Although much is known about the proteins and processes within the plant cell required for efficient virus transmission, up to now, little was known about the requirements and mechanisms from the insect point of view. In this issue of PNAS, Uzest et al. (1) tackle that problem and trace the receptor for the cauliflower mosaic virus (CaMV) movement protein to a protein imbedded into the chitin matrix at the tip of the stylet of the aphid vector.
In terms of epidemiology, insects are the most important factors in plant virus disease. Approximately 80% of the plant viruses depend on insect vectors for transmission (other vectors can be nematodes and fungi), and the plant virus vector interactions are very specific. Thus, the recent spreading of begomoviruses throughout America might be caused by the introduction of the old world vector Bemisia tabaci. This spreading might have provided the opportunity of preexisting viruses to be transmitted to a variety of crop plants (2).
Plant viruses can be transmitted by insects in various ways. These have been classified as nonpersistent, semipersistent, and persistent, depending on the length of the period the vector can harbor infectious particles, which can range from minutes to hours (nonpersistent) to days (semipersistent) and to live-time and even inheritance by the insect progeny (persistent). Another classification distinguishes stylet-borne, foregut-borne, and circulative transmission, usually corresponding to non-, semi-, and full persistence. In circulative transmission, viruses move from the foregut further to the mid- and hindgut, from where they are transported to the hemolymph and further to the salivary gland, from where they are released into the plant tissue during feeding. In some cases, plant viruses are further replicated in the insect hemolymph, e.g., reoviruses in leaf hoppers (3), and these viruses can therefore also be considered doubly as plant and as insect viruses.
An example of circulative (nonreplicative) transmission is given by the begomo geminiviruses, which are transmitted by whiteflies, e.g., B. tabaci. Interestingly, this transmission involves a third partner, namely insect-symbiontic bacteria. These produce a chaperonin: “symbiontin,” also known as GroEl protein (4). Symbiontin binds to the viral capsid and is required to pass the midgut/hemolymph barrier and to stabilize the virion (5, 6). Interestingly, when introduced into plant cells, symbiontin can inhibit virus replication, presumably by inhibiting the nucleic acid release or the cell-to-cell transport (7). Geminiviruses have no special insect transmission factor (or “helper component”). All properties required for geminivirus transmission, including groel interaction, rely on the viral capsid protein.
In contrast, the caulimoviruses (type member CaMV) with icosahedral symmetry are transmitted semipersistently by aphids, such as Mycus persicae. For CaMV transmission, three proteins have been shown to be required (Fig. 1), the viral capsid protein (GAG), the loosely bound virion associated protein (VAP), and the aphid transmission factor (ATF; ref. 8). VAP forms a network around the virion with its C terminus anchored in the inner shell (9) and the N-terminal extremity facing out of the capsid, forming dimers by coiled-coil interactions (10). In addition to the movement protein (MOV), GAG and VAP and are also required for cell-to-cell movement (11).
Fig. 1.
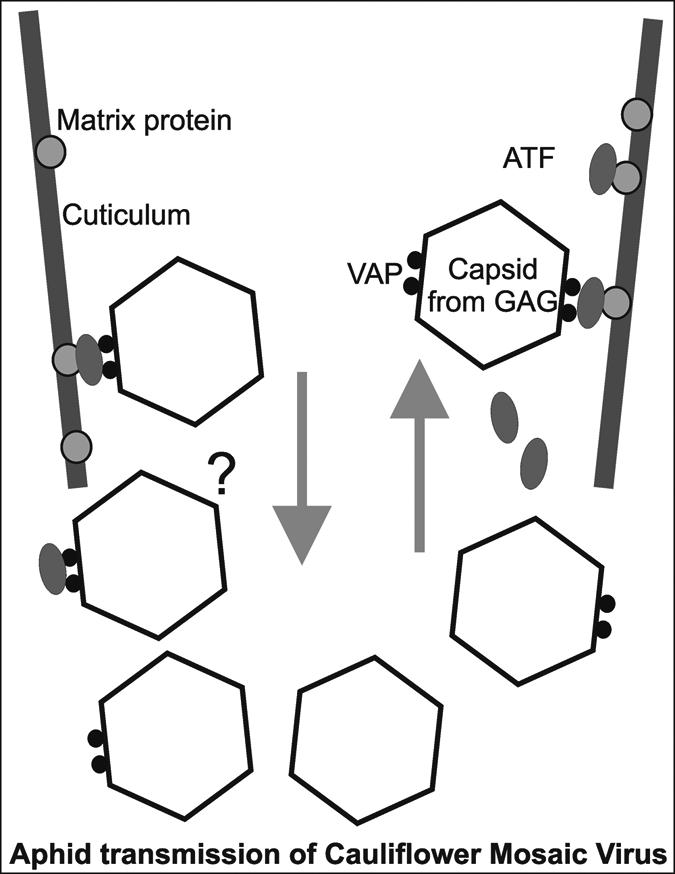
Schematic representation of the tip of the aphid stylet showing uptake and release of CaMV particles. The aphid transmission factor (ATF, P2) is taken up by the insect and binds specifically to a specific nongycosylated protein imbedded in chitin matrix of the cuticulum. Virus particles bind to the ATF/matrixprotein complex via the virion associated protein (VAP, P3). It is not known how the release is initiated and whether virions with or without VAP and ATF are released.
The ATF can be taken up by the insect independently from virus particles, and insects prefed with ATF can subsequently transmit ATF-defective viruses. It was suggested that independent uptake is the norm and, interestingly, virions including VAP and ITF accumulate in different inclusion bodies within the infected plant cell, allowing the separate uptake (12). Such a mode of transmission can support multiple virus uptake and lead to recombinant viruses in the next progeny.
But what happens during virus uptake by the insect vector? Uzest et al. (1) found, by imaging using GVP-fused ATF and microscopy, that ATF binds to the very tip of the maxillary stylet (Fig. 1). The interaction does not occur with aphids that are not vectors for the virus or with mutated ATF incapable of transmitting CaMV. The authors also showed that semipersistent virus transmission is not connected to foregut-borne transmission. Furthermore, the authors gave first indications to the nature of the receptor. It is stable to trypsin, pronase E, and subtilisin, but pretreatment with proteinase K abolishes the activity, suggesting that it is nonglycosylated proteinaceous and partly protected by deep imbedding into the chitin matrix of the cuticulum. Furthermore, EM studies reveal that virus particles accumulate within the stylet coinciding with the location of the ATF receptor.
The work will provide the basis for further interesting research to answer questions such as: what regulates binding vs. release of the virus particles, whether the release is spontaneous or caused by properties of the medium flowing through the stylet, such as plant sap vs. saliva and their pH values, or whether the release is even coupled to degradation of one of the proteins involved in virion binding. Are the virions released by loosening the interaction between cuticulum-anchored protein and ATF, ATF and VAP, or even VAP and virion (Fig. 1)? The present findings will certainly be relevant for interfering with plant virus epidemics through targeting the transmission.
Footnotes
The author declares no conflict of interest.
See companion article on page 17959.
References
- 1.Uzest M, Gargani D, Drucker M, Hébrard E, Garzo E, Candresse T, Fereres A, Blanc S. Proc Natl Acad Sci USA. 2007;104:17959–17964. doi: 10.1073/pnas.0706608104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Power AG. Curr Opin Plant Biol. 2000;3:336–340. doi: 10.1016/s1369-5266(00)00090-x. [DOI] [PubMed] [Google Scholar]
- 3.Black LM. Curr Top Vector Res. 2007;2:1–30. [Google Scholar]
- 4.Banerjee S, Hess D, Majumder P, Roy D, Das S. J Biol Chem. 2004;279:23782–23789. doi: 10.1074/jbc.M401405200. [DOI] [PubMed] [Google Scholar]
- 5.Morin S, Ghanim M, Zeidan M, Czosnek H, Verbeek M, van den Heuvel JF. Virology. 1999;256:75–84. doi: 10.1006/viro.1999.9631. [DOI] [PubMed] [Google Scholar]
- 6.Hogenhout SA, van der WF, Verbeek M, Goldbach RW, van den Heuvel JF. J Virol. 2000;74:4541–4548. doi: 10.1128/jvi.74.10.4541-4548.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akad F, Eybishtz A, Edelbaum D, Gorovits R, Dar-Issa O, Iraki N, Czosnek H. Arch Virol. 2007;152:1323–1339. doi: 10.1007/s00705-007-0942-0. [DOI] [PubMed] [Google Scholar]
- 8.Hebrard E, Drucker M, Leclerc D, Hohn T, Uzest M, Froissart R, Strub JM, Sanglier S, van Dorsselaer A, Padilla A, et al. J Virol. 2001;75:8538–8546. doi: 10.1128/JVI.75.18.8538-8546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leclerc D, Stavolone L, Meier E, Guerra-Peraza O, Herzog E, Hohn T. Virus Genes. 2001;22:159–165. doi: 10.1023/a:1008121228637. [DOI] [PubMed] [Google Scholar]
- 10.Plisson C, Uzest M, Drucker M, Froissart R, Dumas C, Conway J, Thomas D, Blanc S, Bron P. J Mol Biol. 2005;346:267–277. doi: 10.1016/j.jmb.2004.11.052. [DOI] [PubMed] [Google Scholar]
- 11.Stavolone L, Villani ME, Leclerc D, Hohn T. Proc Natl Acad Sci USA. 2005;102:6219–6224. doi: 10.1073/pnas.0407731102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khelifa M, Journou S, Krishnan K, Gargani D, Esperandieu P, Blanc S, Drucker M. J Gen Virol. 2007;88:2872–2880. doi: 10.1099/vir.0.83009-0. [DOI] [PubMed] [Google Scholar]


