Abstract
Excess production of reactive oxygen species (ROS) is an important mechanism underlying the pathogenesis of a number of neurodegenerative diseases including Parkinson’s disease (PD) which is characterized by a progressive loss of dopaminergic neurons in the substantia nigra. Exposure to paraquat, an herbicide with structure similar to the dopaminergic neurotoxin, 1-methyl-4-phenylpyridinium (MPP+), has been shown to produce PD-like symptoms. Despite previous focus on the dopaminergic neurons and signaling pathways involved in their cell death, recent studies have implicated microglial cells as a major producer of ROS for damaging neighboring neurons. In this study, we examined the source of ROS and the underlying signaling pathway for paraquat-induced cytotoxicity to BV-2 microglial cells. Paraquat-induced ROS production (including superoxide anions) in BV-2 cells was accompanied by translocation of the p67phox cytosolic subunit of NADPH oxidase to the membrane. Paraquat-induced ROS production was inhibited by NADPH oxidase inhibitors, apocynin and diphenylene iodonium (DPI), but not the xanthine/xanthine oxidase inhibitor, allopurinol. Apocynin and DPI also rescued cells from paraquat-induced toxicity. The inhibitors for protein kinase C delta (PKCδ) or extracellular signal-regulated kinases (ERK1/2) could partially attenuate paraquat-induced ROS production and cell death. Rottlerin, a selective PKCδ inhibitor, also inhibited paraquat-induced translocation of p67phox. Taken together, this study demonstrates the involvement of ROS from NADPH oxidase in mediating paraquat cytotoxicity in BV-2 microglial cells and this process is mediated through PKCδ- and ERK-dependent pathways.
Keywords: paraquat, NADPH oxidase, apocynin, microglia, cytotoxicity, ROS
1. Introduction
Parkinson’s disease (PD) is one of the most common neurodegenerative diseases in the elderly and is characterized by the loss of dopaminergic neurons in the substantia nigra pars compacta (Schober, 2004). Although genetic mutations have been suggested for the juvenile onset of PD, for most cases, the cause is unclear and a number of environmental factors have been implicated in idiopathic PD (Firestone et al., 2005). Studies with MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine), a toxin known to target dopaminergic neurons, demonstrated the involvement of oxidative stress in mediating the neuronal damage that resembled PD (Chun et al., 2001). Paraquat, an herbicide structurally similar to MPTP, has also been implicated in a variety of epidemiological studies (Peng et al., 2005). Similar to other dopaminergic toxins, paraquat has been shown to cause oxidative stress and selective death of dopaminergic neurons (Herrera et al., 2005; Peng et al., 2005).
Earlier studies have demonstrated a significant increase in markers for oxidative stress in the brains of PD patients as compared to controls (Beal, 2003; Koutsilieri et al., 2002; Shavali et al., 2006). In many instances, oxidative stress occurs before appearance of clinical manifestations (Koutsilieri et al., 2002). In studies with cellular models, paraquat was shown to increase oxidative stress and neurotoxicity in PC12 cells, and polyphenolic antioxidants could ameliorate the cytotoxicity (Koutsilieri et al., 2002; Yang and Sun, 1998a; Yang and Sun, 1998b).
Aside from oxidative stress, studies with animal models have implicated microglia activation in the progression of PD (Croisier et al., 2005). Activated microglial cells were observed in primates given MPTP and humans accidentally exposed to MPTP (Barcia et al., 2004; Langston et al., 1999). However, despite the increase in microglia in the PD brain as compared to normal controls, mechanism(s) linking activation of microglia to neuronal damage has not been elucidated.
Recent studies have provided strong evidence for microglia to produce ROS through activation of NADPH oxidase (Casarejos et al., 2006; Choi et al., 2005) and involvement of this enzyme in a number of neurodegenerative diseases (Sun et al., 2007). This enzyme is comprised of multiple subunits in the cytosol and plasma membrane and assembly of the subunits is initiated by phosphorylation of the cytosolic subunits, e.g., p47phox, p67phox, p40phox, and translocation to the membrane subunits, e.g., gp91phox, p22phox (Choi et al., 2005; Sumimoto et al., 2004). Paraquat has been shown to induce ROS in microglia through activation of NADPH oxidase (Bonneh-Barkay et al., 2005; Wu et al., 2005). However, mechanisms whereby paraquat stimulates ROS production and elicits cell damage remain unclear. Since our preliminary data indicated the ability of paraquat to induce cytotoxicity on BV-2 microglial cells, this study is aimed at investigating the role of NADPH oxidase and its signaling pathways in mediating paraquat-induced ROS production and cytotoxicity in microglial cells.
2. Results
Paraquat induced morphological changes, ROS production, and cytotoxicity in BV-2 microglial cells
Under light microscopic examination, exposure of BV-2 microglial cells to paraquat (50 μM) resulted in shape change and cell condensation starting from 24 h (data not shown). In this study, paraquat-induced ROS production was assessed using the DCF-DA assay and cytotoxicity using the MTT assay. Paraquat exposure caused a dose- and time-dependent increase in ROS production (Fig. 1A) and decrease in cell survival (Fig. 1B). The increase in ROS production was readily observed by 24 h but with 50 μM of paraquat, significant cell death was not observed until 48 hours, suggesting a delayed cell death phenomenon. In subsequent studies, paraquat treatment at 50 μM for 24 h was used for determination of ROS production and cell survival was determined at 48 h.
Fig. 1.

Paraquat induced cytotoxicity and increased ROS production in BV-2 microglia cells. A: Paraquat induced a time and dose-dependent increase in ROS production as determined by DCF-DA assay. B: Paraquat induced a time and dose-dependent decrease in cell viability as determined by MTT reduction assay. See Methods for detail description of the protocols. Data are normalized to control and results are mean ± SD from 3 individual experiments.
Paraquat induced production of superoxide anions
In order to better determine whether paraquat induces production of superoxide anions, the dihydroethidium (DHE) method was used. DHE is oxidized to oxoethidium by superoxide anion and this highly fluorescent product binds with DNA (Miyata et al., 2005). Data in Fig. 2A showed DHE fluorescent intensity in microglial cells after treatment with paraquat with and without diethyldithiocarbamate (DDC), a selective inhibitor of superoxide dismutase (SOD). DDC has been shown to block the conversion of superoxide anions to H2O2 in a number of cell systems (Andresen, et al. 2004; Pagano, et al. 1999; Siwik, et al. 2001). Little or no increase in DHE intensity was observed after treating BV-2 microglial cells with paraquat (50 μM) for 4 h (comparing Fig. 2A-1 with 2A-2). However, an increase in DHE fluorescent intensity was observed upon adding DDC to the cells (comparing Fig. 2A-3 to Fig. 2A-2). Addition of DDC together with paraquat further increased DHE fluorescence as compared with controls and cells treated with paraquat alone (Fig. 2B). Upon addition of DDC to cells to block SOD, increase in paraquat-mediated cell cytotoxicity could be observed as early as 4 h (data not shown). These results suggest that under normal conditions, superoxide anions are continuously produced and degraded by existing SOD and catalase but a blockage of this pathway can result in enhanced cell cytotoxicity.
Fig. 2.

Paraquat caused the increase in superoxide anion as determined by the DHE assay protocol as described in Method. A. Cells were treated with paraquat and/or DDC, a SOD inhibitor, at 37°C for 4 h. Results represent typical fluorescent micrographs depicting BV-2 microglial cells exposed to A-1: control; 2: paraquat (50 μM); 3: DDC (10 μM), and 4: DDC and paraquat. B. Quantitative measurements of paraquat-induced ROS production by DHE in the presence and absence of DDC. Results are mean ± SD from three individual experiments. One-way ANOVA followed by Newman-Keul post-tests indicated significant difference (p < 0.05): a comparing treatment with other groups.
Paraquat-induced ROS is linked to activation of NADPH oxidase
NADPH oxidase subunits are highly expressed in microglia cells (Jekabsone et al., 2006) and functional activity of this enzyme requires the assembly of cytosolic and membrane subunits. As a first step, we examined translocation of the cytosolic subunit, e.g., p67phox, to the membrane upon exposure of microglial cells to paraquat. After treating cells with paraquat (50 μM) for 5 and 10 min, cells were harvested and subjected to subcellular fractionation and the cytosol and membrane fractions were used for Western blot analysis. Paraquat treatment for 5 and 10 min resulted in significant increase in immunoreactivity of p67phox in the membrane fraction (Fig. 3A), indicating the translocation of p67phox to the membrane fraction. High levels of p67phox were found in the cytosol, and there were no apparent differences in p67phox to β-actin ratios in this fraction due to paraquat treatment (Fig 3B). We also tested translocation of other NADPH oxidase cytosolic subunits, e.g., the p47phox or p40phox. However, we had difficulty in finding suitable antibodies for these subunits. Since the cytosolic subunits were translocated as a complex, it is reasonable to assume that the other subunits are assembled together with the p67phox subunit prior to translocation.
Fig 3.

Western blot analysis of p67phox subunit of NADPH oxidase in (A) membrane and (B) cytosol fractions. Microglial cells were exposed to paraquat (50 μM) for 0, 5 and 10 min after which they were subjected to fractionation to separate cytosol and membrane fractions as described in text. Beta-actin was used as loading controls (n=3). Results are representative blots from three independent experiments.
In order to further test the involvement of NADPH oxidase in paraquat-mediated ROS production, inhibitors such as apocynin and diphenylene iodonium (DPI) were used. Apocynin is a methoxy-substituted catechol extracted from the root of Picrorhiza kurroa plant. This compound has been shown to block translocation of the cytosolic subunits to the membrane (Dodd-O and Pearse, 2000). DPI is not a selective inhibitor of NADPH oxidase as it can also inhibit electron transport through the cytochrome b (O’Donnell et al., 1993) as well as other redox cycling reactions. However, DPI has been extensively used as a supporting evidence for the presence of flavan catalyzed oxido/reduction reactions including NADPH oxidase (O’Donnell et al., 1994). As shown in Figs. 4A and B, apocynin significantly inhibited paraquat-induced ROS production (from 0.6 + 0.17 to 0.3 + 0.13) and rescued the cells from cytotoxicity (from 0.5 + 0.10 to 0.9 + 0.15). Pretreatment of cells with DPI (1 μM) completely abolished paraquat-induced ROS production (0.8 + 0.17 to 0.1 + 0.09) (Fig. 4C). Due to interference of DPI with MTT, its effect on paraquat-induced cytotoxicity was not determined (O’Donnell et al., 1993). We also tested the effects of gp91ds-tat, a specific peptide inhibitor for NADPH oxidase, (Krotz et al., 2007). However, for unknown reasons, this peptide compound failed to work in microglial cells (data not shown).
Fig. 4.

Effects of apocynin and DPI on paraquat-induced ROS production and cytotoxity. Microglial cells were treated with paraquat (50 μM) and/or apocynin (1 mM) for (A) ROS determination by DCF at 24 h (n = 6) and (B) assessment of cytotoxicity by MTT at 48 h (n = 3). (C) Treatment of microglial cells with paraquat (50 μM) and/or DPI (1 μM) for 24 h for ROS determination by DCF (n = 5). Results are mean ± SD from number of experiments indicated. One-way ANOVA followed by Newman-Keul post-tests indicated significant difference, p< 0.05: a comparing with control and b comparing with paraquat.
Xanthine Oxidase was not involved in paraquat cytotoxicity
Xanthine oxidase is another enzyme known to generate superoxide and hydrogen peroxide (Biagi and Abate, 2005; Pacher et al., 2006). This enzyme is expressed widely in various organs including the brain (Pacher et al., 2006) and increased levels in the plasma and peripheral organs have been used as indications of tissue damage (Biagi and Abate, 2005; Pacher et al., 2006). In our study, we used two concentrations (10 and 50 μM) of allopurinol, a specific xanthine oxidase inhibitor, to evaluate effects on paraquat-induced production of ROS and cell death. However, neither concentration was able to alter paraquat-induced ROS production and cell death (data not shown).
Role of PKCδ in paraquat-induced ROS production and cytotoxicity
PKC isoforms have been implicated in phosphorylation of the cytosolic subunits of NADPH oxidase (Grandvaux et al., 2001; Serezani et al., 2005; Waki et al., 2006; Zhao et al., 2005). In this study, we tested the possible involvement of PKC in paraquat-induced ROS production and cytotoxicity. Treatment of microglial cells with GF109203x (5 μM), a general PKC inhibitor, partially inhibited paraquat-induced ROS production (Fig. 5A) and cell death (Fig. 5B), although the decrease in ROS production did not reach significance due to the large variance in the data. Since rottlerin is a more selective inhibitor for PKCδ (Zhao et al., 2005), almost complete inhibition paraquat-induced ROS production (0.6 + 0.19 versus 0.2 + 0.15) and cell death (0.4 + 0.07 versus 0.9 + 0.13) was also observed with 1 μM of rottlerin (Fig 5D).
Fig. 5.

The role of PKC on paraquat-induced ROS production and cytotoxicity. Microglial cells were treated with paraquat (50 μM), GF109203x (5 μM), and rottlerin (1 μM) for 24 or 48 h as described above. (A) Treatment with paraquat and/or GF109203x for 24 h for ROS determination; (B) treatment with paraquat and/or GF109203x for 48 h for assessment of cytotoxicity; (C) treatment with paraquat and/or rottlerin for 24 h for ROS determination; and (D) treatment with paraquat and/or rottlerin for 48 h for cytotoxicity. Results are mean ± SD from 3–5 independent experiments. One-way ANOVA followed by Newman-Keul post-tests indicated significant difference, p< 0.05: a comparing with control and b comparing with paraquat.
If PKCδ is essential for phosphorylating the NADPH oxidase subunits, it is reasonable that inhibition of PKCδ phosphorylation by rottlerin would prevent translocation of the cytosolic subunits. As shown in Fig. 6, paraquat induced translocation of the p67phox subunit to membrane was completely inhibited by rottlerin. These results further support the notion that paraquat-induced cytotoxicity through phosphorylation of the cytosolic subunits of NADPH oxidase by PKCδ.
Fig. 6.
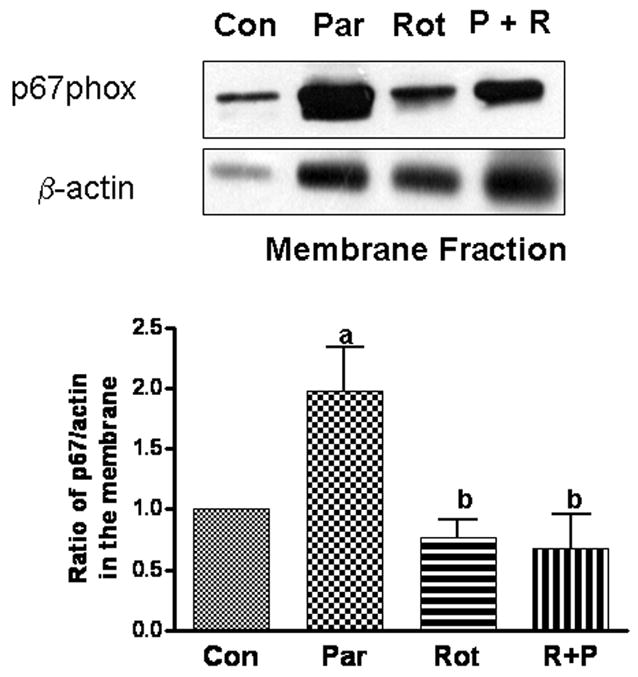
Effects of rottlerin on paraquat-induced translocation of p67 subunit to membranes. Microglial cells were exposed to paraquat and/or rottlerin for 24 h prior to separation of cytosol and membrane fractions for Western blot analysis. Blots were used for densitometer scanning and ratios of p67phox to β-actin in controls were normalized to 1. Results are expressed as mean ± SD (n = 5). One-way ANOVA followed by Newman-Keul post-tests indicated significant difference, p< 0.05: a comparing with control and b comparing with paraquat.
Paraquat induced NADPH oxidase activity is dependent on ERK 1/2
Mitogen activated protein kinases (MAPKs) also have been implicated in the activation of NADPH oxidase subunits, particularly the p47phox subunit (Dewas et al., 2000; Karlsson et al., 2000). In order to test whether paraquat increases phosphorylation of ERK 1/2, we examined both p-ERK1/2 and total ERK1/2 in cells after paraquat treatment. As shown in Fig. 7A, treatment of microglial cells with paraquat caused the increase in phosphorylation of ERK1/2 within 5 and 10 min. The U0126, an inhibitor for the MEK that phosphorylates ERK1/2, significantly inhibited paraquat-induced ROS production (0.9 + 0.24 versus 0.6 + 0.20) and ameliorated cell death (0.52 + 0.08 versus 0.8 + 0.08) (Figs. 7B and C). Our results are in agreement with other studies showing ERK1/2 dependency in the activation of NADPH oxidase and cell death (Dewas et al., 2000; Karlsson et al., 2000).
Fig. 7.

Paraquat induces phosphorylation of ERK1/2 and effects of MEK inhibitor on paraquat-induced ROS production and cytotoxicity. (A) For demonstration of ERK1/2 activation, microglial cells were exposed to paraquat (50 μM) for 0, 5 and 10 min prior to lysis for Western blot using antibodies for p-ERK1/2 and total ERK1/2 (n = 3). (B) Cells were exposed to paraquat (50 μM) and/or U0126 (10 μM) for 24 h for assessment of ROS (n = 5); (C) cells were exposed to paraquat (50 μM) and/or U0126 (10 μM) for 48 h for assessment of cytotoxicity (n = 3). One-way ANOVA followed by Newman-Keul post-tests indicated significant difference, p< 0.05: a comparing with control and b comparing with paraquat.
3. Discussion
Microglia activation is known to play an important role in a number of neurodegenerative disorders including PD (Block et al., 2006; Li et al., 2005a; Li et al., 2005b; Qin et al., 2005; Zhang et al., 2005). Microglia are present throughout the brain and are higher in the substantia nigra (12% of cells) than in the cortex (only 5%) (Kim et al., 2000; Lawson et al., 1990). In PD patients, the substantia nigra was found to have more than 6-time the number of reactive microglia as compared to the control brains (McGeer et al., 1988a; McGeer et al., 1988b). Microglia activation is involved in the cytotoxicity of neurotoxins such as MPTP, rotenone, substance P, and methamphetamine (Block et al., 2006; Delgado, 2003; Gao et al., 2002; Gao et al., 2003b; Scheller et al., 2005; Thomas et al., 2004; Wu et al., 2003). In the substantial nigra, a brain region enriched in dopaminergic neurons, microglial activation is associated with neurochemical changes such as the decrease in dopamine synthesis (Barcia et al., 2004; Scheller et al., 2005; Wu et al., 2003). In fact, changes in neurochemical parameters are observed before development of obvious lesions, suggesting that a short exposure to an insult may initiate a continuous process leading to neurodegeneration (Langston et al., 1999). Minocycline, an antibiotic known to inhibit microglial proliferation, attenuated the neurotoxicity due to 6-hydroxydopamine, MPTP and rotenone (Casarejos et al., 2006; Croisier et al., 2005). These studies support the important role of microglia in the initiation and progression of PD. Using paraquat as a model compound, our study demonstrated time- and dose- effects for this compound to increase ROS production and cytotoxicity in microglial cells. In fact, paraquat-induced cytotoxicity was more pronounced in microglial cells as compared to neuronal cells such as the SH-SY5Y neuroblastoma cells (data not shown).
Recent studies have demonstrated NADPH oxidase as an important source of ROS produced by activated microglia (Li et al., 2005b). In addition, ROS produced by glial cells can cause deleterious effects to neighboring cells including neurons (Abramov et al., 2004; Wilkinson and Landreth, 2006). Neurotoxicity induced by other neurotoxins such as substance P, rotenone and MPTP, can be attenuated upon inhibition of NADPH oxidase by compounds such as DPI and apocynin or by using microglial cells deficient in NADPH oxidase (Block et al., 2006; Gao et al., 2002; Gao et al., 2003a; Wu et al., 2003). In agreement with these earlier studies, DPI and apocynin also inhibited paraquat-induced ROS production and cell death in BV-2 microglial cells. In addition, negative results from testing with the xanthine oxidase inhibitor, allopurinol, further suggest that xanthine oxidase is not a major contributor of the ROS produced by paraquat in microglial cells.
Increases in ROS production by oxidases in the mitochondrial respiratory chain have been regarded as an important process for toxin-induced apoptosis (Gonzalez-Polo et al., 2004; McCarthy et al., 2004). Mitochondrial dysfunction and mitochondrial-induced apoptosis have been demonstrated in paraquat toxicity in dopaminergic cells (Gomez et al., 2007; Gonzalez-Polo et al., 2004; McCarthy et al., 2004; Richardson et al., 2005). Our results show that paraquat-induced cytotoxicity is preceded by the increase in ROS production. This type of delayed cell death together with cell condensation is in line with cell death by apoptosis triggered by mitochondrial dysfunction. Since NADPH oxidase inhibitors can completely block ROS production and rescue paraquat-mediated cell death pathways, it is reasonable to suggest that the pool of ROS produced by NADPH oxidase is an early event leading to mitochondrial dysfunction and cell death. Obviously, more studies are needed to detail the link between ROS produced by NADPH oxidase and mitochondria-mediated cell death mechanism. Our results support the hypothesis that intricate mechanisms regulate different pools of ROS in cells and in paraquat cytotoxicity, and the pool of ROS produced by NADPH oxidase is an early event preceding production of mitochondrial ROS and cell death. In a recent study with lung cells, paraquat was shown to cause a 2–4 fold increase in cellular oxygen consumption, which was neither due to NADPH oxidase nor mitochondrial respiration (Gray et al., 2007). This study further indicated the presence of a specific NADPH:paraquat oxidoreductase in the lung cells for initiating the redox mechanism (Gray et al., 2007). Whether similar mechanism exists in microglial cells is not known and remains to be investigated.
Among more than 20 different PKC isoforms present in mammalian cells, PKCδ has been most directly linked to activation of NADPH oxidase (Zhao et al., 2005). In mouse adypocytes, overexpression of PKCδ has been shown to increase generation of superoxide anions by NADPH oxidase (Talior et al., 2005). Other studies demonstrated attenuation of superoxide anions production by rottlerin, a specific inhibitor for PKCδ, and by expression of dominant negative PKCδ in the cells (Talior et al., 2005; Waki et al., 2006; Zhao et al., 2005). Futhermore, rottlerin and antisense oligodeoxyribonucleotides also inhibited phosphorylation of the p67phox and p40phox subunits (Bey et al., 2004; Grandvaux et al., 2001; Zhao et al., 2005). In our previous study using a special Western blot protocol to analyze PKC isoforms (Xu et al., 2002), we observed 5 major PKC isoforms in BV-2 microglial cells (namely, PKCα, δ, ε, ι and λ and PKCδ is the most highly expressed isoform (data not shown). The high abundance of PKCδ in BV-2 microglial cells clearly supports its role in kinase activity and explains the effects of rottlerin in inhibiting paraquat-induced translocation of the p67phox subunit, ROS production and cell death. Future experiments using more molecular tools to verify site of action of PKCδ to phosphorylate specific NADPH oxidase subunit(s) will help in the development of specific pharmacological compounds targeting the signaling pathway for activation of NADPH oxidase.
Our study also demonstrated the involvement of ERK1/2 in paraquat-mediated ROS production and cell cytotoxicity in microglial cells. ERK1/2 activation has been shown in transgenic animal model of PD (Thiruchelvam et al., 2005). In our study, exposure of microglial cells to paraquat resulted in a rapid phosphorylation of ERK1/2 and inhibition of MEK by U0126 attenuated paraquat induced ROS production and cell death. Although antibodies for the p47phox subunit did not work in our hands for Western analysis (data not shown), other studies have demonstrated ability for MEK inhibitors to block phosphorylation of this subunit (Dewas et al., 2000; Karlsson et al., 2000). In agreement with results from a study by Dewas et al. (2000), our results show that inhibition of both PKC and ERK1/2 by GF109203x and U0126, respectively, could completely abrogate paraquat-induced ROS production and cytotoxic effects (data not shown).
The ability for apocynin to inhibit NADPH oxidase-mediated ROS production has generated special interest. This botanical compound is derived from the root of Picrorhiza kurroa (Dodd-O and Pearse, 2000) and has been used as a Chinese medicinal herb for treatment of a number of infectious and inflammatory diseases (Hougee et al., 2006; Kim-Mitsuyama et al., 2005; Lafeber et al., 1999). Studies from our laboratory showed the ability for apocynin to protect against delayed neuronal death induced by global cerebral ischemia in the gerbil (Wang et al., 2006). Future studies with PD animal models may provide information regarding whether apocynin can be a therapeutic compound for ameliorating paraquat cytotoxicity and PD-like symptoms induced by neurotoxins.
In summary, results from this study demonstrated cytotoxicity of paraquat to microglial cells through ROS produced from NADPH oxidase (Fig. 8). Our data also show that agents inhibiting NADPH oxidase not only inhibited ROS production but also rescued cells from cytotoxic effects of paraquat. Thus, generation of superoxide anions from microglial NADPH oxidase is regarded an important initiator and factor for the progression of neurodegeneration in Parkinson’s disease. Results from this study underline the importance of pharmacological agents targeting NADPH oxidase as therapeutic strategy for treatment and retardation of Parkinson’s disease.
Fig. 8.

A scheme depicting the signaling pathways for paraquat-induced ROS production through activation of NADPH oxidase in microglial cells.
4. Experimental procedure
Cell Culture and Chemicals
Microglia cells (BV-2) were obtained from R. Donato (University of Purugia, Italy) and were routinely cultured in 75 ml flasks containing DMEM (Gibco) with 5% FBS and 500 units/ml Penicillin/Streptomycin (Gibco). After confluent, cells were subcultured in 6 or 24-well plates for experiments. The chemicals used in the treatments include: U0126 (promega), GF109203x (Biosource), dichlorodihydrofluorescein diacetate (DCF-DA) (Invitrogen), and dihydroethidium (DHE) (Invitrogen). Chemicals from Sigma included paraquat, rottlerin, 4-hydroxy-3-methoxy-acetophenone (apocynin), diphenylene iodonium (DPI), allopurinol, sodium diethyldithiocarbamate trihydrate (DDC), and 2′,7′-3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyl tetrazolium bromide (MTT). Cells were routinely checked for contaminations and other morphological abnormalities prior to use in experiments.
Determination of ROS production
For assessment of ROS produced by paraquat in the cell cytotoxicity experiment, cells were grown on 24 well plates until confluent and the DCF-DA assay was used. After removing serum, cells were placed in an isotonic Krebs-Ringer-Hepes (KRH) buffer containing 1.3 mM CaCl2, 131 mM NaCl, 1.3 mM MgSO4, 5 mM KCl, 0.4 mM KH2PO4, 6 mM glucose and 20 mM Hepes (pH 7.4). After equilibration for 2 h, DCF-DA (10 μg/ml) was added. After 1 hr the KRH buffer containing excess DCF was removed from the cells and replaced with fresh KRH and were treated by paraquat and inhibitors as described in figure legends. Fluorescent intensity was measured at 485 nm excitation and 520 nm emission on a Packard Fusion plate reader (Sheehan et al., 1997; Wang and Joseph, 1999). The KRH buffer was used in this assay because of high fluorescent background when using other medium e.g. DMEM. Testing the viability of cells with MTT assay comparing the KRH buffer to DMEM medium showed no significant difference in cell viability after 24 or 48 hours (data not shown).
DCF-DA is known to detect a number of ROS species, including superoxide anions, H2O2 and hydroxyl radical (Gomes et al., 2005). To better detect the superoxide anion, we also used the DHE protocol. DHE is oxidized to oxoethidium by superoxide anion and this highly fluorescent product binds to DNA (Miyata et al., 2005). Because the background fluorescence of DMEM:F12 did not interfere with the results in the DHE protocol, cells were grown on glass slides in serum-free and phenol red-free DMEM:F12 (1:1, by vol) and were incubated with 10μM DHE at 37°C for 30 minutes. After removing excess DHE and replacement with fresh phenol red-free DMEM:F12 medium, paraquat and/or inhibitor were added and cells were incubated at 37°C for 4 hours. For quantitation of DHE fluorescence the glass slides were placed in an inverted fluorescent microscope (Nikon Eclipse TE2000-U) outfitted with a × 40 PlanFluor objective and a rhodamine filter set. Images were acquired using the MetaMorph software with a × 4 neutral density filter engaged. More than four separate images were taken per well. For determination of fluorescent intensity in individual cells, the MetaMorph software was used with proper background subtraction. Average intensity per cell was determined for more than 10 cells in each well and experiment was repeated three times.
Assessment of cell viability
The MTT assay was used for assessment of cell viability. Before treatment with paraquat and inhibitors, cells were placed in serum-free DMEM. Cells were incubated with paraquat and other agents for different periods up to 48 hours. After various treatments, culture medium was removed and 1 mg/ml MTT dissolved in DMEM was added to the cells and incubated for 2 hours. MTT was converted to blue formazan crystals by the dehydrogenases in active mitochondria in live cells. After incubation, medium was discarded, crystals dissolved in dimethyl sulfoxide:ethanol (1:1, by vol) and absorbance of the solution read at 560 nm (Li and Sun, 1999).
Immunoblot assay
In experiments requiring separation of cytosol from membrane fractions, cells were suspended in 0.25M sucrose, 5mM MgCl2, 2mM EGTA, 2mM EDTA, 10mM Tris (pH7.5), 10 μg/ml leupeptin, 1mM phenlymethylsulfonyl fluoride, 10 μg/ml pepstatin, and 10 μg/ml aprotinin. The cells were sonicated 3 times for 10 seconds and centrifuged at 1000g for 5 minutes at 4°C to remove unbroken cells and nuclei. Supernatant was removed and centrifuged at 100,000g for 1 hour. The pellet was resuspended in the membrane separation buffer with Triton-x 100 (Min et al., 2003).
For Western blot analysis, cells were lysed with lysis buffer containing 150 mM NaCl, 50 mM tris pH 8, 2 mM EDTA, 1% Triton, 0.1% sodium dodecyl sulfate (SDS) plus protease inhibitors 1 mM phenylmethylsulfonyl fluoride and 10 μg/ml leupeptin (Ruiz-Leon and Pascul, 2001). The lysate was then centrifuged for 15 minutes at 14,000 rpm to remove debris. The supernatant was used to determine proteins using the DC protein assay protocol (Bio-Rad). Proteins were separated on 10 % dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) at 100 V and subsequently transferred to nitrocellulose membranes at 200 mAmps. Membranes were blocked with dry non-fat milk (5% w/v) dissolved in Tris-buffered saline with Tween 20 (TBST) for one hour at room temperature. Primary antibodies were diluted in 2% milk in TBST. Primary antibodies include the following: polyclonal anti-p67phox 1:1000 (Upstate), monoclonal anti-β-actin 1: 2000 (Cytoskeleton), polyclonal anti-ERK1/2 1:1000 (Cell Signaling), and monoclonal anti-phospho-ERK 1/2 1:1000 (Cell Signaling). After incubation for 1 h at room temperature, excess primary antibodies were removed by washing with TBST. Secondary antibodies, either anti-rabbit goat HRP conjugated (Santa Cruz) or anti-mouse goat HRP conjugated (Santa Cruz), were diluted in 2% milk in TBST. After incubation for 1 h at room temperature, excess secondary antibody was removed by washing with TBST. Bands were visualized with enhanced chemiluminescence (Pierce) and autoradiograph film (SciMart). Density of bands was measured using the Quantity One software.
Statistics
All graphs were created using GraphPad Prism. Due to photo-bleaching and color and/or fluorescence contributed by some inhibitors that may interfere with the DCF assay, it was necessary to subtract fluorescent contributions by the inhibitors alone and normalize results to fold increase of control. Statistics were performed using One-way ANOVA with Newman-Keuls post test to compare, all pairs of columns and a 95% confidence interval.
Acknowledgments
I would like to thank Dr. James Lee and Dr. Ronald Korthuis laboratories for help in use of the microscopes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abramov AY, Canevari L, Duchen MR. Beta-amyloid peptides induce mitochondrial dysfunction and oxidative stress in astrocytes and death of neurons through activation of NADPH oxidase. Journal of Neuroscience. 2004;24:565–75. doi: 10.1523/JNEUROSCI.4042-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andresen JJ, Faraci FM, Heistad DD. Vasomotor responses in MnSOD-deficient mice. American Journal of Physiology - Heart & Circulatory Physiology. 2004;287(3):H1141–8. doi: 10.1152/ajpheart.01215.2003. [DOI] [PubMed] [Google Scholar]
- Barcia C, Bahillo AS, Fernandez-Villalba E, Bautista V, Poza MP, Fernandez-Barreiro A, Hirsch EC, Herrero M-T. Evidence of the active microglia in substantia nigra pars compacta of Parkinsonian monkeys 1 year after MPTP exposure. GLIA. 2004;46:402–9. doi: 10.1002/glia.20015. [DOI] [PubMed] [Google Scholar]
- Beal MF. Mitochondria, oxidative damage, and inflammation in Parkinson’s disease. Annals of New York Academy of Sciences. 2003;991:120–31. doi: 10.1111/j.1749-6632.2003.tb07470.x. [DOI] [PubMed] [Google Scholar]
- Bey EA, Xu B, Bhattacharjee A, Oldfield CM, Zhao X, Li Q, Subbulakshmi V, Feldman GM, Wientjes FB, Cathcart MK. Protein kinase C delta is required for p47phox phosphorylation and translocation in activated human monocytes. Journal of Immunology. 2004;173:5730–8. doi: 10.4049/jimmunol.173.9.5730. [DOI] [PubMed] [Google Scholar]
- Biagi P, Abate L. Heart failure, oxidative stress and allopurinol. Monaldi Archives for Chest Disease. 2005;64:33–7. doi: 10.4081/monaldi.2005.609. [DOI] [PubMed] [Google Scholar]
- Block ML, Li G, Qin L, Wu X, Pei Z, Wang T, Wilson B, Yang J, Hong JS. Potent regulation of microglia-derived oxidative stress and dopaminergic neuron survival: substance P vs. dynorphin. FASEB Journal. 2006;20:251–8. doi: 10.1096/fj.05-4553com. [DOI] [PubMed] [Google Scholar]
- Bonneh-Barkay D, Reaney SH, Langston WJ, Di Monte DA. Redox cycling of the herbicide paraquat in microglial cultures. Brain Research Molecular Brain Research. 2005;134:52–6. doi: 10.1016/j.molbrainres.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Casarejos MJ, Menendez J, Solano RM, Rodriguez-Navarro JA, Garcia de Yebenes J, Mena MA. Susceptibility of rotenone is increased in neurons from parkin null mice and is reduced by minocycline. Journal of Neurochemistry. 2006;97:934–46. doi: 10.1111/j.1471-4159.2006.03777.x. [DOI] [PubMed] [Google Scholar]
- Choi SH, Lee da Y, Kim SU, Jin BK. Thrombin-induced oxidative stress contributes to the death of hippocampal neurons in vivo: role of microglial NADPH oxidase. Journal of Neuroscience. 2005;25:4082–90. doi: 10.1523/JNEUROSCI.4306-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun HS, Gibson GE, DeGiorgio LA, Zhang H, Kidd VJ, Son JH. Dopaminergic cell death induced by MPP+, oxidant and specific neurotoxicants shares the common molecular mechanism. Journal of Neurochemistry. 2001;76:1010–21. doi: 10.1046/j.1471-4159.2001.00096.x. [DOI] [PubMed] [Google Scholar]
- Croisier E, Moran LB, Dexter DT, Pearce RKB, Graeber MB. Microglial inflammation in the parkinsonian substantia nigra: relationship to alpha-synuclein deposition. Journal of Neuroinflammation. 2005;2:14–22. doi: 10.1186/1742-2094-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado M, Ganea D. Neuroprotective effect of vasoactive intestinal peptide (VIP) in a mouse model of Parkinson’s disease by blocking microglial activation. FASEB Journal. 2003;17:944–6. doi: 10.1096/fj.02-0799fje. [DOI] [PubMed] [Google Scholar]
- Dewas C, Fay M, Gougerot-Pocidalo MA, El-Benna J. The mitogen-activated protein kinase extracellular signal-regulated kinase 1/2 pathway is involved in formyl-methionyl-leucyl-phenylalanine-induced p47phox phosphorylation in human neutrophils. Journal of Immunology. 2000;165:5238–44. doi: 10.4049/jimmunol.165.9.5238. [DOI] [PubMed] [Google Scholar]
- Dodd-O JM, Pearse DB. Effect of the NADPH oxidase inhibitor apocynin on ischemia-reperfusion lung injury. American Journal of Physiology-Heart Circulation Physiology. 2000;279:H303–12. doi: 10.1152/ajpheart.2000.279.1.H303. [DOI] [PubMed] [Google Scholar]
- Firestone JA, Smith-Weller T, Franklin G, Swanson P, Lonstreth WT, Checkoway H. Pesticides and risk of Parkinson disease. Arch Neurology. 2005;62:91–5. doi: 10.1001/archneur.62.1.91. [DOI] [PubMed] [Google Scholar]
- Gao HM, Hong JS, Zhang W, Liu B. Distinct role for microglia in rotenone-induced degeneration of dopaminergic neurons. Journal of Neuroscience. 2002;22:782–90. doi: 10.1523/JNEUROSCI.22-03-00782.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao HM, Liu B, Hong JS. Critical role for microglial NADPH oxidase in rotenone-induced degeneration of dopamingeric neurons. Journal of Neuroscience. 2003a;23:6181–7. doi: 10.1523/JNEUROSCI.23-15-06181.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao HM, Liu B, Zhang W, Hong JS. Critical role of microglial NADPH oxidase-derived free radicals in the in vitro MPTP model of Parkinson’s disease. FASEB Journal. 2003b;17:1954–6. doi: 10.1096/fj.03-0109fje. [DOI] [PubMed] [Google Scholar]
- Gomes A, Fernandes E, Lima JL. Fluorescence probes used for detection of reactive oxygen species. Journal of Biochemical & Biophysical Methods. 2005;65:45–80. doi: 10.1016/j.jbbm.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Gomez C, Bandez MJ, Navarro A. Pesticides and impairment of mitochondrial function in relation with the parkinsonian syndrome. Frontiers in Bioscience. 2007;12:1079–93. doi: 10.2741/2128. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Polo RA, Rodriguez-Martin A, Moran JM, Niso M, Soler G, Fuentes JM. Paraquat-induced apoptotic cell death in cerebellar granule cells. Brain Research. 2004;1011:170–6. doi: 10.1016/j.brainres.2004.02.078. [DOI] [PubMed] [Google Scholar]
- Grandvaux N, Elsen S, Vignais PV. Oxidant-dependent phosphorylation of p40phox in B lymphocytes. Biochemical & Biophysical Research Communications. 2001;287:1009–16. doi: 10.1006/bbrc.2001.5665. [DOI] [PubMed] [Google Scholar]
- Gray JP, Heck DE, Mishin V, Smith PJS, Hong J-Y, Thiruchelvam M, Cory-Slechta DA, Laskin DL, Laskin JD. Paraquat increases cyanide-insensitive respiration in murine lung epithelial cells by activating an NAD(P)H: Paraquat Oxidoreductase: Identification of the enzyme as thioredoxin reductase. Journal of Biolological Chemistry. 2007 doi: 10.1074/jbc.M611817200. [DOI] [PubMed] [Google Scholar]
- Herrera AJ, Tomas-Camardiel M, Venero JL, Cano J, Machado A. Inflammatory process as a determinant factor for the degeneration of substantia nigra dopaminergic neurons. journal of Neural Transmission. 2005;112:111–119. doi: 10.1007/s00702-004-0121-3. [DOI] [PubMed] [Google Scholar]
- Hougee S, Hartog A, Sanders A, Graus YM, Hoijer MA, Garssen J, van den Berg WB, van Beuningen HM, Smit HF. Oral administration of the NADPH-oxidase inhibitor apocynin partially restores diminished cartilage proteoglycan synthesis and reduces inflammation in mice. European Journal of Pharmacology. 2006;531:264–9. doi: 10.1016/j.ejphar.2005.11.061. [DOI] [PubMed] [Google Scholar]
- Jekabsone A, Mander PK, Tickler A, Sharpe M, Brown GC. Fibrillar beta-amyloid peptide Aβ1–40 activates microglial proliferation via stimulating TNF-α release and H2O2 derived from NADPH oxidase: a cell culture study. Journal of Neuroinflammation. 2006:3. doi: 10.1186/1742-2094-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson A, Nixon JB, McPhail LC. Phorbol myristate acetate induces neutrophil NADPH-oxidase activity by two separate signal transduction pathways: dependent or independent of phosphatidylinositol 3-kinase. Journal of Leukocyte Biology. 2000;67:396–404. doi: 10.1002/jlb.67.3.396. [DOI] [PubMed] [Google Scholar]
- Kim-Mitsuyama S, Yamamoto E, Tanaka T, Zhan Y, Izumi Y, Izumiya Y, Ioroi T, Wanibuchi H, Iwao H. Critical Role of Angiotensin II in Excess Salt-Induced Brain Oxidative Stress of Stroke-Prone Spontaneously Hypertensive Rats. Stroke. 2005;36:1077. doi: 10.1161/01.STR.0000163084.16505.e3. [DOI] [PubMed] [Google Scholar]
- Kim W-G, Mohney RP, Wilson B, Jeohn G-H, Liu B, Hong J-S. Regional difference in susceptibility of lipopolysaccharide-induced neurotoxicity in the rat brain: role of microglia. Journal of Neuroscience. 2000;20:6309–16. doi: 10.1523/JNEUROSCI.20-16-06309.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsilieri E, Scheller C, Grunblatt E, Nara K, Li J, Riederer P. Free radicals in Parkinson’s disease. Journal of Neurology. 2002;249:II/1–II/5. doi: 10.1007/s00415-002-1201-7. [DOI] [PubMed] [Google Scholar]
- Krotz F, Keller M, Derflinger S, Schmid H, Gloe T, Bassermann F, Duyster J, Cohen CD, Schuhmann C, Klauss V, Pohl U, Stempfle HU, Sohn HY. Mycophenolate acid inhibits endothelial NAD(P)H oxidase activity and superoxide formation by a Rac1-dependent mechanism. Hypertension. 2007;49:201–8. doi: 10.1161/01.HYP.0000251162.14782.d4. [DOI] [PubMed] [Google Scholar]
- Lafeber FP, Beukelman CJ, van den Worm E, van Roy JL, Vianen ME, van Roon JA, van Dijk H, Bijlsma JW. Apocynin, a plant-derived, cartilage-saving drug, might be useful in the treatment of rheumatoid arthritis. Rheumatology. 1999;38:1088–93. doi: 10.1093/rheumatology/38.11.1088. [DOI] [PubMed] [Google Scholar]
- Langston JW, Forno LS, Tetrud J, Reeves AG, Kaplan JA, Karluk D. Evidence of active nerve cell degeneration in the substantia nigra of humans years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine exposure. Annals of Neurology. 1999;46:598–605. doi: 10.1002/1531-8249(199910)46:4<598::aid-ana7>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Lawson LJ, Perry VH, Dri P, Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39:151–70. doi: 10.1016/0306-4522(90)90229-w. [DOI] [PubMed] [Google Scholar]
- Li G, Cui G, Tzeng NS, Wei SJ, Wang T, Block ML, Hong JS. Femtomolar concentrations of dextromethorphan protect mesencephalic dopaminergic neurons from inflammatory damage. FASEB Journal. 2005a;19:489–96. doi: 10.1096/fj.04-2555com. [DOI] [PubMed] [Google Scholar]
- Li J, Baud O, Vartanian T, Volpe JJ, Rosenberg PA. Peroxynitrite generated by inducible nitric oxide synthase and NADPH oxidase mediates microglial toxicity to oligodendrocytes. PNAS. 2005b;102:9936–41. doi: 10.1073/pnas.0502552102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Sun AY. Paraquat induced activation of transcription factor AP-1 and apoptosis in PC12 cells. Journal of Neural Transmission. 1999;106:1–21. doi: 10.1007/s007020050137. [DOI] [PubMed] [Google Scholar]
- McCarthy S, Somayajulu M, Sikorska M, Borowy-Borowski H, Pandey S. Paraquat induces oxidative stress and neuronal cell death; neuroprotection by water-soluble Coenzyme Q10. Toxicology & Applied Pharmacology. 2004;201:21–31. doi: 10.1016/j.taap.2004.04.019. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Akiyama H, McGeer EG. Rate of cell death in parkinsonism indicates active neuropathological process. Annals of Neurology. 1988a;24:574–6. doi: 10.1002/ana.410240415. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology. 1988b;38:1285–91. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- Min KJ, Pyo HK, Yang MS, Ji KA, Jou I, Joe EH. Gangliosides activate microglia via protein kinase C and NADPH oxidase. GLIA. 2004;48(3):197–206. doi: 10.1002/glia.20069. [DOI] [PubMed] [Google Scholar]
- Miyata K, Rahman M, Shokoji T, Nagai Y, Zhang G-X, Sun G-P, Kimura S, Yukimura T, Kiyomoto H, Kohno M, Abe Y, Nishiyama A. Aldosterone stimulates reactive oxygen species production through activation of NADPH oxidase in rat mesangial cells. Journal of the American Society of Nephrology. 2005;16:2906–12. doi: 10.1681/ASN.2005040390. [DOI] [PubMed] [Google Scholar]
- O’Donnell VB, Smith GC, Jones OT. Involvement of phenyl radicals in iodonium inhibition of flavoenzymes. Molecular pharmacology. 1994;46:778–85. [PubMed] [Google Scholar]
- O’Donnell VB, Tew DG, Jones OT, England PJ. Studies on the inhibitory mechanism of iodonium compounds with special reference to neutrophil NADPH oxidase. Biochemistry Journal. 1993;290:41–49. doi: 10.1042/bj2900041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Nivorozhkin A, Szabo C. Therapeutic effects of xanthine oxidase inhibitors: renaissance half a century after the discovery of allopurinol. Pharmacology Reviews. 2006;59:87–114. doi: 10.1124/pr.58.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano PJ, Griswold MC, Najibi S, Marklund SL, Cohen RA. Resistance of endothelium-dependent relaxation to elevation of O2- levels in rabbit carotid artery. American Journal of Physiology. 1999;277(5 Pt 2):H2109–14. doi: 10.1152/ajpheart.1999.277.5.H2109. [DOI] [PubMed] [Google Scholar]
- Peng J, Stevenson FF, Doctrow SR, Andersen JK. Superoxide dismutase/catalase mimetics are neuroprotective against selective paraquat-mediated dopaminergic neuron death in the substantial nigra. Journal of Biological Chemistry. 2005;280:29194–8. doi: 10.1074/jbc.M500984200. [DOI] [PubMed] [Google Scholar]
- Qin L, Block ML, Liu Y, Bienstock RJ, Pei Z, Zhang W, Wu X, Wilson B, Burka T, Hong JS. Microglial NADPH oxidase is a novel target for femtomolar neuroprotection against oxidative stress. FASEB Journal. 2005;19:550–7. doi: 10.1096/fj.04-2857com. [DOI] [PubMed] [Google Scholar]
- Richardson JR, Quan Y, Sherer TB, Greenamyre JT, Miller GW. Paraquat neurotoxicity is distinct from that of MPTP and rotenone. Toxicological Sciences. 2005;88:193–201. doi: 10.1093/toxsci/kfi304. [DOI] [PubMed] [Google Scholar]
- Ruiz-Leon Y, Pascul A. Brain-derived neurotrophic factor stimulates β-amyloid gene promoter activity by a ras-dependent/AP-1-independent mechanism in SH-SY5Y neuroblastoma cells. Journal of Neurochemistry. 2001;79:278–85. doi: 10.1046/j.1471-4159.2001.00547.x. [DOI] [PubMed] [Google Scholar]
- Scheller C, Sopper S, Jenuwein M, Neuen-Jacob E, Tatschner T, Grunblatt E, ter Meulen V, Riederer P, Koutsilieri E. Early impairment in dopaminergic neurotransmission in brains of SIV-infected rhesus monkeys due to microglia activation. Journal of Neurochemistry. 2005;95:377–87. doi: 10.1111/j.1471-4159.2005.03373.x. [DOI] [PubMed] [Google Scholar]
- Schober A. Classic toxin-induced animal models of Parkinson’s disease: 6-OHDA and MPTP. Cell Tissue Research. 2004;318:215–24. doi: 10.1007/s00441-004-0938-y. [DOI] [PubMed] [Google Scholar]
- Serezani CH, Aronoff DM, Jancar S, Mancuso P, Peters-Golden M. Leukotrienes enhance the bactericidal activity of alveolare macrophages against Klebsiella pneumoniae through the activation of NADPH oxidase. Blood. 2005;106:1067–75. doi: 10.1182/blood-2004-08-3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shavali S, Combs CK, Ebadi M. Reactive macrophages increase oxidative stress and alpha-synuclein nitration during death of dopaminergic neuronal cells in co-culture: relevance to Parkinson’s disease. Neurochemical Research. 2006;31:85–94. doi: 10.1007/s11064-005-9233-x. [DOI] [PubMed] [Google Scholar]
- Sheehan JP, Swerdlow RH, Miller SW, Davis RE, Parks JK, Parker WD, Tuttle JB. Calcium homeostasis and reactive oxygen species production in cells transformed by mitochondria from individuals with sporadic Alzheimer’s disease. Journal of Neuroscience. 1997;17:4612–22. doi: 10.1523/JNEUROSCI.17-12-04612.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siwik DA, Pagano PJ, Colucci WS. Oxidative stress regulates collagen synthesis and matrix metalloproteinase activity in cardiac fibroblasts. American Journal of Physiology -Cell Physiology. 2001;280(1):C53–60. doi: 10.1152/ajpcell.2001.280.1.C53. [DOI] [PubMed] [Google Scholar]
- Sumimoto H, Ueno N, Yamasaki T, Taura M, Takeya R. Molecular mechanism underlying activation of superoxide-producing NADPH oxidases: roles for their regulatory proteins. Japanese Journal of Infectious Diseases. 2004;57:S24–5. [PubMed] [Google Scholar]
- Sun GY, Horrocks LA, Farooqui AA. The roles of NADPH oxidase and phospholipases A(2) in oxidative and inflammatory responses in neurodegenerative diseases. Journal of Neurochemistry. 2007 doi: 10.1111/j.1471-4159.2007.04670.x. in press. [DOI] [PubMed] [Google Scholar]
- Talior I, Tennebaum T, Kuroki T, Eldar-Finkelman H. PKC-delta-dependent activation of oxidative stress in adipocytes of obese and insulin-resistant mice: role for NADPH oxidase. American Journal of Physiology-Endocrinology & Metabolism. 2005;288:E405–11. doi: 10.1152/ajpendo.00378.2004. [DOI] [PubMed] [Google Scholar]
- Thiruchelvam M, Prokopenko O, Cory-Slechta DA, Richfield EK, Buckley B, Mirochnitchenko O. Overexpression of superoxide dismutase or glutathione peroxidase protects against the paraquat + maneb-induced Parkinson disease phenotype. Journal of Biological Chemistry. 2005;280:22530–9. doi: 10.1074/jbc.M500417200. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Walker PD, Benjamins JA, Geddes TJ, Kuhn DM. Methamphetamine neurotoxicity in dopamine nerve endings of the striatum is associated with microglial activation. Journal of Pharmacology and Experimental Therapeutics. 2004;311:1–7. doi: 10.1124/jpet.104.070961. [DOI] [PubMed] [Google Scholar]
- Waki K, Inanami O, Yamamori T, Nagahata H, Kuwabara M. Involvement of protein kinase Cdelta in the activation of NADPH oxidase and the phagocytosis of neutrophils. Free Radical Research. 2006;40:359–67. doi: 10.1080/10715760500539121. [DOI] [PubMed] [Google Scholar]
- Wang H, Joseph JA. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radical Biology & Medicine. 1999;27:612–6. doi: 10.1016/s0891-5849(99)00107-0. [DOI] [PubMed] [Google Scholar]
- Wang Q, Tompkins KD, Simonyi A, Korthuis RJ, Sun AY, Sun GY. Apocynin protects against global cerebral ischemia-reperfusion-induced oxidative stress and injury in the gerbil hippocampus. Brain Research. 2006;1090:182–9. doi: 10.1016/j.brainres.2006.03.060. [DOI] [PubMed] [Google Scholar]
- Wilkinson BL, Landreth GE. The microglial NADPH oxidase complex as a source of oxidative stress in Alzheimer’s disease. Journal of Neuroinflammation. 2006:3. doi: 10.1186/1742-2094-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu DC, Teismann P, Tieu K, Vila M, Jackson-Lewis V, Ischiropoulos H, Przedborski S. NADPH oxidase mediates oxidative stress in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. PNAS. 2003;100:6145–50. doi: 10.1073/pnas.0937239100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu XF, Block ML, Zhang W, Qin L, Wilson B, Zhang WQ, Veronesi B, Hong JS. The of microglia in paraquat-induced dopaminergic neurotoxicity. Antioxidants & Redox Signaling. 2005;7:654–61. doi: 10.1089/ars.2005.7.654. [DOI] [PubMed] [Google Scholar]
- Xu J, Weng Y-I, Simonyi A, Krugh BW, Liao Z, Weisman GA, Sun GY. Role of PKC and MAPK in cytosolic PLA2 phosphorylation and arachadonic acid release in primary murine astrocytes. Journal of Neurochemistry. 2002;83:259–70. doi: 10.1046/j.1471-4159.2002.01145.x. [DOI] [PubMed] [Google Scholar]
- Yang W-L, Sun AY. Paraquat-induced cell death in PC12 cells. Neurochemical Research. 1998a;23:1387–94. doi: 10.1023/a:1020750706762. [DOI] [PubMed] [Google Scholar]
- Yang W-L, Sun AY. Paraquat-induced free radical reaction in mouse brain microsomes. Neurochemical Research. 1998b;23:47–53. doi: 10.1023/a:1022497319548. [DOI] [PubMed] [Google Scholar]
- Zhang W, Wang T, Pei Z, Miller DS, Wu X, Block ML, Wilson B, Zhang W, Zhou Y, Hong JS, Zhang J. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. FASEB Journal. 2005;19:533–42. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]
- Zhao X, Xu B, Bhattacharjee A, Oldfield CM, Wientjes FB, Feldman GM, Cathcart MK. Protein kinase Cdelta regulates p67phox phosphorylation in human monocytes. Journal of Leukocyte Biology. 2005;77:414–20. doi: 10.1189/jlb.0504284. [DOI] [PubMed] [Google Scholar]


