation rate
To compare activation rates among the isoforms, the 10-90% risetime was measured for the 400 msec duration responses to 1 or 3 mM GABA (Table 2). The α1 subtype conferred the most rapid activation, consistent with some other reports for this isoform (Haas and Macdonald, 1999). α3-containing receptors had the slowest activation rate, while the other receptor isoforms all had activation rates near 1 ms. The slow activation of α3 receptors has been found in previous studies (Gingrich et al., 1995), although our results show faster rates than others have found for α1- and α4-containing receptors (McClellan and Twyman, 1999; Lagrange et al., 2007). The slow activation rate associated with the α3 subtype could also influence our ability to accurately measure the GABA EC50 and to detect fast components of desensitization for these receptors. Slow activation might blunt the peak current in whole-cell and excised patch recordings by reducing synchronization of channel activation.
2.5. Recovery from activation - pairs of 5 msec pulses
To examine the effect of the α subtype on the kinetics of recovery from activation, pairs of 5 msec applications of 1 or 3 mM GABA were given with a varying interval between applications (Figure 4, Table 4). The amplitude of the response to the 2nd application will be reduced by the population of receptors that are in a GABA-bound but closed state (i.e. desensitized). The recovery data were fit with the sum of two exponentials for all receptor isoforms, similar to previous reports (Jones and Westbrook, 1995). The α3-, α4-, and α5-containing receptors all showed rapid recovery, giving a nearly full amplitude response within 100 msec. Receptors with α1 ad α2 subunits were intermediate in their recovery kinetics. The α6-containing receptors exhibited very slow recovery, with >3 sec required for return of the full amplitude response. This suggests that long-lived desensitized states may be partially responsible for the slow deactivation kinetics associated with the α6 subunit. Entry into desensitized states can lead to slower deactivation for single responses, but receptors containing these subunits would likely exhibit a large decrease in current amplitude with repeated stimulation by fast-firing neuronal populations. Although the α4-containing receptors recovered quickly from brief stimulation, their relatively complete entry into desensitized states observed with longer applications (Figure 3) suggests that prolonged stimulation would substantially reduce their activity. However, populations with rapid recovery from activation combined with slow entry into desensitized states (such as the α3- or α5-containing GABARs) would be expected to maintain their maximal amplitude, even with rapid firing input. These receptors may also be well-suited to produce tonic current in extra-synaptic locations.
Fig. 4. Recovery from activation.
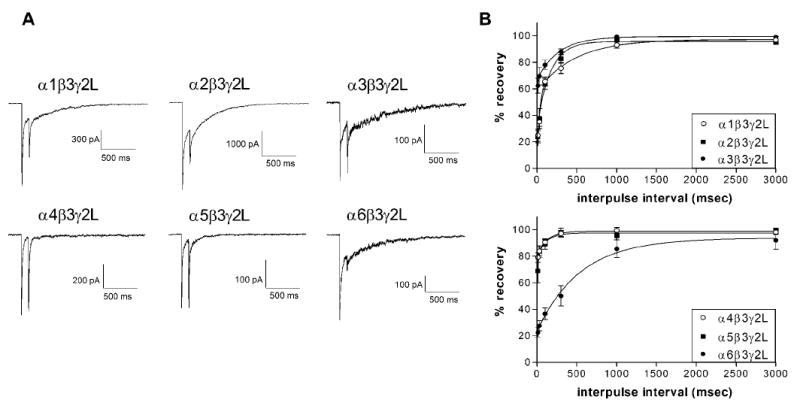
A. Representative traces are shown for responses to 5 msec pulses of 1 or 3 mM GABA applied 100 msec apart. Excised macropatches were obtained from cells expressing each of the six isoforms, as indicated and currents were obtained with a holding potential of -70 mV.
B. Paired 5 msec pulses of GABA were applied to macropatches at intervals of 10, 30, 100, 300, 1000 or 3000 msec. Recovery was calculated as (peak of first response minus onset) divided by (peak of second response minus onset) where onset is the current amplitude at the beginning of the second application. Symbols show mean ± SEM (n=5). Data was fit with the sum of two exponential components for all isoforms.
Table 4.
Recovery from activation – 5 msec paired pulse
| Subtype | τfast (msec) | areafast | τslow (msec) |
|---|---|---|---|
| α1
(n= 5) |
30.0 | 60% | 473.9 |
| α2
(n= 5) |
10.8 | 33% | 165.6 |
| α3
(n=5) |
4.0 | 67% | 265.7 |
| α4
(n=5) |
2.8 | 81% | 120.8 |
| α5
(n=5) |
5.2 | 81% | 112.7 |
| α6
(n=5) |
4.2 | 25% | 539.1 |
3. DISCUSSION
This study compared the effect of differences in α subunit subtype composition on the GABA sensitivity and macroscopic kinetic properties of recombinant GABARs. We found distinct properties associated with each subtype (summarized in Table 5), suggesting that, as might be expected, the large structural heterogeneity of the GABARs can lead to substantial functional heterogeneity.
Table 5.
Summary of functional properties
| Subtype | GABA
sensitivity |
Activation | Deactivation | Desensitization
Rate and Extent |
Recovery |
|---|---|---|---|---|---|
| α1 | Moderate | Fast | Moderate | Fast, intermediate | Moderate |
| α2 | Moderate | Fast | Moderate | Fast, intermediate | Moderate |
| α3 | Low | Slow | Slow | Slow, incomplete | Fast |
| α4 | Moderate | Moderate | Fast | Fast, complete | Fast |
| α5 | Moderate | Moderate | Fast | Slow, incomplete | Fast |
| α6 | High | Moderate | Slow | Fast, complete | Slow |
How might the differences that we observed in kinetic properties affect GABAergic transmission in neurons expressing different α subunit subtypes? The impact on brain function would obviously depend upon the role of the post-synaptic neuron in the network, and on whether the increase in chloride current was hyperpolarizing or depolarizing. Rates of deactivation would alter the decay rate of post-synaptic currents in response to phasic neurotransmitter release. Shifts in expression from isoforms with slower deactivation rates to faster rates would be expected to lead to less effective GABAergic neurotransmission, and likely reduced inhibitory tone. This pattern is observed in models of hyperexcitability, in which faster IPSC decay rates are associated with an increased seizure susceptibility (Smith et al., 1998). The increase in seizure activity could be reduced by decreasing α4 subunit expression (Smith et al., 1998) or by increasing α1 subunit expression (Raol et al., 2006). Our results suggest that either of these changes in subunit expression would slow the deactivation rate. In addition, two GABAR mutations associated with inheritance of epilepsy have been found to accelerate the deactivation rate, also correlating rapid deactivation with conditions of hyperexcitability (Bianchi et al., 2002b; Fisher, 2004a).
Differences in desensitization kinetic properties might have more complex effects on neuronal activity. Changes in onset of as well as recovery from desensitization would affect the ability of the post-synaptic neuron to maintain responsiveness to repetitive firing, and influence the reliability of neurotransmission (Mody and Pearce, 2004). Even slow phases of desensitization have been predicted to influence post-synaptic responses to repeated stimulation (Bianchi and Macdonald, 2002). Drugs that alter desensitization rates of GABARs have been shown to dramatically alter oscillation patterns of inhibitory networks (Baker et al, 2002). Therefore, changes in desensitization kinetics due to changes in subunit composition could have significant functional consequences. The impact of desensitization properties will also depend upon the location of the GABAR. The extrasynaptic receptors that contribute to the tonic current are exposed to constant low agonist concentrations (Farrant and Nusser, 2005). Most extrasynaptic populations contain the δ subunit, which confers high sensitivity to GABA and minimal desensitization (Saxena and Macdonald, 1997, Bianchi et al., 2002a; Brown et al., 2002), properties ideally suited for maintenance of a tonic current in response to ambient levels of GABA. The α5βxγ2 receptors are also primarily located extrasynaptically in the CA regions of the hippocampus (Caraiscos et al., 2004). Our findings that these receptors show minimal levels of desensitization are consistent with their contribution to a long-lasting tonic current.
Our work utilized transient expression of recombinant GABARs in non-neuronal cell lines. As a result, there is the potential for differences in channel characteristics compared to native receptors because of neuron-specific processes that regulate receptor function. Neuronal receptors are likely to be subject to modulation by post-translational modifications such as phosphorylation (Brandon et al., 2002), interactions with cytoskeletal proteins (Chen and Olsen, 2007), and differences in assembly and membrane targeting (Fritschy et al., 1998; Brünig et al., 2002; Klausberger et al., 2002). However, virtually all neurons and neuronal cell lines that express GABARs produce multiple subunit subtypes (Wisden et al., 1992; Laurie et al., 1992a; Tyndale et al., 1994; Neelands et al., 1998) and neuronal populations which express only one or two of the α subtypes are extremely rare. Therefore, in order to control the subunit composition of the receptors and describe the characteristics of a homogeneous population of receptors, these studies must be done in cell lines that do not normally express functional GABARs. Whether the predictions suggested by our results hold true in native receptors might be tested in future studies which manipulate α subunit expression or in which the subunit composition of the receptors can be clearly defined. Most of the studies in neurons with well-described changes in subunit expression have reported functional effects consistent with our findings. One example is the faster decay rate associated in many types of neurons with a shift from α2/α3 subunit expression to α1 subunit expression (Brussaard and Herbison, 2000; Takahashi, 2005). These functional changes are not observed in α1 knock-out animals, suggesting they are indeed mediated though alterations in subunit expression (Vicini et al., 2001; Bosman et al., 2005). Our results showing that the α4 subtype confers a very rapid deactivation rate are also consistent with several neuronal studies in which pathological conditions of hyperexcitability cause an increase in the relative expression of the α4 subunit and a more rapid IPSC decay rate (Smith et al., 1998; Follesa et al., 2004). Again, the change in kinetic properties could be prevented by reducing expression of the α4 subunit (Smith et al., 1998).
Our study examined the properties of receptors containing a single α subtype in combination with the same β (β3) and γ (γ2L) subunits. We maintained constant β and γ subunit subtypes in order to focus on the α subunit. It is very likely that the nature of the β and γ subtypes also influences kinetic properties of the receptor, as they are known to change pharmacological characteristics (Korpi et al., 2002) but their impact on macroscopic kinetic properties has only rarely been examined (Hinkle and Macdonald, 2003; Huntsman and Huguenard, 2006). The effect of the other tertiary subunits (δ, ε, π, and θ) on the properties of receptors containing different α subunit subtypes would also be of interest. Adding another layer of complexity is the relatively common occurrence of native receptors containing two different α subtypes within a single receptor (Araujo et al., 1996, 1999; Jechlinger et al., 1998; Benke et al., 2004). Would the kinetic properties of these receptors be intermediate, or might they be dominated by the characteristics of one of the subtypes? Characterization of all these possible combinations will add to our understanding of the regulation of GABAergic neurotransmission through variations in subunit expression.
The structural differences among the α subtypes responsible for their distinct functional properties are largely unknown. A variety of heterogeneous sites within the extracellular N-terminal domain of α subunits have been reported to influence GABA sensitivity (Böhme et al., 2004; Drafts and Fisher, 2004) but few studies have examined the structural basis for differences in macroscopic kinetics. It is perhaps not surprising that complex interactions among different regions of the subunits appear to control deactivation and desensitization kinetics (Bianchi et al., 2001, 2002a; Bianchi and Macdonald 2002; Fisher, 2004b). A comparison of the structural heterogeneity among all the α subtypes in view of the functional characteristics we have described may help to identify some of the common structures that influence channel gating.
It is clear that changes in expression of GABAR α subunit subtypes are associated with both normal development and pathological conditions. It is important to understand the functional implications of these changes. Are they adaptive changes that help to repair the circuits and permit normal plasticity, or do these changes contribute to the development and maintenance of abnormal and destructive hyperexcitability? It is also clear that members of the other GABAR subunit families, in addition to the α subtypes, influence the properties of the receptors. A complete description of the functional differences associated with the GABAR subunits may lead the way toward predicting the effects of changes in subunit expression and developing targeted treatments for a variety of neurological disorders (Rudolph and Möhler, 2006).
4. EXPERIMENTAL PROCEDURES
4.1. Transient expression of recombinant receptors
Full-length cDNAs for the wild-type rat or human (α2) GABAR subunits in mammalian expression vectors were obtained from Dr. Robert Macdonald (Vanderbilt University). Recombinant receptors were transiently expressed in the mouse fibroblast L929 or human endothelial HEK-293T cell lines. The cells were maintained in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% horse serum (L929) or 10% fetal bovine serum (HEK-293T) along with 100 IU/ml penicillin, and 100 μg/ml streptomycin. The L929 cells were used for whole-cell recordings while the HEK-293T cells were used for rapid application experiments with excised macropatches. The L929 cells are well-suited for whole-cell studies as they grow individually without forming gap junctions and they do not express endogenous GABAR subunits. However, their protein expression level is relatively low, and therefore they are not suitable for macropatch studies which require a higher receptor density. The HEK-293T cells have the disadvantages of an endogenous β3 GABAR subunit (Davies et al., 2000) as well as their tendency to form electrical connections with one another through gap junctions. However, they have a high transfection efficiency and can replicate plasmids containing an SV40 origin of replication, allowing very high protein expression levels. We have used both cell lines for many years and they are commonly used by many investigators to study GABARs. We have not observed any differences in the properties of receptors expressed in these two lines.
Cells were transfected using calcium phosphate precipitation (Angelotti et al., 1993). Plasmids encoding the selected GABAR subunit cDNAs were added to the cells in 1:1:1 ratios of 2-4 μg each. To isolate the transfected cells, 1-2 μg of the Capture-Tec pHook-1 (Invitrogen) plasmid encoding a surface antibody, sFv, were also transfected.
The isolation procedure for the transfected cells was conducted 20-28 hours later. The cells were first passaged with trypsin and then mixed for 40-50 min. with magnetic beads (approximately 7.5 × 105 beads) coated with antigen specific for the pHook antibody (Chesnut et al. 1996). Bead-bound cells were separated with a magnetic stand, resuspended into DMEM, and plated onto coverslips coated with poly-lysine and collagen. Cells were used for recording 20-28 hours later.
4.2. Electrophysiological recording techniques
The external bath solution contained 142 mM NaCl, 8.1 mM KCl, 6 mM MgCl2, 1 mM CaCl2, and 10 mM HEPES with a pH of 7.4 and osmolarity adjusted to 295-305 mOsm. Recording electrodes were filled with internal solution composed of 153 mM KCl, 1 mM MgCl2, 5 mM K-EGTA, and 10 mM HEPES (pH =7.4, 295-305 mOsm). For macropatch recordings 4 mM MgATP was added to the internal solution on the day of recording. A Narishige PP-830 electrode puller was used to pull recording electrodes to a resistance of 5-10 MΩ from thick-walled borosilicate glass with an internal filament (World Precision Instruments). GABA was diluted into external solution from a fresh or frozen stock in water.
The recordings were stored on a computer hard drive for off-line analysis. For whole-cell recordings GABA was applied to cells using a stepper solution exchanger with a complete exchange time of <50 msec (open tip, SF-77B, Warner Instruments). For macropatch recordings the 3-barrel square glass was pulled to a final size near 200 μm. 10-90% rise times of the junction potential at the open tip were consistently faster than 400 μsec and were tested after each patch recording using a diluted external solution. There was a continuous flow of external solution through the chamber. Currents were recorded with an Axon 200B patch clamp amplifier
4.3. Data analysis
Whole-cell current recordings were analyzed using the pClamp8.0 suite (Axon Instruments Inc.) and GraphPad Prism (GraphPad Software Inc.). To determine GABA concentration response relationships, peak current amplitudes were normalized to the maximum current elicited by 1 mM GABA for each cell. Normalized concentration-response data were fit with a four-parameter logistic equation: current = [minimum current + (maximum current − minimum current)] / [1 + (10̂(log EC50 − log [GABA]) × n), where n represents the hill number. Statistical comparisons were performed using the Tukey-Kramer multiple comparisons test (Instat, GraphPad) with a significance level of p < 0.05.
Macropatch currents were digitized at 10 kHz and analyzed with the pClamp8.0 suite of programs (Axon Instruments). The deactivation or desensitization rate was determined by fitting the decay current with the Levenberg-Marquardt least squares method with one or two exponential functions, as determined by a significant improvement of the fit with additional components (F test of the sum of squared residuals).
Table 3.
Desensitization properties – 2 sec application
| Subtype | τfast (msec) | areafast | τint (msec) | areaint | τslow (msec) | areaslow | residual current |
|---|---|---|---|---|---|---|---|
| α1
(n=6) |
18.02 ± 2.58 | 55.8 ± 3.7% | 201.13 ± 73.62 | 15.2 ± 2.4% | 1323.18 ± 160.35 | 29.0 ± 4.6% | 17.4 ± 1.8% |
| α2
(n=6) |
22.31 ± 6.67 | 45.2 ± 4.2% | 213.60 ± 59.98 | 22.8 ± 6.5% | 1035.67 ± 233.57 | 32.1 ± 6.5% | 25.2 ± 2.8% |
| α3
(n=5) |
28.85 ± 5.06 | 41.8 ± 7.2% | 249.63 ± 28.48 | 19.4 ± 9.6% | 1647.52 ± 203.17 | 38.9 ± 7.5% | 32.5 ± 3.5% |
| α4
(n=5) |
25.72 ± 3.48 | 47.0 ± 6.1% | 283.48 ± 73.41 | 29.0 ± 8.3% | 1307.12 ± 156.77 | 24.0 ± 4.2% | 8.7 ± 2.5% |
| α5
(n=6) |
N/A | N/A | 105.86 ± 32.89 | 21.9 ± 7.3% | 1654.05 ± 537.82 | 78.1 ± 7.3% | 35.4 ± 4.4% |
| α6
(n=6) |
24.75 ± 8.66 | 44.4 ± 6.3% | 235.05 ± 33.36 | 11.4 ± 2.5% | 1263.88 ± 243.37 | 44.2 ± 5.8% | 10.l ± 2.3% |
Acknowledgments
This work was supported by NIH-NINDS (RO1-NS045950), the PhRMA Foundation, the Epilepsy Foundation and the University of South Carolina School of Medicine Research Development Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Angelotti TP, Uhler MD, Macdonald RL. Assembly of GABAA receptor subunits: analysis of transient single-cell expression utilizing a fluorescent substrate/marker gene technique. J Neurosci. 1993;13:1418–1428. doi: 10.1523/JNEUROSCI.13-04-01418.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo F, Tan S, Ruano D, Schoemaker H, Benavides J, Vitorica J. Molecular and pharmacological characterization of native cortical γ-aminobutyric acidA receptors containing both α1 and α3 subunits. J Biol Chem. 1996;271:27902–27911. doi: 10.1074/jbc.271.44.27902. [DOI] [PubMed] [Google Scholar]
- Araujo F, Ruano D, Vitorica J. Native γ-aminobutyric type A receptors from rat hippocampus, containing both α1 and α5 subunits, exhibit a single benzodiazepine site with α5 pharmacological properties. J Pharm Exp Ther. 1999;290:989–997. [PubMed] [Google Scholar]
- Baker PM, Pennefather PS, Orser BA, Skinner FK. Disruption of coherent oscillation in inhibitory networks with anesthetics: role of GABAA receptor desensitization. J Neurophys. 2002;88:2821–2833. doi: 10.1152/jn.00052.2002. [DOI] [PubMed] [Google Scholar]
- Banks MI, Pearce RA. Kinetic differences between synaptic and extrasynaptic GABAA receptors in CA1 pyramidal cells. J Neurosci. 2000;20:937–948. doi: 10.1523/JNEUROSCI.20-03-00937.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann SW, Baur R, Sigel E. Forced subunit assembly in α1β2γ2 GABAA receptors. J Biol Chem. 2002;277:46020–46025. doi: 10.1074/jbc.M207663200. [DOI] [PubMed] [Google Scholar]
- Benke D, Fakitsas P, Roggenmoser C, Michel C, Rudolph U, Mohler H. Analysis of the presence and abundance of GABAA receptors containing two different types of α subunits in murine brain using point-mutated α subunits. J Biol Chem. 2004;279:43654–43660. doi: 10.1074/jbc.M407154200. [DOI] [PubMed] [Google Scholar]
- Bianchi MT, Haas KF, Macdonald RL. Structural determinants of fast desensitization and desensitization-deactivation coupling in GABAA receptors. J Neurosci. 2001;21:1127–1136. doi: 10.1523/JNEUROSCI.21-04-01127.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi MT, Macdonald RL. Slow phases of GABAA receptor desensitization: structural determinants and possible relevance for synaptic function. J Physiol. 2002;544:3–18. doi: 10.1113/jphysiol.2002.020255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi MT, Haas KF, Macdonald RL. α1 and α6 subunits specify distinct desensitization, deactivation and neurosteroid modulation of GABAA receptors containing the δ subunit. Neuropharm. 2002a;43:492–502. doi: 10.1016/s0028-3908(02)00163-6. [DOI] [PubMed] [Google Scholar]
- Bianchi MT, Song L, Zhang H, Macdonald RL. Two different mechanisms of disinhibition produced by GABAA receptor mutations linked to epilepsy in humans. J Neurosci. 2002b;22:5321–5327. doi: 10.1523/JNEUROSCI.22-13-05321.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhme I, Rabe H, Lüddens H. Four amino acids in the α subunits determine the γ-aminobutyric acid sensitivities of GABAA receptor subtypes. J Biol Chem. 2004;279:35193–35200. doi: 10.1074/jbc.M405653200. [DOI] [PubMed] [Google Scholar]
- Bosman LWJ, Heinen K, Spijker S, Brussaard AB. Mice lacking the major adult GABAA receptor subtype have normal number of synapses, but retain juvenile IPSC kinetics until adulthood. J Neurophys. 2005;94:338–346. doi: 10.1152/jn.00084.2005. [DOI] [PubMed] [Google Scholar]
- Brandon N, Jovanovic J, Moss S. Multiple roles of protein kinases in the modulation of γ-aminobutyric acidA receptor function and cell surface expression. Pharm Ther. 2002;94:113–122. doi: 10.1016/s0163-7258(02)00175-4. [DOI] [PubMed] [Google Scholar]
- Brooks-Kayal AR, Shumate MD, Jin H, Rikhter TY, Coulter DA. Selective changes in single cell GABAA receptor subunit expression and function in temporal lobe epilepsy. Nature Medicine. 1998;4:1166–1172. doi: 10.1038/2661. [DOI] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing human α4β3δ GABAA receptors. Br J Pharm. 2002;136:965–974. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne SH, Kang J, Akk G, Chiang LW, Schulman H, Huguenard JR, Prince DA. Kinetic and pharmacological properties of GABAA receptors in single thalamic neurons and GABAA subunit expression. J Neurophys. 2001;86:2312–2322. doi: 10.1152/jn.2001.86.5.2312. [DOI] [PubMed] [Google Scholar]
- Brünig I, Scotti E, Sidler C, Fritschy J-M. Intact sorting, targeting, and clustering of γ-aminobutyric acid A receptor subtypes in hippocampal neurons in vitro. J Comp Neurol. 2002;443:43–55. doi: 10.1002/cne.10102. [DOI] [PubMed] [Google Scholar]
- Brussaard AB, Herbison AE. Long-term plasticity of postsynaptic GABAA-receptor function in the adult brain: insights from the oxytocin neurone. Trends in Neurosci. 2000;23:190–195. doi: 10.1016/s0166-2236(99)01540-4. [DOI] [PubMed] [Google Scholar]
- Burgard EC, Haas KF, Macdonald RL. Channel properties determine the transient activation kinetics of recombinant GABAA receptors. Mol Brain Res. 1999;73:28–36. doi: 10.1016/s0169-328x(99)00230-2. [DOI] [PubMed] [Google Scholar]
- Caraiscos VB, Elliott EM, You T, Cheng VY, Belelli D, Newell JG, Jackson MF, Lambert JJ, Rosahl TW, Wafford KA, MacDonald JF, Orser BA. Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by α5 subunit-containing γ-aminobutyric acid type A receptors. Proc Natl Acad Sci. 2004;101:3662–3667. doi: 10.1073/pnas.0307231101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z-W, Olsen RW. GABAA receptor associated proteins: a key factor regulating GABAA receptor function. J Neurochem. 2007;100:279–294. doi: 10.1111/j.1471-4159.2006.04206.x. [DOI] [PubMed] [Google Scholar]
- Chesnut JD, Baytan AR, Russell M, Chang MP, Bernard A, Maxwell IH, Hoeffler JP. Selective isolation of transiently transfected cells from a mammalian cell population with vectors expressing a membrane anchored single-chain antibody. J Immunol Methods. 1996;193:17–27. doi: 10.1016/0022-1759(96)00032-4. [DOI] [PubMed] [Google Scholar]
- Colquhoun D. Binding, gating, affinity and efficacy: the interpretation of structure-activity relationships for agonists and of the effects of mutating receptors. Br J Pharm. 1998;125:924–947. doi: 10.1038/sj.bjp.0702164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PA, Hoffmann EB, Carlisle HJ, Tyndale RF, Hales TG. The influence of an endogenous β3 subunit on recombinant GABAA receptor assembly and pharmacology in WSS-1 cells and transiently transfected HEK293 cells. Neruropharm. 2000;39:611–620. doi: 10.1016/s0028-3908(99)00163-x. [DOI] [PubMed] [Google Scholar]
- Drafts BC, Fisher JL. Structural determinants of the pharmacological properties of the GABAA receptor α6 subunit. J Pharm Exp Ther. 2004;309:1108–1115. doi: 10.1124/jpet.103.064360. [DOI] [PubMed] [Google Scholar]
- Dučić I, Caruncho HJ, Zhu WJ, Vicini S, Costa E. γ-Aminobutyric acid gating of Cl- channels in recombinant GABAA receptors. J Pharm Exp Ther. 1995;272:438–445. [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nature Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Fisher JL. A mutation in the GABAA receptor α1 subunit linked to human epilepsy affects channel gating properties. Neuropharm. 2004a;46:629–637. doi: 10.1016/j.neuropharm.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Fisher JL. The α1 and α6 subunit subtypes of the mammalian GABAA receptor confer distinct channel gating kinetics. J Physiol. 2004b;561:433–448. doi: 10.1113/jphysiol.2003.051839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follesa P, Biggio F, Caria S, Gorini G, Biggio G. Modulation of GABAA receptor gene expression by allopregnanolone and ethanol. Eur J Pharmacol. 2004;500:413–425. doi: 10.1016/j.ejphar.2004.07.041. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Benke D, Johnson DK, Mohler H, Rudolph U. GABAA-receptor α-subunit is an essential prerequisite for receptor formation in vivo. Neurosci. 1997;81:1043–53. doi: 10.1016/s0306-4522(97)00244-3. [DOI] [PubMed] [Google Scholar]
- Fritschy J-M, Johnson DK, Mohler H, Rudolph U. Independent assembly and subcellular targeting of GABAA receptor subtypes demonstrated in mouse hippocampal and olfactory neurons in vivo. Neurosci Lett. 1998;249:99–102. doi: 10.1016/s0304-3940(98)00397-8. [DOI] [PubMed] [Google Scholar]
- Gingrich KJ, Roberts WA, Kass RS. Dependence of the GABAA receptor gating kinetics on the α-subunit isoform: Implications for structure-function relations and synaptic transmission. J Physiol. 1995;489:529–543. doi: 10.1113/jphysiol.1995.sp021070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas KF, Macdonald RL. GABAA receptor subunit γ2 and δ subtypes confer unique kinetic properties on recombinant GABAA receptor currents in mouse fibroblasts. J Physiol. 1999;514:27–45. doi: 10.1111/j.1469-7793.1999.027af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkle DJ, Macdonald RL. β subunit phosphorylation selectively increases fast desensitization and prolongs deactivation of α1β1γ2L and α1β3γ2L GABAA receptor currents. J Neurosci. 2003;23:11698–11710. doi: 10.1523/JNEUROSCI.23-37-11698.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntsman MM, Huguenard JR. Fast IPSCs in rat thalamic reticular nucleus require the GABAA receptor β1 subunit. J Physiol. 2006;572:459–475. doi: 10.1113/jphysiol.2006.106617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jechlinger M, Peiz R, Tretter V, Klausberger T, Sieghart W. Subunit composition and quantitative importance of hetero-oligomeric receptors: GABAA receptors containing α6 subunits. J Neurosci. 1998;18:2449–2457. doi: 10.1523/JNEUROSCI.18-07-02449.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MV, Westbrook GL. Desensitized states prolong GABAA channel responses to brief agonist pulses. Neuron. 1995;15:181–191. doi: 10.1016/0896-6273(95)90075-6. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Roberts JDB, Somogyi P. Cell type- and input-specific differences in the number and subtypes of synaptic GABAA receptors in the hippocampus. J Neurosci. 2002;22:2513–2521. doi: 10.1523/JNEUROSCI.22-07-02513.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpi ER, Gründer G, Lüddens H. Drug interactions at GABAA receptors. Prog Neurobiol. 2002;67:113–159. doi: 10.1016/s0301-0082(02)00013-8. [DOI] [PubMed] [Google Scholar]
- Lagrange AH, Botzolakis EJ, Macdonald RL. Enhanced macroscopic desensitization shapes the response of α4 subtype-containing GABAA receptors to synaptic and extrasynaptic GABA. J Physiol. 2007;578:655–676. doi: 10.1113/jphysiol.2006.122135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie AM, Tingey JJ, Harrison NL, Pritchett DB, Twyman RE. Activation and deactivation rates of recombinant GABAA receptor channels are dependent on α-subunit isoform. Biophys J. 1997;73:2518–2526. doi: 10.1016/S0006-3495(97)78280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie DJ, Seeburg PH, Wisden W. The distribution of thirteen GABAA receptor subunits mRNAs in the rat brain. II. Olfactory bulb and cerebellum. J Neurosci. 1992a;12:1063–1076. doi: 10.1523/JNEUROSCI.12-03-01063.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie DJ, Wisden W, Seeburg PH. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J Neurosci. 1992b;12:4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maric D, Maric I, Wen X, Fritschy J-M, Sieghart W, Barker JL, Serafini R. GABAA receptor subunit composition and functional properties of Cl- channels with differential sensitivity to zolpidem in embryonic rat hippocampal cells. J Neurosci. 1999;19:4921–4937. doi: 10.1523/JNEUROSCI.19-12-04921.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClellan AML, Twyman RE. Receptor system response kinetics reveal functional subtypes of native murine and recombinant human GABAA receptors. J Physiol. 1999;515:711–727. doi: 10.1111/j.1469-7793.1999.711ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKernan RM, Whiting PJ. Which GABAA-receptor subtypes really occur in the brain? Trends in Neurosci. 1996;19:139–143. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- Mehta AK, Ticku MK. An update on GABAA receptors. Brain Res Rev. 1999;29:196–217. doi: 10.1016/s0165-0173(98)00052-6. [DOI] [PubMed] [Google Scholar]
- Mody I, Pearce RA. Diversity of inhibitory neurotransmission through GABAA receptors. Trends in Neurosci. 2004;27:569–575. doi: 10.1016/j.tins.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Neelands TR, Greenfield LJ, Jr, Zhang J, Turner RS, Macdonald RL. GABAA receptor pharmacology and subtype mRNA expression in human neuronal NT2-N cells. J Neurosci. 1998;18:4993–5007. doi: 10.1523/JNEUROSCI.18-13-04993.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulter MO, Brown LA, Tynan S, Willick G, William R, McIntyre DC. Differential expression of α1, α2, α3, and α5 GABAA receptor subunits in seizure-prone and seizure-resistant rat models of temporal lobe epilepsy. J Neurosci. 1999;19:4654–4661. doi: 10.1523/JNEUROSCI.19-11-04654.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raol YH, Lund IV, Bandyopadhyay S, Zhang G, Roberts DS, Wolfe JH, Russek SJ, Brooks-Kayal AR. Enhancing GABAA receptor α1 subunit levels in hippocampal dentate gyrus inhibits epilepsy development in an animal model of temporal lobe epilepsy. J Neurosci. 2006;26:11342–11346. doi: 10.1523/JNEUROSCI.3329-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Möhler H. GABAA receptor subtypes: dissecting their pharmacological functions. Trends in Pharm Sci. 2001;22:188–194. doi: 10.1016/s0165-6147(00)01646-1. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Möhler H. GABA-based therapeutic approaches: GABAA receptor subtype functions. Curr Opin Pharm. 2006;6:18–23. doi: 10.1016/j.coph.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Saxena NC, Macdonald RL. Properties of putative cerebellar γ-aminobutyric acidA receptor isoforms. Mol Pharm. 1996;49:567–579. [PubMed] [Google Scholar]
- Smith SS, Gong QH, Hus FC, Markowitz RS, ffrench-Mullen JM, Li X. GABAA receptor α4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature. 1998;392:926–930. doi: 10.1038/31948. [DOI] [PubMed] [Google Scholar]
- Smith AJ, Oxley B, Malpas S, Pillai GV, Simpson PB. Compounds exhibiting selective efficacy for different β subunits of human recombinant γ-aminobutyric acidA receptors. J Pharm Exp Ther. 2004;311:601–609. doi: 10.1124/jpet.104.070342. [DOI] [PubMed] [Google Scholar]
- Takahashi T. Postsynaptic receptor mechanisms underlying developmental speeding of synaptic transmission. Neurosci Res. 2005;53:229–240. doi: 10.1016/j.neures.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Tia S, Wang JF, Kotchabhakdi N, Vicini S. Developmental changes of inhibitory synaptic currents in cerebellar granule neurons: role of GABAA receptor α6 subunit. J Neurosci. 1996a;16:3630–3640. doi: 10.1523/JNEUROSCI.16-11-03630.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tia S, Wang JF, Kotchabhakdi N, Vicini S. Distinct deactivation and desensitization kinetics of recombinant GABAA receptors. Neuropharm. 1996b;35:1375–1382. doi: 10.1016/s0028-3908(96)00018-4. [DOI] [PubMed] [Google Scholar]
- Tyndale RF, Hales TG, Olsen RW, Tobin AJ. Distinctive patterns of GABAA receptor subunit mRNAs in 13 cell lines. J Neurosci. 1994;14:5417–5428. doi: 10.1523/JNEUROSCI.14-09-05417.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicini S, Ferguson C, Prybylowski K, Kralic J, Morrow AL, Homanics GE. GABAA receptor α1 subunit deletion prevents developmental changes of inhibitory synaptic currents in cerebellar neurons. J Neurosci. 2001;21:3009–3016. doi: 10.1523/JNEUROSCI.21-09-03009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting PJ, Bonnert TP, McKernan RM, Farrar S, Le Bourdelles B, Heavens RP, Smith DW, Hewson L, Rigby MR, Sirinathsinghji JS, Thompson SA, Wafford KA. Molecular and functional diversity of the expanding GABAA receptor gene family. Ann NY Acad Sci. 1999;868:645–653. doi: 10.1111/j.1749-6632.1999.tb11341.x. [DOI] [PubMed] [Google Scholar]
- Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Z, Huguenard JR, Prince DA. GABAA receptor-mediated currents in interneurons and pyramidal cells of rat visual cortex. J Physiol. 1998;506:715–730. doi: 10.1111/j.1469-7793.1998.715bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


