Abstract
Endosialin/TEM1 was originally discovered as a human embryonic fibroblast-specific antigen and was later found to be differentially expressed in tumor stroma and endothelium. Endosialin/TEM1 overexpression has been observed in many cancers of various tissue origin, including colon, breast, pancreatic, and lung. The knockout (KO) mouse model showed the absence of endosialin/TEM1 expression reduced growth, invasion, and metastasis of human tumor xenografts. In addition, lack of endosialin/TEM1 led to an increase in small immature blood vessels and decreased numbers of medium and large tumor vessels. This abnormal angiogenic response could be responsible for the reduced tumor growth and invasion observed in endosialin/TEM1 KO mice, suggesting a role for endosialin/TEM1 in controlling the interaction among tumor cells, endothelia, and stromal matrix. Here we report the identification of fibronectin (FN) and collagen types I and IV as specific ligands for endosialin/TEM1. More importantly, cells expressing endosialin/TEM1 exhibit enhanced adhesion to FN as well as enhanced migration through matrigel, although these properties could be blocked by a humanized antibody directed against human endosialin/TEM1. Our results pinpoint to a molecular mechanism by which expression of endosialin/TEM1 in the tumor stroma and endothelium may support tumor progression and invasion.
Keywords: CD248, fibronectin, stroma, collagen
The process of angiogenesis, which is critical for physiological tissue growth, wound healing, and embryonic development, also is required for the formation of large solid tumors (1). Within the developing capillary, extracellular matrix (ECM) proteins serve as a structural scaffold for proliferating endothelial and tumor tissues and, more important, provide support for the growth of tumor cells. Therefore, anticancer strategies aimed at disrupting these processes could result in effective therapies.
Endosialin/TEM1 (CD248) originally discovered as a human embryonic fibroblast-specific antigen was later found to be differentially expressed in tumor stroma and endothelium. The monoclonal antibody FB5, generated through immunization of mice with human embryonic fibroblasts, was found to recognize an antigen, named endosialin, present on tumor stromal cells (2). Subsequently, examination of gene expression patterns in normal and neoplastic tissue indicated up-regulation of endosialin/TEM1 mRNA expression in tumor neovessels (3). Similar endosialin/TEM1 expression levels were noted in human colorectal cancer (4), breast cancer tissues (5), and histiocytomas (6). Moreover, human endosialin expression has been observed in highly invasive glioblastoma, anaplastic astrocytomas, and metastatic carcinomas, including melanomas (7, 8).
Tem1 knockout (KO) mice develop normally and exhibit normal wound healing, suggesting that endosialin/TEM1 is not required for neovascularization during fetal development or wound repair (9). However, when colorectal cancer cells were implanted in the abdominal sites of Tem1 KO mice, the loss of endosialin/TEM1 expression did correlate with a drastic reduction in tumor growth, invasion, and metastases, compared with parental animals. These results suggest that stromal- and/or endothelial-associated cells expressing endosialin/TEM1 support tumor growth and invasion perhaps by the interaction with ECM structures.
In an effort to elucidate endosialin/TEM1 functions, we generated a fusion protein containing a murine γ-heavy chain fused inframe with the endosialin/TEM1 extracellular domain (Fc-TEM1) to identify possible interactions with ECM proteins. We also generated stable cell lines overexpressing endosialin/TEM1 and examined the ability of endosialin/TEM1 to mediate adhesion to ECM proteins and migration of cells through matrigel.
Results
Identification of Endosialin/TEM1 Ligands.
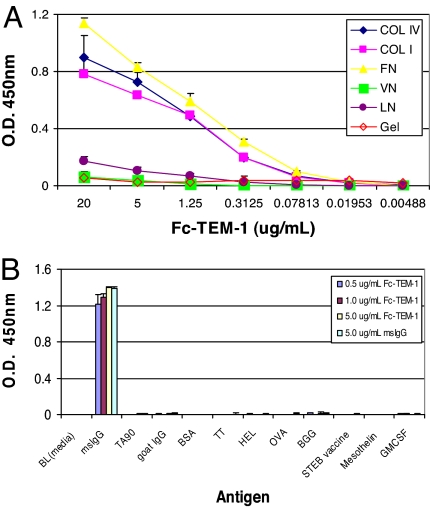
Endosialin/TEM1 is a C-type, lectin-like, integral membrane receptor exhibiting a high degree of O-linked glycosylation (10). Based on the observation that the expression of endosialin/TEM1 is preferentially associated with tumor vascular cells and that Tem1 KO mice displayed a reduced capacity to promote tumor invasion (9), we investigated whether endosialin/TEM1 preferentially adheres to proteins comprising the ECM. To measure the direct binding of endosialin/TEM1 with ECM proteins, we generated a soluble endosialin/TEM1 by fusing the N-terminal leader sequence and the entire extracellular domain of endosialin/TEM1 to a murine γ-heavy chain (Fc-TEM1) [see supporting information (SI) Materials and Methods and SI Fig. 10]. As shown in Fig. 1A, Fc-TEM1 bound to collagen (Col) I, Col IV, and fibronectin (FN) in a dose-dependent fashion, whereas no binding was detected within the entire dose range to laminin and vitronectin. Interestingly, although Fc-TEM1 was able to bind to collagen, no detectable interaction was observed on gelatin. None of four murine isotype IgG antibodies tested could bind to any of the ECM proteins, ruling out the possibility that these interactions were mediated by the murine Fc backbone of the Fc-TEM1 fusion protein (data not shown). To confirm the selectivity of the Fc-TEM1 and ECM protein interaction, we applied Fc-TEM1 to ELISA plates coated with different purified proteins. Fc-TEM1 did not bind to any of the proteins tested except for the anti-mouse Fc used as a positive control (Fig. 1B). Taken together, these results strongly suggested a specific interaction between Fc-TEM1 and Col I, Col IV, and FN.
Fig. 1.
Fc-TEM1 binds to ECM proteins. (A) ELISA plates precoated with different ECM proteins were blocked with ELISA buffer before the addition of purified Fc-TEM1 protein at the indicated concentrations. After 2 h of incubation, the plates were washed, and HRP-conjugated goat anti-mouse antibody was added to detect bound Fc-TEM1. FN, fibronectin; Col I/IV, collagen I/IV; VN, vitronectin; LN, laminin; Gel, gelatin. (B) An ELISA plate was precoated overnight with the following antigens: Staphylococcus Enterotoxin B (STEB), ovalbumin (OVA), bovine γ-globulin (BGG), tumor-associated 90-kDa glycoprotein antigen expressed on most melanoma cells (TA90), hen egg lysozyme (HEL), tetanus toxoid (TT), 1% BSA, human mesothelin, human GM-CSF, goat IgG, and mouse IgG. Fc-TEM1 was added at increasing amounts (5, 10, and 50 μg/ml) and allowed to adhere for 2 h. Plates were then washed, and HRP-conjugated goat anti-mouse antibody was added to detect bound Fc-TEM1.
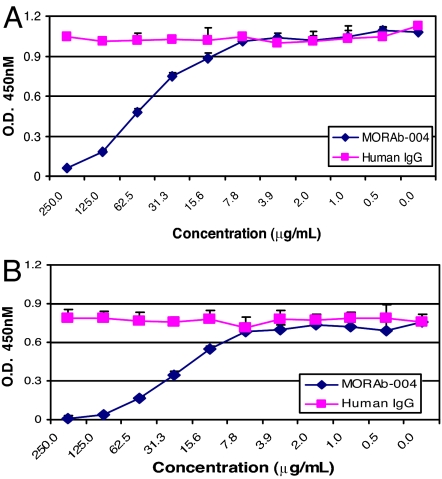
We developed a humanized anti-endosialin/TEM1 antibody (MORAb-004) that does not bind to other species homologs, with the exception of nonhuman primates, and its binding epitope has been mapped within the extracellular lectin domain of endosialin/TEM1. A fusion protein containing the murine γ-heavy chain and only the lectin domain of endosialin/TEM1 also could bind to FN (data not shown). We reasoned that MORAb-004 might be able to inhibit the specific interactions between Fc-TEM1 and Col I or FN. Indeed, MORAb-004, but not human IgG1 isotype control, blocked the binding of Fc-TEM1 to both FN (Fig. 2A) and Col I (Fig. 2B) in a dose-dependent fashion. Similar to MORAb-004, a rabbit polyclonal anti-endosialin/TEM1 antibody also inhibited Fc-TEM1 binding to Col I and FN (data not shown). These results indicate that the interaction between endosialin/TEM1 and Col I or FN is (i) specific, (ii) mediated by the endosialin/TEM1 lectin domain, and (iii) blocked by anti-endosialin/TEM1-specific antibodies.
Fig. 2.
MORAb-004 Inhibits Fc-TEM1 binding to FN and Col I. First, 1.25 μg/ml Fc-TEM1 was preincubated for 1 h at 4°C with increasing concentrations of MORAb-004 or human IgG. The protein–antibody complex was then added to an ELISA plate precoated with FN (A) or Col I (B). After 2 h of incubation at room temperature, the plate was washed, and HRP-conjugated goat anti-mouse antibody was added to detect bound Fc-TEM1.
Identification of FN Domains Involved in the Endosialin/TEM1 Interaction.
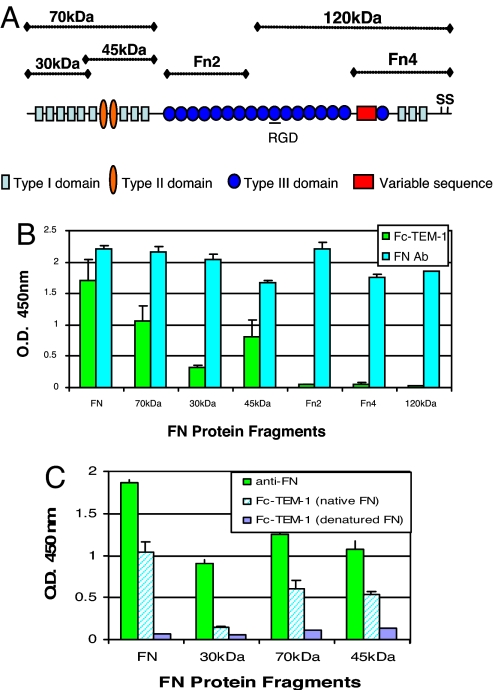
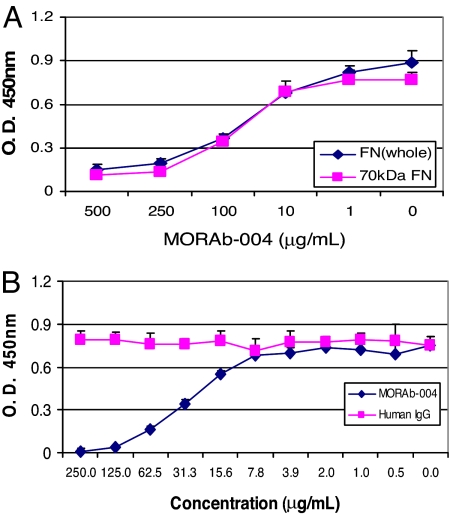
FN is a large complex glycoprotein that exists as a dimer covalently linked by a disulfide bridge at the C terminus of the protein (11–13). FN fragments either derived from enzymatic degradation or alternative splicing have been reported to be associated with certain disease states and possess distinct biological functions (13–15). To further characterize the domains within FN that mediate endosialin/TEM1 binding, we examined the ability of Fc-TEM1 to bind to several proteolytic FN fragments as illustrated in Fig. 3A. As shown in Fig. 3B, Fc-TEM1 could bind to the amino-terminal 70-kDa fragment and its proteolytic cleavage products (45- and 30-kDa fragments). The extent of binding varied among the different fragments and was less than that seen with the whole FN molecule. In contrast, Fc-TEM1 did not bind to the 120-kDa FN fragment or to the recombinant fragments Fn2 or Fn4. This lack of binding was unlikely due to uneven coating or degradation because all FN fragments were strongly detected by an anti-FN polyclonal antibody (Fig. 3B). These data suggest that the FN domain involved with the interaction with endosialin/TEM1 resides within the 70-kDa amino-terminal portion. The reduced binding capacity upon further digestion of the 70-kDa fragment indicates that Fc-TEM1 may bind to a region located in close proximity to the proteolytic cleavage site or that TEM1 recognizes conformationally dependent epitopes within the amino terminus of FN, which is altered after further digestion. To test this notion, we examined the ability of Fc-TEM1 to bind to reduced forms of FN proteins. Although anti-FN antibody was able to recognize reduced FN, indicating equivalent coating, the binding of Fc-TEM1 was completely ablated (Fig. 3C). Similar to FN whole protein, MORAb-004 could block Fc-TEM1 binding to the 70-kDa fragment in a dose-dependent manner (Fig. 4A), whereas an isotype control antibody had no effect (Fig. 4B). Based on these observations, we conclude that Fc-TEM1 recognizes conformationally dependent epitopes located within the amino terminus of FN that could be impaired upon further proteolytic degradation.
Fig. 3.
Mapping of FN/Fc–TEM1 interaction. (A) Proteolytic and recombinant fragments derived from FN include the N-terminal 70-kDa fragment, the 30-kDa heparin-binding fragment, the 45-kDa gelatin-binding fragment (both obtained from cryptic digestion of the 70-kDa fragment), the 120-kDa fragment containing the cell-attachment domain, and two recombinant fragments: Fn2, which contains the first seven FN type III domains, and Fn4, which contains the site of interchain-disulfide bonds and the α4β1 integrin-binding domain. (B and C) The protein fragments derived from native FN (B) or denatured FN (C) were coated onto an ELISA plate at the indicated concentrations. Detection of intact, bound proteins was done by using an anti-FN polyclonal antibody, followed by the addition of HRP-conjugated goat anti-rabbit secondary antibody. Then 1.25 μg/ml Fc-TEM1 was added and allowed to bind for 2 h. Plates were then washed, and HRP-conjugated goat anti-mouse antibody was added to detect bound Fc-TEM1. Hatched bars (Fc-TEM1 native) represent Fc-TEM1 binding to nondenatured FN.
Fig. 4.
MORAb-004 inhibits Fc-TEM1 binding to the 70-kDa FN fragment. Whole FN and the 70-kDa FN protein fragment were coated onto an ELISA plate at a fixed concentration of ≈15 nmol per well for both proteins. Then 1.25 μg/ml Fc-TEM1 was preincubated at 4°C for 1 h, with increasing amounts of MORAb-004 or isotype control IgG. The Fc-TEM1/MORAb-004 (A) or Fc-TEM1/IgG (B) complexes were then added to FN and 70-kDa-coated wells and incubated for 2 h at RT. Bound Fc-TEM1 protein was detected by the addition of HRP-conjugated goat anti-mouse secondary antibody.
Cell-Surface FN Is Found Associated with Endosialin/TEM1.
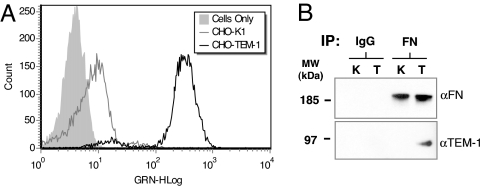
We generated CHO-TEM1 cells stably expressing endosialin/TEM1 (verified by FACS with MORAb-004 antibody) (Fig. 5A). We examined the level of cell-surface FN by flow cytometry in parental CHO-K1 and CHO-TEM1 cells by using a polyclonal anti-FN antibody. We observed 15–20% higher levels of surface FN in CHO-TEM1 cells, compared with CHO-K1 cells constantly (data not shown). Next, we tested whether the cell-surface FN could be associated with endosialin/TEM1. Using an anti-FN antibody, we immunoprecipitated FN from both CHO-K1 and CHO-TEM1 lysates, followed by Western blot using the same antibody (Fig. 5B Upper) or an anti-TEM1 antibody (Fig. 5B Lower), and found that FN could coimmunoprecipitate endosialin/TEM1 from CHO-TEM1 cell lysates. In contrast, in cell lysates immunoprecipitated with a normal IgG that did not pull down FN, no endosialin/TEM1 could be detected. In summary, two independent approaches (ELISA and coimmunoprecipitation) provide strong evidence of the FN and endosialin/TEM1 interaction. Similar results were obtained by using HEK293T cells ectopically expressing endosialin/TEM1 (data not shown).
Fig. 5.
Endosialin/TEM1 expressed in CHO cells interacts with FN. (A) Stable CHO-K1 transfectants were examined by FACS for surface expression of endosialin/TEM1. Cells were suspended in PBS plus 1.0% FBS and incubated with 10 μg/ml MORAb-004 for 1 h at 4°C, followed by addition of FITC-conjugated goat anti-mouse secondary antibody. (B) For immunoprecipitation (IP), polyclonal antibodies against FN were absorbed to protein G Sepharose beads. We subjected 107 cells to overnight IP at 4°C, followed by Western blot analysis with either the anti-FN (Upper) or anti-endosialin/TEM1 (Lower) antibodies.
CHO Cells Expressing Endosialin/TEM1 Cultured on Matrigel Form Web-Like Structures.
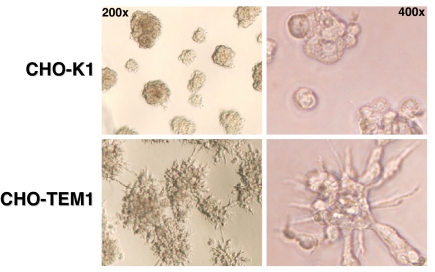
Although no differences in growth or survival were observed between parental CHO-K1 and CHO-TEM1 cells cultured on plastic surface, a drastically different morphology was seen when these cells were cultured on matrigel. Parental CHO-K1 cells grew into isolated cell clusters with minimal protrusions after 2 days of culturing (Fig. 6Upper), whereas CHO-TEM1 cells grew into clusters forming a web-like network (Fig. 6 Lower Left). In addition, CHO-TEM1 cells within the cluster exhibited protrusions reaching out to other clusters (Fig. 6 Lower Right). Interestingly, over time, CHO-TEM1 cells, but not CHO-K1 cells, moved closer to each other to form larger clusters (data not shown).
Fig. 6.
Expression of endosialin/TEM1 changes cell morphology on matrigel. We seeded 8 × 104 cells of either CHO-K1 (Upper) or CHO-TEM1 (Lower) onto a 96-well plate coated with matrigel and incubated them at 37°C. After overnight incubation, the wells were photographed for macroscopic examination of tubule formation.
Expression of Endosialin/TEM1 Enhances Cell Adhesion to FN.
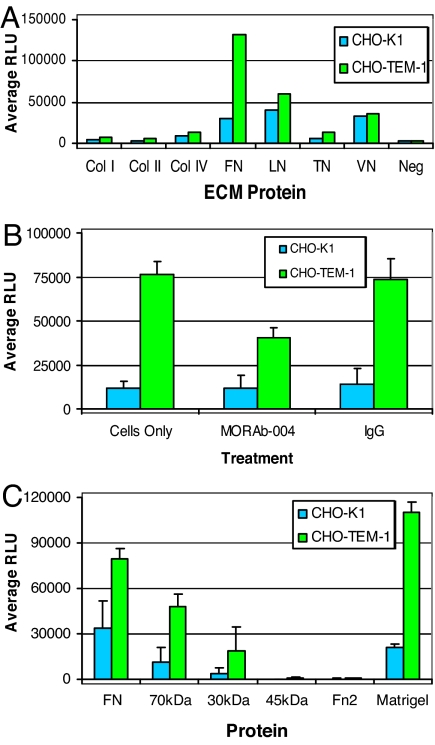
Because Fc-TEM1 showed the ability to bind to FN and collagens, we next assessed the potential effect of endosialin/TEM1 expression on cell adhesion by using an array of ECM proteins. We harvested both CHO-K1 and CHO-TEM1 cells with nonenzymatic dissociation buffer and added an equal number of cells to wells precoated with ECM proteins. After 1 h, the number of adherent CHO-TEM1 cells was 6-fold higher than the number of parental CHO-K1 cells in wells coated with FN (Fig. 7A). We did not observe significant differences in adhesion between CHO-K1 and CHO-TEM1 on surfaces coated with laminin or vitronectin, whereas adhesion to collagens and tenascin was too weak to assess any valuable differences (Fig. 7A). Pretreatment of CHO-TEM1 cells with MORAb-004 resulted in a 50% reduction of TEM1/FN-dependent cell adhesion, whereas IgG control antibody had no effect (Fig. 7B). Expectedly, MORAb-004 treatment did not affect the FN-dependent, endosialin/TEM1-independent cell adhesion (baseline adhesion) of parental CHO-K1 cells. Next, we precoated plates with equimolar amounts of whole FN, FN proteolytic fragments, and matrigel. CHO-TEM1 cells showed a 3- to 5-fold increased adhesion to FN, 70-, or 30-kDa fragments, compared with parental CHO-K1 cells, whereas no significant adhesion was seen to 45-kDa or Fn2 fragments. Expectedly, CHO-TEM1 cells also bound to matrigel five times better than CHO-K1 (Fig. 7C). Overall, these data indicate that endosialin/TEM1 enhances the adhesion of cells to extracellular matrices and that the amino terminus of FN is involved with these interactions.
Fig. 7.
Expression of endosialin/TEM1 enhances FN-dependent cell adhesion. (A) Cells were added to a precoated 96-well plate containing various ECM proteins. The cells were allowed to adhere for 1 h at 37°C, and wells were washed extensively to remove any loosely bound cells. The number of attached cells was determined by using the CellTiter-Glo Luminescent Cell Viability Assay. Col, collagen; FN, fibronectin; LN, laminin; TN, tenascin; VN, vitronectin; Neg, BSA. (B) To determine whether MORAb-004 blocks endosialin/TEM1-mediated cell adhesion to FN, cells (1.5 × 105) were preincubated for 1 h at 4°C with 100 μg/ml MORAb-004 or 100 μg/ml IgG isotype control antibody. After pretreatment, cells were added to a 96-well plate coated with FN and incubated for 1 h at 37°C. (C) The region within FN that mediates adhesion of endosialin/TEM1-positive cells was determined by precoating 96-well plates with equimolar amounts of protein fragments derived from whole FN, and the number of attached cells was determined as described before. Matrigel served as a positive control.
Expression of Endosialin/TEM1 Enhances Cell Migration Through Matrigel and FN.
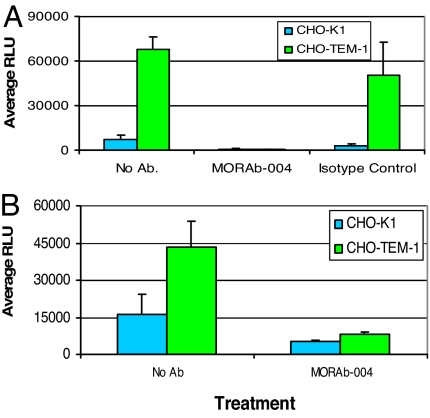
Because CHO-TEM1 cells displayed a different morphology (Fig. 6) and adhere better to matrigel (Fig. 7), we assessed whether endosialin/TEM1 changed the migration characteristics of CHO-TEM1 cells versus parental CHO-K1 cells seeded on transwell chambers coated with matrigel. Under these conditions, CHO-K1 cells exhibited modest migration, whereas CHO-TEM1 cells showed >10-fold enhanced migration (Fig. 8A). MORAb-004 treatment, but not control IgG, abolished CHO-TEM1 cell migration. Similar results were observed in migration experiments by using transwell chambers coated with FN (Fig. 8B).
Fig. 8.
MORAb-004 inhibits endosialin/TEM1-mediated cell migration. The ability of MORAb-004 to inhibit the migration of CHO-TEM1 and CHO-K1 cells through matrigel (A) or FN-coated (B) membranes was determined. Cells were added to the top chamber and allowed to migrate for 48 h at 37°C. The membrane was removed, and the number of migrated cells was determined by using the CellTiter-Glo Luminescence Cell Viability Assay. Where indicated, cells were treated with MORAb-004 or IgG isotype control for the duration of experiment.
Expression of Endosialin/TEM1 Enhances Matrix Metalloproteinase 9 (MMP 9) Activity.
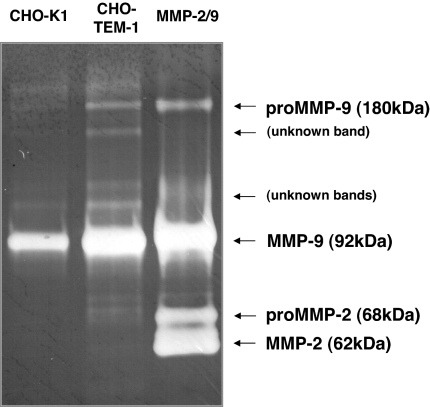
Endosialin/TEM1, MMP 2, and Col IV have been shown to colocalize in tissue areas characterized by finger-like protrudence of early angiogenic processes (16). This finding suggests a possible functional association between endosialin/TEM1 expression and MMP activity in early angiogenesis and cell-migration processes. We sought to investigate whether MMP activity was changed in CHO-TEM1 cells. Fig. 9 shows that MMP 9 activity was indeed significantly enhanced in CHO-TEM1 cells, compared with parental CHO-K1 cells in an MMP zymography assay. The enhanced MMP 9 activity correlated with increased secretion of MMP 9 protein in the supernatant of CHO-TEM1 cells as measured by an MMP-specific ELISA (data not shown). Taken together, these data suggest that induced MMP 9 secretion may contribute to the enhanced migration capability of CHO-TEM1 cells through matrigel and FN-coated transwells.
Fig. 9.
Expression of endosialin/TEM1 enhances MMP 9 activity. Supernatants from serum-starved CHO-TEM1 or CHO-K1 cells were subjected to gelatin zymography to assess the activation state of MMPs. Equal amounts of protein supernatant collected after 48 h were loaded onto a gelatin-containing gel and subjected to electrophoresis under nonreducing/nondenaturing conditions. The positive MMP 2/9 marker (right lane) served as a control for MMP 2/9 migration and activity. Arrows indicate pro and active forms of MMP 2/9 and protease bands of unknown origin.
Discussion
The Tem1 KO mouse model unveiled a biological role of endosialin/TEM1 in tumor progression. Our study aimed at elucidating the molecular mechanism by which endosialin/TEM1 supports this function. The data we generated demonstrate that FN, and specifically its 70-kDa amino terminus, as well as Col I and Col IV serve as ligands of endosialin/TEM1; expression of endosialin/TEM1 in cells increases their ability to adhere to extracellular matrices; expression of endosialin/TEM1 increases cell migration through the extracellular matrices; endosialin/TEM1-dependent cell adhesion and migration can be blocked by an anti-TEM1 monoclonal antibody; and expression of endosialin/TEM1 enhances MMP 9 activity.
Integrins (e.g., α4β1, α5β1) are well characterized receptors that mediate FN-dependent cell adhesion (12, 13). In addition, an unidentified cellular receptor has been functionally described that binds the N-terminal 70-kDa region (17) and is required to expose the cryptic integrin-binding site (RGD motif) of soluble FN involved with FN-integrin and FN–FN interactions (18, 19). We have now identified endosialin/TEM1 as a receptor that interacts with the N-terminal 70-kDa FN region and enhances FN-dependent cell adhesion. Therefore, the enhanced FN binding measured in our cellular systems in vitro could be the result of a sequential interaction with endosialin/TEM1 and integrins. We hypothesize that the interaction between endosialin/TEM1 and the N-terminal 70-kDa portion of soluble FN could be responsible for initiating the assembly of FN into a multimeric, high-affinity form able to bind integrins. Future studies would have to confirm the presence of this cooperation between integrins and endosialin/TEM1 in binding FN in vivo.
We have not yet identified a common motif in FN, Col I, or Col IV required for endosialin/TEM1 binding. However, we have observed that Fc-TEM1 could no longer bind collagen if this finding has been denatured by heat (gelatin). Similarly, further digestion of the N-terminal 70-kDa fragment into its proteolytic 30- and 45-kDa fragments drastically reduced Fc-TEM1 binding. These observations suggest that the presence of a conformation-dependent motif in both the FN and Col molecules is responsible for the endosialin/TEM1 interaction.
FN is a multifunctional protein capable of interacting with various cell-surface receptors, as well as with other components of the ECM, which in turn mediate cell adhesion, migration, survival, and proliferation (12, 13). The N-terminal 70-kDa fragment of FN can bind cells with the same affinity as intact FN, but cannot polymerize into the ECM (19). It has been shown that both intact soluble FN and its proteolytic fragment containing the gelatin-binding domain, which resides in the N-terminal 70-kDa fragment, possess the ability to stimulate fibroblast cell migration through a Col I-coated transmembrane (20). Gray et al. (21) isolated a protein named migration-stimulating factor from fetal tissue and breast cancer patients, which they later characterized as being expressed in carcinomas and tumor-associated stromal cells and resembled an FN isoform encompassing the entire sequence of the N-terminal 70-kDa fragment of FN (22). Because endosialin/TEM1 overexpression in cells strongly increases their ability to migrate through FN- or matrigel-coated transwells, one could speculate that this effect is mediated by the interaction of endosialin/TEM1 with a putative soluble 70-kDa FN isoform. However, we speculate that the endosialin/TEM1-mediated migration is the result of multiple effects, including cell adhesion, cell protrusion (see Fig. 6), chemotaxis, and/or induction of gelatinases, consistent with the observation that protease activity, specifically MMP 9, is enhanced in cells overexpressing endosialin/TEM1.
Recently, endosialin/TEM1 expression also has been found to be associated with pericytes during fetal and tumor angiogenesis (16, 23). The function of pericytes is to provide physiological and structural support to mature blood vessels as well as the framework to link the neovessel to the basement membrane (24, 25). In fact, disruption of pericyte development and recruitment through ablation of critical signaling pathways, such as PDGFβ and NG2, dramatically reduced normal and pathological angiogenesis (26). It is interesting to note that the absence of endosialin/TEM1 expression in Tem1 KO mice resulted in a significant increase in the number of small and immature tumor vessels and a decrease of larger and mature vessels, indicating an involvement of endosialin/TEM1 in tumor microvasculature maturation (9). Our findings suggest that endosialin/TEM1 and FN interaction may regulate some of the events leading to pericyte/endothelium interactions required for tumor vessel maturation.
Based on the results presented here, a model for endosialin/TEM1 function during tumor progression can be proposed. The expression of endosialin/TEM1 is first induced (by yet unknown factors) in cells associated with endothelium and stromal fibroblasts present in actively remodeling tissues, such as invasive tumor. The interaction between endosialin/TEM1 and ECM components, such as FN, modulates tissue proteases activity; creates an interface among stromal fibroblast, pericytes, and endothelial cells within the tumor tissue microenvironment; and, in turn, mediates cell attachment and migration during tumor invasion and metastasis. Based on this model, endosialin/TEM1-blocking antibodies, such as MORAb-004, could serve as useful therapeutic agents to counteract cancer growth and progression.
Materials and Methods
Cells and Reagents.
CHO-K1 cells from American Type Culture Collection (Manassas, VA) and LA1–5S from the Sloan–Kettering Institute were maintained in RPMI supplemented with l-glutamine, 1% minimal essential amino acids, sodium pyruvate, nonessential amino acids, and 10% heat-inactivated FBS (Invitrogen, Carlsbad, CA). Full-length human plasma-purified FN and 120-kDa cell attachment FN fragment proteins were purchased from Chemicon International (Temecula, CA); proteolytic 30-, 45-, and 70-kDa fragments were purchased from Sigma–Aldrich (St. Louis, MO); and the recombinant human FN fragments 2 and 4 were purchased from R&D Systems (Minneapolis, MN). The human IgG1 monoclonal antibody used as an isotype control for MORAb-004 was purchased from Sigma–Aldrich.
Generation of Stable Cell Lines.
We electroporated 3 × 106 cells with 10 μg of linearized plasmid DNA in a 0.4-mm electroporation cuvette. A pulse of 170 V/1,000 μF was delivered by using a Gene Pulser (Bio-Rad, Hercules, CA). Electroporated cells were allowed to recover for 24 h, after which 5 μg/ml Blasticidin-resistant clones (Invitrogen) were selected. Endosialin/TEM1 expression was verified by FACS, and cells were sorted for high expression.
ELISA.
Precoated FN, Col I, Col IV, laminin, gelatin (BD Biosciences, San Diego CA), or vitronectin (Millipore Corporation, Billerica, MA) on 96-well plates were used to assess Fc-TEM1 binding. The binding of TEM1 to Col I, Col IV, and FN was not due to trace contaminants in the purified protein from human plasma because neither Col I nor Col IV was detected in FN by anti-Col antibody ELISA, nor was FN detected in Col (data not shown). In experiments using FN fragments, equimolar amounts of proteins were diluted in coating buffer [50 mM carbonate-bicarbonate (pH 9.4)], added to an ELISA plate (Greiner Bio-One, Monroe, NC), and incubated overnight at 4°C. All plates were blocked with assay buffer (0.5% BSA/0.5% Tween 20 in PBS) for 2 h before the addition of fusion proteins. After 1 h of incubation at room temperature, the plates were washed, and HRP-goat anti-human IgG (H+L) antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) was added for 1 h. Color development was assessed by using the SureBlue TMB Peroxidase Substrate (KPL, Gaithersburg, MD). For inhibition experiments, protein was pretreated with MORAb-004 or human IgG (Jackson ImmunoResearch Laboratories) at the indicated concentrations for 1 h before being added to the ELISA plates. Antigen-specific ELISAs were performed by coating TPP Immunomini ELISA plates with 1 μg/ml Staphylococcus Enterotoxin B vaccine, 2 μg/ml bovine γ-globulin, 2 μg/ml tumor-associated 90-kDa glycoprotein antigen expressed on most melanoma cells (TA90), 2 μg/ml hen egg lysozyme, 1:500 dilution of tetanus toxoid, 1% BSA, 0.2 μg/ml human mesothelin, 2 μg/ml ovalbumin, 1 μg/ml human GM-CSF, 2 μg/ml goat IgG, and 2 μg/ml mouse IgG dissolved in bicarbonate-coating buffer (pH 9.6) (Sigma–Aldrich) overnight at 4°C. The plates were washed three times with washing buffer (containing 0.5% Tween 20) and blocked with 1× assay buffer for 2 h at room temperature, and ELISA was performed as described above.
Flow Cytometry.
Cells were harvested in cell dissociation buffer (Invitrogen), washed, and resuspended in ice-cold PBS plus 1% FBS. Cells were incubated for 1 h on ice with the primary antibody, 10 μg/ml MORAb-004, washed, incubated with FITC-conjugated goat anti-human secondary antibody (Southern Biotech, Birmingham, AL), and then analyzed on an EasyCyte Flow Cytometer (Guava Technologies, Hayward, CA).
Immunoprecipation and Western Blot Analysis.
We lysed 107 cells in radioimmunoprecipitation (RIPA) buffer [50 mM Tris·HCl (pH 7.4)/1% Nonidet P-40/0.5% sodium deoxycholate/150 mM NaCl/0.1% SDS] supplemented with Complete Mini protease inhibitor mixture (Roche Diagnostics, Indianapolis, IN) and centrifuged them at 13,000 × g for 15 min to remove debris. Protein G Sepharose 6 Fast Flow Beads (Amersham Biosciences, Piscataway, NJ) were washed three times with PBS, and 1 μg of anti-FN antibody was captured by gentle rocking at 4°C. Equal amounts of protein per sample were precleared by the addition of unbound protein G. After 2 h of incubation, the protein G was removed, and the supernatant was added to the antibody–Sepharose complex and incubated overnight at 4°C. After extensive washing with RIPA buffer, the bound protein was removed by boiling for 10 min in NuPAGE LDS sample buffer (Invitrogen) containing 5% 2-mercaptoethanol. Proteins were separated by using SDS/PAGE on a 4–12% Bis-Tris gel (Invitrogen) and transferred to PVDF membrane. Immunoblotting was conducted by using rabbit polyclonal antibodies specific for FN (Abcam, Cambridge, MA) or TEM1 (in house), detected with a goat anti-rabbit HRP-conjugated antibody, and visualized using SuperSignal West Pico chemiluminescent substrate (Pierce, Rockford, IL). The integrity and purity of soluble Fc-TEM1 also was monitored by Western blot. Five micrograms of protein was boiled for 5 min in 4× NuPage LDS Sample buffer (Invitrogen) containing 5% 2-mercaptoethanol subjected to electrophoresis on a NuPage 4–12% Bis-Tris gel (Invitrogen) and transferred to PVDF membrane, and immunoblotting was performed as described before.
FN-Mediated Adhesion assays.
We washed 1.5 × 105 cells per well and suspended them in PBS containing Mg2+/Ca2+, added in quadruplicate to an ECM Cell Adhesion Array Kit (Millipore Corporation), plated on individually coated FN, laminin, gelatin, and Col I plates (BD Biosciences), and allowed to adhere for 1 h. To assess adhesion to FN fragments, equimolar amounts of protein fragments were precoated overnight and then blocked for 2 h with PBS containing 10 mg/ml BSA. Cells were added for the indicated periods of time and, after incubation, each well was washed five times with PBS. The number of cells in each well was measured by using CellTiter-Glo (Promega, Madison, WI) according to the manufacturer's instructions. Where indicated, cells were preincubated with antibody for 1 h before the start of the assays.
Migration Assay.
The BD BioCoat Tumor Invasion System and Human FN-coated cell culture inserts (BD Bioscience) were used to assess TEM1-mediated migration of cells. Briefly, cells were harvested with nonenzymatic cell dissociation buffer and diluted to a concentration of 4 × 105 cells per ml in growth media supplemented with 2% FBS, and 500 μl of cell suspension was added to the top chamber of the membrane insert. To create a gradient, growth media containing 20% FBS was added to the bottom chamber. Cells were incubated for 48 h, after which the insert was removed and cells that had migrated through the coated membrane were counted by using CellTiter-Glo (Promega). Cells were pretreated with antibody as indicated in the figure legend, and migration was assessed in the continual presence of antibody. To examine the formation of tubules on matrigel, 8 × 104 cells per well were added to a 96-well plate coated with matrigel (BD Bioscience), incubated overnight, and photographed at ×200–400 magnification.
MMP Zymography.
MMP activity was assessed from culture supernatants of cells. Briefly, 4 × 105 cells per milliliter were seeded into a six-well plate (Costar, Cambridge, MA) and allowed to adhere, after which time they were washed and serum free-media was added. After 48 h, culture supernatants were collected, and equal volumes were subjected to gelatin and casein zymography (Invitrogen) under nonreducing conditions as indicated by the manufacturer's instructions. The gelatinase zymography standards, human MMP 2 and MMP 9 (Chemicon International), were used as a control to assess activity.
Supplementary Material
Acknowledgments
We thank Drs. Lloyd J. Old and Bert Vogelstein for critical review of the manuscript.
Abbreviations
- Col
collagen
- ECM
extracellular matrix
- FN
fibronectin
- KO
knockout
- MMP
matrix metalloproteinase.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0705647104/DC1.
References
- 1.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 2.Rettig WJ, Garin-Chesa P, Healey JH, Su SL, Jaffe EA, Old LJ. Proc Natl Acad Sci USA. 1992;89:10832–10836. doi: 10.1073/pnas.89.22.10832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.St Croix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E, Lal A, Riggins GJ, Lengauer C, Vogelstein B, Kinzler KW. Science. 2000;289:1197–1202. doi: 10.1126/science.289.5482.1197. [DOI] [PubMed] [Google Scholar]
- 4.Rmali KA, Puntis MC, Jiang WG. World J Gastroenterol. 2005;11:1283–1286. doi: 10.3748/wjg.v11.i9.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies G, Cunnick GH, Mansel RE, Mason MD, Jiang WG. Clin Exp Metastasis. 2004;21:31–37. doi: 10.1023/b:clin.0000017168.83616.d0. [DOI] [PubMed] [Google Scholar]
- 6.Dolznig H, Schweifer N, Puri C, Kraut N, Rettig WJ, Kerjaschki D, Garin-Chesa P. Cancer Immun. 2005;5:10. [PubMed] [Google Scholar]
- 7.Brady J, Neal J, Sadakar N, Gasque P. J Neuropathol Exp Neurol. 2004;63:1274–1283. doi: 10.1093/jnen/63.12.1274. [DOI] [PubMed] [Google Scholar]
- 8.Huber MA, Kraut N, Schweifer N, Dolznig H, Peter RU, Schubert RD, Scharffetter-Kochanek K, Pehamberger H, Garin-Chesa P. J Cutan Pathol. 2006;33:145–155. doi: 10.1111/j.0303-6987.2006.00446.x. [DOI] [PubMed] [Google Scholar]
- 9.Nanda A, Karim B, Peng Z, Liu G, Qiu W, Gan C, Vogelstein B, St Croix B, Kinzler KW, Huso DL. Proc Natl Acad Sci USA. 2006;103:3351–3356. doi: 10.1073/pnas.0511306103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christian S, Ahorn H, Novatchkova M, Garin-Chesa P, Park JE, Weber G, Eisenhaber F, Rettig WJ, Lenter MC. J Biol Chem. 2001;276:48588–48595. doi: 10.1074/jbc.M106152200. [DOI] [PubMed] [Google Scholar]
- 11.Ruoslahti E, Hayman EG, Engvall E, Cothran WC, Butler WT. J Biol Chem. 1981;256:7277–7281. [PubMed] [Google Scholar]
- 12.Wierzbicka-Patynowski I, Schwarzbauer JE. J Cell Sci. 2003;116:3269–3276. doi: 10.1242/jcs.00670. [DOI] [PubMed] [Google Scholar]
- 13.Magnusson MK, Mosher DF. Arterioscler Thromb Vasc Biol. 1998;18:1363–1370. doi: 10.1161/01.atv.18.9.1363. [DOI] [PubMed] [Google Scholar]
- 14.Labat-Robert J. Semin Cancer Biol. 2002;12:187–195. doi: 10.1016/S1044-579X(02)00022-6. [DOI] [PubMed] [Google Scholar]
- 15.Homandberg GA. Front Biosci. 1999;4:D713–D730. doi: 10.2741/homandberg. [DOI] [PubMed] [Google Scholar]
- 16.Virgintino D, Girolamo F, Errede M, Capobianco C, Robertson D, Stallcup WB, Perris R, Roncali L. Angiogenesis. 2007;10:35–45. doi: 10.1007/s10456-006-9061-x. [DOI] [PubMed] [Google Scholar]
- 17.McKeown-Longo PJ, Mosher DF. J Cell Biol. 1983;97:466–472. doi: 10.1083/jcb.97.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tomasini-Johansson BR, Annis DS, Mosher DF. Matrix Biol. 2006;25:282–293. doi: 10.1016/j.matbio.2006.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKeown-Longo PJ, Mosher DF. J Cell Biol. 1985;100:364–374. doi: 10.1083/jcb.100.2.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schor SL, Ellis I, Dolman C, Banyard J, Humphries MJ, Mosher DF, Grey AM, Mould AP, Sottile J, Schor AM. J Cell Sci. 1996;109:2581–2590. doi: 10.1242/jcs.109.10.2581. [DOI] [PubMed] [Google Scholar]
- 21.Grey AM, Schor AM, Rushton G, Ellis I, Schor SL. Proc Natl Acad Sci USA. 1989;86:2438–2442. doi: 10.1073/pnas.86.7.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schor SL, Ellis IR, Jones SJ, Baillie R, Seneviratne K, Clausen J, Motegi K, Vojtesek B, Kankova K, Furrie E, et al. Cancer Res. 2003;63:8827–8836. [PubMed] [Google Scholar]
- 23.MacFadyen JR, Haworth O, Roberston D, Hardie D, Webster MT, Morris HR, Panico M, Sutton-Smith M, Dell A, van der Geer P, et al. FEBS Lett. 2005;579:2569–2575. doi: 10.1016/j.febslet.2005.03.071. [DOI] [PubMed] [Google Scholar]
- 24.Shepro D, Morel NM. FASEB J. 1993;7:1031–1038. doi: 10.1096/fasebj.7.11.8370472. [DOI] [PubMed] [Google Scholar]
- 25.Armulik A, Abramsson A, Betsholtz C. Circ Res. 2005;97:512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 26.Ozerdem U, Stallcup WB. Angiogenesis. 2004;7:269–276. doi: 10.1007/s10456-004-4182-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.