Abstract
Background
The effects of black tea consumption on cardiovascular risk factors have been inconsistent in previous randomized trials, all of which have been limited to a few weeks duration.
Methods
We conducted a pilot parallel-design randomized controlled trial among 31 adults aged 55 years and older with either diabetes or two other cardiovascular risk factors but no established clinical cardiovascular disease. Participants were randomized to drink three glasses daily of either a standardized black tea preparation or water for six months. Cardiovascular risk factors were measured at the beginning and conclusion of the study.
Results
Three participants dropped out of the study, leaving 14 participants assigned to tea and 14 assigned to water eligible for analyses. We found no statistically significant effects of black tea on cardiovascular biomarkers, including lipids, inflammatory markers, hemoglobin, adhesion molecules, prothrombotic and fibrinolytic parameters, and lipoprotein oxidizability. Assignment to tea did not appreciably influence blood pressure, and heart rate among participants assigned to tea was marginally higher than among control participants at three months (p=0.07) but not six months.
Conclusions
In this randomized trial of black tea intake over six months among older adults with known cardiovascular risk factors, black tea did not appreciably influence any traditional or novel biomarkers of cardiovascular risk. Longer randomized trials are needed to verify the inverse association of tea with risk of cardiovascular disease seen in cohort studies and identify potential candidate mechanisms for such an association.
Tea consumption is highly prevalent worldwide, and has been postulated to have a variety of health benefits, thought chiefly to be related to its complex polyphenolic antioxidants, such as theaflavin and thearubigin in black tea.1, 2 Indeed, a meta-analysis of 17 observational studies found a 11% lower incidence of myocardial infarction associated with intake of three cups of tea per day compared with abstention from tea.3
Randomized trials to identify effects of tea on cardiovascular risk factors have yielded mixed results. In the few studies of markers of endothelial function, tea consumption lowered levels of soluble P-selectin but not ICAM-1 or VCAM-14 or von Willebrand factor (vWF).5 Likewise, most human studies have found no effect of short-term tea intake on oxidizability of LDL in ex vivo experiments6, 7 or on biomarkers of lipid oxidation,8 although studies that have assessed lipoprotein oxidizability without isolating lipoproteins from hydrophilic antioxidants have suggested otherwise.9, 10
The effects of tea on other cardiovascular risk factors have also been mixed. Some studies have shown that tea lowers levels of plasminogen activator inhibitor-1 (PAI-1) in some individuals,11 while others have not;4, 12 similar inconsistency exists for fibrinogen.4, 9, 12 Both animal models and epidemiological studies suggest that tea consumption can lower total cholesterol levels,9, 13 but feeding studies in humans have not consistently shown such an effect.6, 14, 15 A few feeding studies have also found no effect of black tea on inflammatory markers.5, 16 Finally, feeding studies suggest black tea consumption may raise homocysteine levels in some individuals,17, 18 although observational data suggest the opposite.19 Virtually all of the randomized feeding trials of tea conducted to date have been limited to four or fewer weeks of follow-up.
To address the inconsistencies in previous studies of tea, and to determine the effects of intermediate-term tea consumption on cardiovascular risk factors, we conducted a six-month, randomized clinical trial of black tea consumption among older adults at increased risk for cardiovascular disease, the longest such trial conducted to our knowledge. This pilot study also sought to determine the feasibility of incorporating magnetic resonance imaging (MRI) into subsequent clinical trials of tea by having participants undergo MRI examinations of the torso six months apart.
Methods
The Tea’s Effect on Atherosclerosis (TEA) Pilot Study was a randomized, parallel-design, assessor-blinded clinical trial of black tea consumption among free-living individuals, with measurements conducted at the General Clinical Research Center (GCRC) of Beth Israel Deaconess Medical Center (BIDMC). All patients provided written informed consent, and the BIDMC Committee on Clinical Investigations approved the protocol. The ClinicalTrials.gov identifier for the study is NCT00120107.
Study Participants
Participants were community-dwelling adults aged 55 years and older with either diabetes or two other cardiovascular risk factors (hypertension, current smoking, LDL cholesterol ≥130 mg/dl, HDL cholesterol <40 mg/dl, or family history of premature coronary heart disease). Exclusion criteria included established cardiovascular disease (congestive heart failure, myocardial infarction, coronary, carotid, or peripheral arterial revascularization procedure, stroke, angina, or intermittent claudication), contraindications to MRI (severe claustrophobia, intolerance to previous MRI examinations, pacemaker, intraauricular implants, or intracranial clips), atrial fibrillation (due to requirement for gated MRI images), severe illness expected to cause death or disability within six months, blood pressure ≥180/110 mmHg, serum creatinine >2.5 mg/dl or dialysis, history of hyponatremia, use of vitamin supplements greater than the recommended daily allowance, inability to speak English, and lack of a working telephone.
Study Materials
Dehydrated soluble black tea powder (Templar Food Products, Inc., New Providence, NJ) was provided to participants in unit-dose containers. Each container included 2.0 gm of powder, and three containers (representing a single day supply) were bagged together. The catechin content of the tea, measured in duplicate on six separate containers, was 106 +/− 7 mg/serving (i.e., 318 mg/day) of catechin equivalents using a standard colorimetric assay.20
Study Protocol
Interested participants were screened for eligibility and then randomized using random permuted blocks of sizes two and four using a SAS macro specifically developed for this purpose. Randomization assignments were placed into opaque, sealed, sequentially-numbered envelopes in a locked, off-site location.
Participants randomized to tea were asked to consume three servings daily. No restrictions were made on addition of milk or sweeteners, reconstitution with hot or cold water, or time of day of consumption, as in previous positive trials.21 Control subjects were asked to consume three glasses of water daily to control for fluid intake.22 The only dietary restrictions were consumption of non-study tea (green, oolong, or black). Participants were also asked to keep their intake of red wine and multivitamins consistent during the trial.
Participants attended the GCRC for a screening/entry visit, followed by visits at two weeks, three months, and six months. At each visit, nurses measured vital signs, ascertained changes in medication, and queried possible side effects. Dieticians collected 3-day dietary records at the second and final visits. At the initial and final visits, participants were asked to fast for at least eight hours beforehand and underwent phlebotomy, with immediate storage of samples at −80 °C. All assays were performed by technicians blinded to treatment assignment.
Measurements
All measurements were performed by technicians or investigators blinded to treatment assignment. Interleukin-6 (IL-6), tissue necrosis factor alpha (TNFα), VCAM-1, and ICAM-1 were analyzed by immunoassays (R & D Systems, Minneapolis, MN). C-reactive protein (CRP) was measured with a high-sensitivity chemiluminescent assay (Diagnostic Products, Los Angeles, CA). Tissue plasminogen activator (tPA) antigen and vWF were measured by enzyme-linked immunosorbent assays (Diagnostica Stago, Parsippany, NJ). Albumin, glucose, and lipids were analyzed on a Roche autoanalyzer using Roche reagents (F. Hoffmann-La Roche, Basel, Switzerland). Homocysteine was measured on an Abbott (Abbott Park, IL) AxSYM using Abbott reagents. Complete blood counts were performed on the Bayer (Bayer Diagnostics, Tarrytown, NY) Advia 120 using Bayer reagents. Factor VIII was assessed on the bioMerieux (bioMérieux SA, Marcy l’Etoile, France) MDA180, using reagents from Precision Biologics. Fibrinogen was analyzed by the Clauss method on the MDA180, with bioMerieux reagents.
We assessed lipoprotein oxidation using an affinity-column to isolate lipoproteins without removing adherent polyphenols.9 We measured lag time in response to 25 μM cupric ion standardized to protein concentration; longer lag time indicates greater resistance to oxidation.
As a planned measure of adherence, urine samples were tested for 4-O-methylgallic acid at baseline and at the three- and six-month visits by high-performance liquid chromatography as described.23 However, freeze-thaw related sample degradation limited these to a subsample of participants.
Participants underwent electrocardiogram-gated T2-weighted spin-echo MRI examination of the abdomen at the two-week and six-month visits using a previously published protocol.24 A single board-certified radiologist (NO) reviewed all scans in a tandem manner at the completion of the study.
Statistical Analyses
We assessed the effect of tea intake as the arithmetic difference between 6-month and baseline values for participants in each of the treatment arms, using t-tests for normally distributed values and Wilcoxon rank sum tests where skewed. Single extreme outlying values for baseline CRP and ICAM-1 were deleted, but their inclusion did not materially affect any of our results. Results using t-tests with logarithmic transformation of skewed values (rather than rank sum tests) were not materially different. For blood pressure and heart rate, which were measured serially, mixed models with compound symmetry were used, with model terms of time, intervention assignment, and their interaction (the primary estimate of the effect of tea through time). In all cases, participants were analyzed on an intention-to-treat basis. SAS v9.0 (Cary, NC) was used for all analyses.
Results
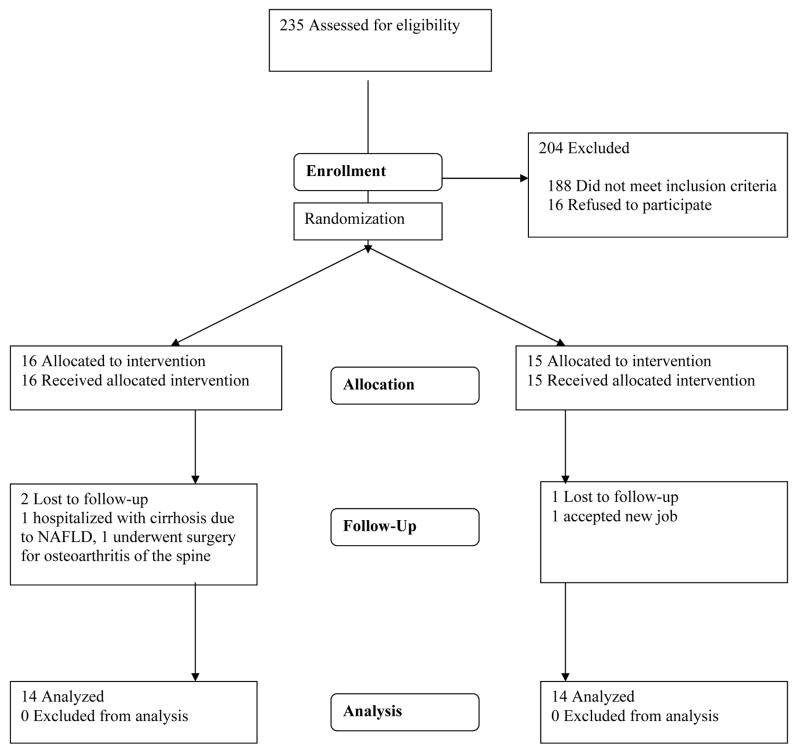
Figure 1 shows the CONSORT flow of participants in the TEA Pilot Study. A total of three participants did not complete the trial, including two who left the study within two weeks of entry for non-study related reasons (change in job and surgery for pre-existing back pain). A third participant assigned to tea developed bacterial peritonitis from unrecognized non-alcoholic fatty liver disease. Characteristics of the 28 participants who completed the study are shown in Table 1. Median baseline tea consumption was 1 cup per week among those assigned to tea and 2 cups per week among those assigned to water, and only 2 subjects reported no tea consumption in the previous year. Caffeine consumption estimated from dietary records at the two-week and final visits was similar in the two groups (two-week: 143 vs. 116 mg per day, p=0.56; final: 128 vs. 165 mg per day, p=0.39).
Figure 1.
CONSORT flowchart of participants in the TEA Pilot Study.
Table 1.
Baseline characteristics of TEA Pilot Study participants.
| Tea (n=14) | Water (n=14) | P-value | |
|---|---|---|---|
| Age (years) | 66.6 ±8.0 | 64.9 ±7.0 | 0.55 |
| Body-Mass Index (kg/m2) | 27.7 ±4.5 | 30.5 ±4.5 | 0.11 |
| Female | 9 (64%) | 9 (64%) | 1.0 |
| White | 14 (100%) | 12 (86%) | 0.48 |
| Married | 9 (64%) | 6 (43%) | 0.45 |
| Education | 0.63 | ||
| High School or Less | 6 (43%) | 4 (29%) | |
| College | 4 (29%) | 7 (50%) | |
| Graduate School | 4 (29%) | 3 (21%) | |
| Current Smoker | 2 (14%) | 0 (0%) | 0.48 |
| Diabetes | 3 (21%) | 1 (7%) | 0.60 |
| Hypertension | 11 (79%) | 13 (93%) | 0.60 |
| Hypercholesterolemia | 12 (86%) | 13 (93%) | 1.0 |
| Statin Use | 11 (79%) | 8 (57%) | 0.42 |
P-values derived from Fisher exact tests.
Table 2 shows the primary results of tea intake on endothelial, inflammatory, metabolic, and hematological factors. We did not find significant differences between participants assigned to tea or to water. There was also no statistically significant difference in lipoprotein oxidizability as measured by oxidation lag time.
Table 2.
Baseline levels and changes over 6-month follow-up in cardiovascular biomarkers among TEA Pilot Study participants.
| Tea (n=14) | Water (n=14) | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | 6-months | Difference | Baseline | 6-months | Difference | P-value | |
| HDL-C (mg/dl) | 62 ±3 | 62 ±2 | −0.5 ±2.0 | 64 ±4 | 64 ±5 | +0.1 ±3.4 | 0.87 |
| Triglycerides (mg/dl) | 148 ±24 | 116 ±13 | −32 ±19 | 160 ±46 | 125 ±20 | −35 ±28 | 0.65 |
| LDL-C (mg/dl) | 111 ±12 | 98 ±9 | −13 ±10 | 116 ±8 | 119 ±9 | +2 ±5 | 0.32 |
| Glucose (mg/dl) | 92 ±4 | 99 ±6 | +7 ±4 | 102 ±14 | 103 ±9 | +1 ±6 | 0.91 |
| WBC (K/μl) | 6.3 ±0.4 | 5.8 ±0.3 | −0.5 ±0.2 | 7.3 ±0.4 | 7.0 ±0.4 | −0.2 ±0.4 | 0.25 |
| Hemoglobin (g/dl) | 13.5 ±0.3 | 13.4 ±0.3 | −0.1 ±0.3 | 13.5 ±0.3 | 13.5 ±0.3 | +0.1 ±0.2 | 0.59 |
| Albumin (g/dl) | 4.31 ±0.08 | 4.36 ±0.06 | +0.05 ±0.07 | 4.26 ±0.07 | 4.41 ±0.06 | +0.15 ±0.06 | 0.29 |
| Homocysteine (μmol/L) | 8.4 ±0.4 | 8.7 ±0.4 | +0.3 ±0.2 | 9.9 ±0.5 | 9.7 ±0.6 | −0.1 ±0.5 | 0.31 |
| Fibrinogen (mg/dl) | 330 ±19 | 319 ±25 | −11 ±23 | 379 ±27 | 391 ±22 | +12 ±29 | 0.52 |
| Factor VIII (%) | 134 ±10 | 135 ±9 | +3 ±7 | 161 ±13 | 152 ±10 | −9 ±9 | 0.31 |
| CRP (mg/dl) | 0.13 ±0.03 | 0.14 ±0.03 | +0.01 ±0.02 | 0.79 ±0.27 | 0.67 ±0.16 | −0.12 ±0.28 | 0.50 |
| IL-6 (pg/ml) | 1.6 ±0.2 | 1.5 ±0.1 | −0.2 ±0.3 | 2.0 ±0.3 | 2.2 ±0.3 | +0.2 ±0.4 | 0.35 |
| TNF-α (pg/ml) | 1.9 ±0.2 | 2.3 ±0.2 | +0.4 ±0.2 | 2.0 ±0.3 | 2.4 ±0.3 | +0.5 ±0.2 | 0.86 |
| sICAM (ng/ml) | 238 ±13 | 235 ±16 | −3 ±11 | 276 ±19 | 268 ±16 | −8 ±8 | 0.72 |
| sVCAM (ng/ml) | 703 ±58 | 819 ±96 | +116 ±57 | 719 ±58 | 764 ±68 | +53 ±26 | 0.42 |
| vWF (%) | 140 ±13 | 136 ±9 | −4 ±7 | 158 ±14 | 165 ±15 | +7 ±7 | 0.28 |
| tPA-antigen (ng/ml) | 8.9 ±0.6 | 8.1 ±1.0 | −0.8 ±0.7 | 10.5 ±1.1 | 10.1 ±0.9 | −0.4 ±0.7 | 0.69 |
| PAI-1-activity (U/ml) | 15 ±5 | 24 ±5 | +9 ±5 | 18 ±4 | 23 ±7 | +5 ±4 | 0.60 |
| Lag time (seconds) | 76 ±11 | 85 ±11 | +9 ±19 | 96 ±8 | 80 ±7 | −16 ±10 | 0.31 |
P-values derived from t-tests or Wilcoxon rank sum tests comparing baseline-to-6-month differences among participants assigned to tea versus water.
Single outlying values deleted for baseline CRP and sICAM.
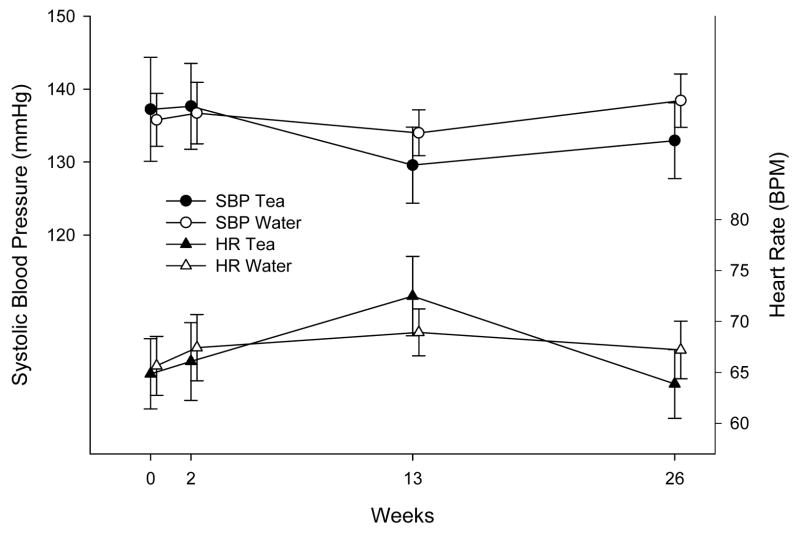
Figure 2 illustrates the change in vital signs over time during the trial. We found no significant effects of assignment to tea on heart rate or systolic blood pressure, although heart rate tended to be higher at the 3 month visit in both groups; the difference in heart rate at that visit between the two groups was of borderline significance (p=0.07). Mean diastolic blood pressure was also uninfluenced by assignment to tea (p=0.76). Mean weight gain was 0.05 ±0.4 kilograms among participants assigned to tea, compared with 0.5 ±0.8 kilograms among participants assigned to water (difference 0.45 ±0.9 kg; p=0.85).
Figure 2.
Systolic blood pressure and heart rate according to tea or water assignment. Means with standard errors are shown.
Among 22 participants with satisfactory samples, mean 4-O-methylgallic acid levels were 13.0 ±8.8 μmol/L in the tea group and 0.3 ±0.1 μmol/L in the water group (p=0.29). Of the 11 participants in each treatment group, six in the tea group and one in the water group had mean levels of at least 1.0 μmol/L (p=0.06). Two participants assigned to water reported consuming any tea during the six months of follow-up.
There were few side effects attributable to tea noted during the trial. A single participant assigned to tea developed chest pain; this was attributed to esophageal reflux from adding large quantities of fresh lemon, and he completed the trial without further symptoms. A second participant noted tooth staining that dental evaluation attributed to concurrent use of stannous fluoride. Other adverse events included a new diagnosis of prostate cancer and a single hospitalization for influenza among participants assigned to tea, and a brief psychiatric hospitalization following cessation of antidepressant use in a participant assigned to water.
All 28 participants completed technically satisfactory MRI examinations. No efficacy analyses of MRI scan findings were planned or performed, but the scans revealed incidental findings in a large proportion of cases. These included seven cases of renal cysts, one hepatic cyst, one splenic cyst, and two cases of cholelithiasis. Two participants were referred for further work-up, including one with generalized lymphadenopathy (found to be chronic lymphocytic leukemia) and one with nonspecific left adrenal gland enlargement. Of the 28 participants, 12 (43%) had at least one incidental finding.
Discussion
In this six-month randomized trial of black tea consumption among adults at higher cardiovascular risk, we found no significant effects of black tea on any biomarkers of advanced cardiovascular risk.
Several possible explanations for our findings should be considered. First, black tea may truly have no important effects on the outcomes we studied, at least over a sustained period of time. Indeed, clinical trials have generally not supported strong effects of black tea on most biomarkers.4–6, 12, 15, 18, 25, 26 If this explanation is correct, then the observed associations of tea with lower risk of cardiovascular disease in cohort studies may either reflect uncontrolled confounding or effects of black tea on other plausible pathways. For example, we did not measure flow-mediated vasodilatation or any other functional measure of vascular function; several clinical trials have shown benefits of tea on such functional parameters.14, 21, 27, 28
Second, the dose of black tea may have been insufficient to produce meaningful effects on these biomarkers. While the dose of three cups/day that we used corresponds well to the levels of intake found to be associated with lower risk in observational studies,3 it is smaller than the doses typically used in those clinical trials that have found benefits of black tea consumption on biomarkers.4, 11 Moreover, because the dose was somewhat smaller than in previous trials, any lack of adherence may have biased our results toward the null findings we observed. Although we undertook extensive efforts to promote adherence, including providing prepackaged containers of a readily solubilized powder and frequent contact with participants, the exact magnitude of adherence is difficult to ascertain in the absence of a long-term biomarker uniformly specific for tea exposure; 4-O-methylgallic acid does not fit this criterion.29, 30
Third, the black tea preparation we chose may not share the benefits of others previously tested, although we have no strong reason to suspect this. We chose a commercially available, representative black tea with ample polyphenol content. While substantial attention has been given to green tea, all forms of tea contain antioxidant polyphenols, and the positive findings in observational studies from the U.S. and Europe clearly reflect the predominately black tea consumption in these populations.
Fourth, the inherent variability in many of these markers – and particularly those related to inflammation and endothelial function – may have obscured any true, modest effect of tea, especially in a population of older adults with cardiovascular risk factors. We intentionally selected this higher risk group based on observational studies,31–33 which have generally supported stronger beneficial effects among higher risk individuals, but it may have introduced additional variability that would overshadow modest effects of tea in a small study such as this. Trials of this duration in younger, healthier subjects will be an important future step.
Fifth, our study was of limited size, and although powered to detect differences similar to those seen in other trials of dietary supplements,34 the confidence limits around our estimates indicate that differences of a potentially important magnitude, at least at the population level, could have been missed. Meta-analyses of small trials like ours may pose the best opportunity to clarify effects of tea with precision.
Although we did not find large benefits of tea, our study provides some reassurance about the safety of long-term tea intake in a clinical trial setting. Despite the inhibitory effect of tea on iron absorption,35 we did not find major changes in hemoglobin levels through the trial, consistent with recent reviews that identified this interaction as being chiefly relevant for individuals with borderline iron status.36, 37 Although some trials have found that tea consumption acutely raises blood pressure14, we found no such effect over 6-months of follow-up, and a recent meta-analysis of five supplementation studies of four-week duration found no significant effect of tea on blood pressure.38 Heart rate tended to rise somewhat more among the tea group than the water group at 3 months, but it also fell more steeply at 6 months in the tea group.
In summary, we found no consistent effects of six months of black tea intake on biomarkers of cardiovascular risk in this study. Our results do not preclude an important effect of tea intake on risk of cardiovascular disease, but they do suggest that alternate mechanisms are apt to be most important, and that long-term randomized trials of tea intake are needed to determine its true effects on cardiovascular risk with certainty.
Acknowledgments
The Tea’s Effect on Atherosclerosis Pilot Study was funded by grants from the American Heart Association (0355638T) and the National Center for Complementary and Alternative Medicine (R21AT01899). This research was also supported in part by grant RR01032 to the BIDMC GCRC from the National Institutes of Health. We thank Wei-Cheng Tung and Najwa Samman for technical assistance in HPLC analyses, and the nurses, technicians, and administrative staff of the BIDMC GCRC for their invaluable assistance throughout the conduct of this trial.
Footnotes
Conflict of Interest
We have cited potential conflicts of interest in the manuscript. Funding for this study was received entirely from the AHA and NCCAM. Templar Foods supplied bulk tea at no charge but provided no other support or funding, had no access to data, and had no involvement with drafting of the manuscript, interpretation or analysis of data, or submission of the manuscript. Dr. Vinson has received previous research funding from Lipton and other food manufacturers for research unrelated to this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mukhtar H, Ahmad N. Tea polyphenols: prevention of cancer and optimizing health. Am J Clin Nutr. 2000;71(6 Suppl):1698S–702S. doi: 10.1093/ajcn/71.6.1698S. discussion 1703S–4S. [DOI] [PubMed] [Google Scholar]
- 2.Riemersma RA, Rice-Evans CA, Tyrrell RM, Clifford MN, Lean ME. Tea flavonoids and cardiovascular health. QJM. 2001;94(5):277–82. doi: 10.1093/qjmed/94.5.277. [DOI] [PubMed] [Google Scholar]
- 3.Peters U, Poole C, Arab L. Does tea affect cardiovascular disease? A meta-analysis. Am J Epidemiol. 2001;154(6):495–503. doi: 10.1093/aje/154.6.495. [DOI] [PubMed] [Google Scholar]
- 4.Hodgson JM, Puddey IB, Mori TA, Burke V, Baker RI, Beilin LJ. Effects of regular ingestion of black tea on haemostasis and cell adhesion molecules in humans. Eur J Clin Nutr. 2001;55(10):881–6. doi: 10.1038/sj.ejcn.1601231. [DOI] [PubMed] [Google Scholar]
- 5.de Maat MP, Pijl H, Kluft C, Princen HM. Consumption of black and green tea had no effect on inflammation, haemostasis and endothelial markers in smoking healthy individuals. Eur J Clin Nutr. 2000;54(10):757–63. doi: 10.1038/sj.ejcn.1601084. [DOI] [PubMed] [Google Scholar]
- 6.Princen HM, van Duyvenvoorde W, Buytenhek R, Blonk C, Tijburg LB, Langius JA, et al. No effect of consumption of green and black tea on plasma lipid and antioxidant levels and on LDL oxidation in smokers. Arterioscler Thromb Vasc Biol. 1998;18(5):833–41. doi: 10.1161/01.atv.18.5.833. [DOI] [PubMed] [Google Scholar]
- 7.van het Hof KH, de Boer HS, Wiseman SA, Lien N, Westrate JA, Tijburg LB. Consumption of green or black tea does not increase resistance of low-density lipoprotein to oxidation in humans. Am J Clin Nutr. 1997;66(5):1125–32. doi: 10.1093/ajcn/66.5.1125. [DOI] [PubMed] [Google Scholar]
- 8.O’Reilly JD, Mallet AI, McAnlis GT, Young IS, Halliwell B, Sanders TA, et al. Consumption of flavonoids in onions and black tea: lack of effect on F2-isoprostanes and autoantibodies to oxidized LDL in healthy humans. Am J Clin Nutr. 2001;73(6):1040–4. doi: 10.1093/ajcn/73.6.1040. [DOI] [PubMed] [Google Scholar]
- 9.Vinson JA, Dabbagh YA. Effect of green and black tea supplementation on lipids, lipid oxidation and fibrinogen in the hamster: mechanisms for the epidemiological benefits of tea drinking. FEBS Lett. 1998;433(1–2):44–6. doi: 10.1016/s0014-5793(98)00880-1. [DOI] [PubMed] [Google Scholar]
- 10.Hodgson JM, Puddey IB, Croft KD, Burke V, Mori TA, Caccetta RA, et al. Acute effects of ingestion of black and green tea on lipoprotein oxidation. Am J Clin Nutr. 2000;71(5):1103–7. doi: 10.1093/ajcn/71.5.1103. [DOI] [PubMed] [Google Scholar]
- 11.Loktionov A, Bingham SA, Vorster H, Jerling JC, Runswick SA, Cummings JH. Apolipoprotein E genotype modulates the effect of black tea drinking on blood lipids and blood coagulation factors: a pilot study. Br J Nutr. 1998;79(2):133–9. doi: 10.1079/bjn19980024. [DOI] [PubMed] [Google Scholar]
- 12.Vorster H, Jerling J, Oosthuizen W, Cummings J, Bingham S, Magee L, et al. Tea drinking and haemostasis: a randomized, placebo-controlled, crossover study in free-living subjects. Haemostasis. 1996;26(1):58–64. doi: 10.1159/000217188. [DOI] [PubMed] [Google Scholar]
- 13.Stensvold I, Tverdal A, Solvoll K, Foss OP. Tea consumption, relationship to cholesterol, blood pressure, and coronary and total mortality. Prev Med. 1992;21(4):546–53. doi: 10.1016/0091-7435(92)90062-m. [DOI] [PubMed] [Google Scholar]
- 14.Duffy SJ, Keaney JF, Jr, Holbrook M, Gokce N, Swerdloff PL, Frei B, et al. Short- and long-term black tea consumption reverses endothelial dysfunction in patients with coronary artery disease. Circulation. 2001;104(2):151–6. doi: 10.1161/01.cir.104.2.151. [DOI] [PubMed] [Google Scholar]
- 15.Bingham SA, Vorster H, Jerling JC, Magee E, Mulligan A, Runswick SA, et al. Effect of black tea drinking on blood lipids, blood pressure and aspects of bowel habit. Br J Nutr. 1997;78(1):41–55. doi: 10.1079/bjn19970117. [DOI] [PubMed] [Google Scholar]
- 16.Widlansky ME, Duffy SJ, Hamburg NM, Gokce N, Warden BA, Wiseman S, et al. Effects of black tea consumption on plasma catechins and markers of oxidative stress and inflammation in patients with coronary artery disease. Free Radic Biol Med. 2005;38(4):499–506. doi: 10.1016/j.freeradbiomed.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 17.Olthof MR, Hollman PC, Zock PL, Katan MB. Consumption of high doses of chlorogenic acid, present in coffee, or of black tea increases plasma total homocysteine concentrations in humans. Am J Clin Nutr. 2001;73(3):532–8. doi: 10.1093/ajcn/73.3.532. [DOI] [PubMed] [Google Scholar]
- 18.Hodgson JM, Burke V, Beilin LJ, Croft KD, Puddey IB. Can black tea influence plasma total homocysteine concentrations? Am J Clin Nutr. 2003;77(4):907–11. doi: 10.1093/ajcn/77.4.907. [DOI] [PubMed] [Google Scholar]
- 19.Hodgson JM, Devine A, Puddey IB, Beilby J, Prince RL. Drinking tea is associated with lower plasma total homocysteine in older women. Asia Pac J Clin Nutr. 2006;15(2):253–8. [PubMed] [Google Scholar]
- 20.Vinson JA, Proch J, Bose P. Determination of quantity and quality of polyphenol antioxidants in foods and beverages. Methods Enzymol. 2001;335:103–14. doi: 10.1016/s0076-6879(01)35235-7. [DOI] [PubMed] [Google Scholar]
- 21.Hodgson JM, Puddey IB, Burke V, Watts GF, Beilin LJ. Regular ingestion of black tea improves brachial artery vasodilator function. Clin Sci (Lond) 2002;102(2):195–201. [PubMed] [Google Scholar]
- 22.Chan J, Knutsen SF, Blix GG, Lee JW, Fraser GE. Water, other fluids, and fatal coronary heart disease: the Adventist Health Study. Am J Epidemiol. 2002;155(9):827–33. doi: 10.1093/aje/155.9.827. [DOI] [PubMed] [Google Scholar]
- 23.Hodgson JM, Chan SY, Puddey IB, Devine A, Wattanapenpaiboon N, Wahlqvist ML, et al. Phenolic acid metabolites as biomarkers for tea- and coffee-derived polyphenol exposure in human subjects. Br J Nutr. 2004;91(2):301–6. doi: 10.1079/BJN20031046. [DOI] [PubMed] [Google Scholar]
- 24.Jaffer FA, O’Donnell CJ, Larson MG, Chan SK, Kissinger KV, Kupka MJ, et al. Age and sex distribution of subclinical aortic atherosclerosis: a magnetic resonance imaging examination of the Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2002;22(5):849–54. doi: 10.1161/01.atv.0000012662.29622.00. [DOI] [PubMed] [Google Scholar]
- 25.McAnlis GT, McEneny J, Pearce J, Young IS. Black tea consumption does not protect low density lipoprotein from oxidative modification. Eur J Clin Nutr. 1998;52(3):202–6. doi: 10.1038/sj.ejcn.1600540. [DOI] [PubMed] [Google Scholar]
- 26.Hodgson JM, Croft KD, Mori TA, Burke V, Beilin LJ, Puddey IB. Regular ingestion of tea does not inhibit in vivo lipid peroxidation in humans. J Nutr. 2002;132(1):55–8. doi: 10.1093/jn/132.1.55. [DOI] [PubMed] [Google Scholar]
- 27.Hodgson JM, Burke V, Puddey IB. Acute effects of tea on fasting and postprandial vascular function and blood pressure in humans. J Hypertens. 2005;23(1):47–54. doi: 10.1097/00004872-200501000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Hirata K, Shimada K, Watanabe H, Otsuka R, Tokai K, Yoshiyama M, et al. Black tea increases coronary flow velocity reserve in healthy male subjects. Am J Cardiol. 2004;93(11):1384–8. A6. doi: 10.1016/j.amjcard.2004.02.035. [DOI] [PubMed] [Google Scholar]
- 29.Mennen LI, Sapinho D, Ito H, Bertrais S, Galan P, Hercberg S, et al. Urinary flavonoids and phenolic acids as biomarkers of intake for polyphenol-rich foods. Br J Nutr. 2006;96(1):191–8. doi: 10.1079/bjn20061808. [DOI] [PubMed] [Google Scholar]
- 30.Hodgson JM, Puddey IB, Burke V, Croft KD. Is reversal of endothelial dysfunction by tea related to flavonoid metabolism? Br J Nutr. 2006;95(1):14–7. doi: 10.1079/bjn20051621. [DOI] [PubMed] [Google Scholar]
- 31.Klatsky AL, Armstrong MA, Friedman GD. Coffee, tea, and mortality. Ann Epidemiol. 1993;3(4):375–81. doi: 10.1016/1047-2797(93)90064-b. [DOI] [PubMed] [Google Scholar]
- 32.Rimm EB, Katan MB, Ascherio A, Stampfer MJ, Willett WC. Relation between intake of flavonoids and risk for coronary heart disease in male health professionals. Ann Intern Med. 1996;125(5):384–9. doi: 10.7326/0003-4819-125-5-199609010-00005. [DOI] [PubMed] [Google Scholar]
- 33.Mukamal KJ, Maclure M, Muller JE, Sherwood JB, Mittleman MA. Tea consumption and mortality after acute myocardial infarction. Circulation. 2002;105(21):2476–81. doi: 10.1161/01.cir.0000017201.88994.f7. [DOI] [PubMed] [Google Scholar]
- 34.Squadrito F, Altavilla D, Crisafulli A, Saitta A, Cucinotta D, Morabito N, et al. Effect of genistein on endothelial function in postmenopausal women: a randomized, double-blind, controlled study. American Journal of Medicine. 2003;114(6):470–6. doi: 10.1016/s0002-9343(03)00059-7. [DOI] [PubMed] [Google Scholar]
- 35.Gabrielli GB, De Sandre G. Excessive tea consumption can inhibit the efficacy of oral iron treatment in iron-deficiency anemia. Haematologica. 1995;80(6):518–20. [PubMed] [Google Scholar]
- 36.Temme EH, Van Hoydonck PG. Tea consumption and iron status. Eur J Clin Nutr. 2002;56(5):379–86. doi: 10.1038/sj.ejcn.1601309. [DOI] [PubMed] [Google Scholar]
- 37.Nelson M, Poulter J. Impact of tea drinking on iron status in the UK: a review. J Hum Nutr Diet. 2004;17(1):43–54. doi: 10.1046/j.1365-277x.2003.00497.x. [DOI] [PubMed] [Google Scholar]
- 38.Taubert D, Roesen R, Schomig E. Effect of cocoa and tea intake on blood pressure: a meta-analysis. Arch Intern Med. 2007;167(7):626–34. doi: 10.1001/archinte.167.7.626. [DOI] [PubMed] [Google Scholar]