Abstract
The consumption of garlic is inversely correlated with the progression of cardiovascular disease, although the responsible mechanisms remain unclear. Here we show that human RBCs convert garlic-derived organic polysulfides into hydrogen sulfide (H2S), an endogenous cardioprotective vascular cell signaling molecule. This H2S production, measured in real time by a novel polarographic H2S sensor, is supported by glucose-maintained cytosolic glutathione levels and is to a large extent reliant on reduced thiols in or on the RBC membrane. H2S production from organic polysulfides is facilitated by allyl substituents and by increasing numbers of tethering sulfur atoms. Allyl-substituted polysulfides undergo nucleophilic substitution at the α carbon of the allyl substituent, thereby forming a hydropolysulfide (RSnH), a key intermediate during the formation of H2S. Organic polysulfides (R-Sn-R′; n > 2) also undergo nucleophilic substitution at a sulfur atom, yielding RSnH and H2S. Intact aorta rings, under physiologically relevant oxygen levels, also metabolize garlic-derived organic polysulfides to liberate H2S. The vasoactivity of garlic compounds is synchronous with H2S production, and their potency to mediate relaxation increases with H2S yield, strongly supporting our hypothesis that H2S mediates the vasoactivity of garlic. Our results also suggest that the capacity to produce H2S can be used to standardize garlic dietary supplements.
Keywords: Allium, aorta, polysulfides, red blood cells, vasorelaxation
Dietary garlic (Allium sativum) has been recognized for its beneficial health effects for centuries. In particular, garlic consumption has been correlated with the reduction in multiple risk factors associated with cardiovascular diseases such as increased reactive oxygen species, high blood pressure, high cholesterol, platelet aggregation, and blood coagulation (1), but the active principles and mechanisms of action remain elusive. Garlic is rich in organosulfur compounds considered responsible for most of its pharmacological activities. Allicin (diallyl thiosulfinate), the main organosulfur compound, is produced from the amino acid alliin by action of the enzyme alliinase when garlic is crushed. Allicin, unstable in aqueous solution, rapidly decomposes mainly to diallyl sulfide (DAS), diallyl disulfide (DADS), diallyl trisulfide (DATS), and ajoene (2). After consumption, neither allicin nor its metabolites have been found in blood or urine (3), indicating that these compounds are rapidly metabolized.
Mounting evidence indicates that hydrogen sulfide (H2S) plays a cell signaling role similar to NO and CO (4, 5). Signaling may be mediated by H2S, HS−, or, less likely, S2−, species related by prototropic equilibrium defined by pK1 of 6.9 (6) and pK2 of 19 ± 2 (7), resulting in molar fractions of H2S, HS−, and S2− at 0.26, 0.74, and 1.7 × 10−12, respectively, at a physiological pH of 7.4. Herein, the term H2S refers to totality of free sulfide in solution. In vivo and in vitro cardiovascular effects of H2S include decreased blood pressure (8), cardioprotection against ischemic reperfusion damage (9), and O2-dependent vasorelaxation (10). In the vascular system, H2S is produced in vascular smooth muscle cells via cysteine metabolism by the pyridoxal 5′-phosphate-dependent enzyme cystathionine-γ-lyase (8). Nonenzymatic H2S production may also occur in the vasculature, as Searcy and Lee (11), corroborated by ourselves (data not shown), have demonstrated that human RBCs produce H2S when provided with elemental sulfur (S8) or inorganic polysulfides (S32− and S52−). However, because inorganic polysulfides are not likely dietary, we predicted that the organic polysulfide compounds found in dietary garlic may react with RBCs in a similar way, perhaps representing a substantial potential source of vascular H2S.
Here we demonstrate that garlic-derived organic polysulfides are H2S donors via glucose-supported and thiol-dependent cellular as well as glutathione (GSH)-dependent acellular reactions, and we propose the chemical mechanism by which H2S is produced leading to vasorelaxation. Taking these observations together, we propose that the major beneficial effects of garlic-rich diets, specifically on cardiovascular disease and more broadly on overall health, are mediated by the biological production of H2S from garlic-derived organic polysulfides.
Results
To define the real-time kinetics of H2S production by RBCs, we used a polarographic H2S sensor (PHSS) in a temperature-controlled closed-chamber respirometer (12). All experiments were performed at pH 7.35 and 37°C. Freshly collected and prepared human RBCs suspended in anoxic PBS containing 50 μM diethylenetriaminepentaacetic acid (DTPA) initiated substantial H2S production upon the addition of 1 mg/ml garlic (Fig. 1A), a concentration equivalent to two garlic cloves (5–6 g) dissolved in the blood volume of a typical adult human (≈5 liters). Because abundant cellular thiols may reduce polysulfides to liberate H2S, GSH, near 2 mM concentration in freshly collected RBCs (13), represents a likely candidate for such a reaction. This led us to predict that, if H2S production is mediated by GSH, then sustained H2S production by RBCs would depend on the presence of glucose to maintain GSH levels. Freshly collected RBCs (20% vol/vol) placed in two identical respirometer chambers, one with glucose and one without glucose, exhibited rapid H2S production at a rate near 12 μM/min and yielded a concentration of ≈8 μM H2S upon first injection of aliquots from a single garlic stock solution (to eliminate interclove variations in organic polysulfide concentrations) (Fig. 1A). The slow decline in H2S after the initial rapid rise suggested ongoing H2S removal from solution, indicating that all H2S production rates should be considered net production rates. After the second and third garlic injections, RBCs with glucose showed sustained H2S production whereas RBCs without glucose gradually exhausted their H2S production capacity (Fig. 1A). PBS and glucose alone did not exhibit garlic-induced H2S production.
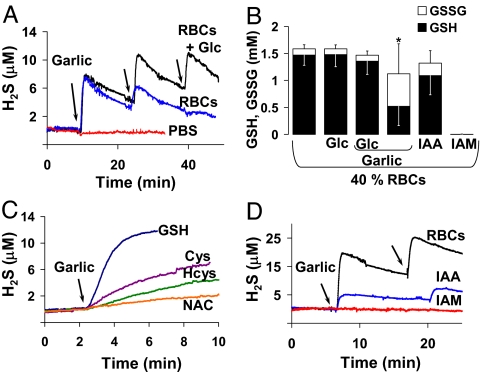
Fig. 1.
Garlic-induced H2S production and GSH and GSSG levels. (A) Representative polarographic traces of garlic-induced H2S production in 20% (vol/vol) RBCs in anoxic 10 mM PBS with 50 μM DTPA, with (black line) and without (blue line) 10 mM glucose (Glc), with sequential 1 mg/ml garlic additions at arrows, compared with the same garlic additions to 10 mM PBS and 50 μM DTPA alone in the absence of RBCs (red line) (pH 7.35) at 37°C. (B) HPLC analysis of GSH (black bars) and GSSG (expressed in GSH equivalents; white bars) in 40% (vol/vol) RBCs subject to no treatment, 10 mM glucose (Glc), 10 mM glucose and 1 mg/ml garlic, 1 mg/ml garlic alone, 10 mM IAA, and 10 mM IAM; each bar represents the mean ± SD of three to five experiments. *, GSH and GSSG levels are statistically different compared with levels in untreated RBCs by Student's t test (P ≤ 0.01). (C and D) Representative polarographic traces of H2S production in anoxic 10 mM PBS with 50 μM DTPA in the absence of RBCs, with 2 mM each GSH, cysteine (Cys), homocysteine (Hcys), or N-acetylcysteine (NAC) (pH 7.35) at 37°C upon addition of 1 mg/ml garlic at arrow (C) and garlic-induced H2S production in 20% (vol/vol) RBCs with 10 mM glucose previously treated with 10 mM IAA (blue line) or 10 mM IAM (red line) compared with untreated RBCs (black line) with 1 mg/ml garlic additions at arrows (D).
We further predicted that, in RBCs without glucose, the cytosolic GSH pool would be depleted after exposure to garlic, compared with RBCs with glucose. Fig. 1B shows that RBCs incubated for 20 min with or without glucose or with glucose and garlic all had statistically similar GSH and glutathione disulfide (GSSG) concentrations. The GSH/GSSG ratios in these RBCs indicated that GSH was largely in its reduced form, consistent with the literature (14, 15). However, RBCs without glucose, after a single bout of garlic-induced H2S production, exhibited decreased GSH and increased GSSG concentrations, leading to a large decrease in the GSH/GSSG ratio compared with control, although total GSH equivalents were conserved (Fig. 1B). In fact, GSH and other thiols (cysteine, homocysteine, and N-acetyl-cysteine) reacted directly, in the absence of RBCs, with garlic to produce H2S (Fig. 1C), with rates and yields that indicate a reaction preference for GSH in the production of H2S.
Other redox active components that may participate in this reaction include membrane protein thiols, perhaps on both membrane faces. To test this, RBCs were pretreated with thiol-blocking reagents, either membrane-impermeable iodoacetic acid (IAA) or membrane-permeant iodoacetamide (IAM), for 20 min at room temperature, then washed before H2S production and GSH and GSSG concentrations were measured. IAA blockade of exofacial thiols did not statistically alter total intracellular GSH equivalents or the GSH/GSSG ratio (Fig. 1B) but inhibited garlic-induced H2S production by 75% compared with control (Fig. 1D). In contrast, IAM blockade of both exofacial and intracellular thiols resulted in elimination of both the entire GSH pool (Fig. 1B) and garlic-induced H2S production (Fig. 1D).
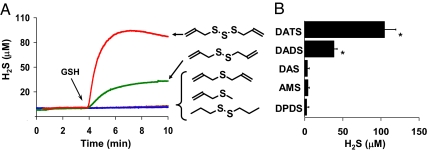
To explore the chemical efficacy of specific garlic-derived organic polysulfides in GSH-mediated H2S production, 2 mM GSH was combined with 100 μM each of DATS, DADS, DAS, allyl methyl sulfide (AMS), and dipropyl disulfide (DPDS) in PBS (Fig. 2A). Total H2S yield (Fig. 2B) was highest with DATS followed by DADS and was lowest with DPDS.
Fig. 2.
Single garlic-derived organic polysulfide-induced (nonenzymatic) H2S production by GSH. (A) Representative polarographic traces of H2S production in anoxic 10 mM PBS with 50 μM DTPA, with 100 μM each DATS (red line), DADS (green line), DAS (brown line), AMS (yellow line), and DPDS (blue line), by addition of 2 mM GSH at arrow. (B) Total H2S yield from each compound under the same conditions as in A; each bar represents the mean ± SD of three to six experiments. *, Yields are statistically different compared with yield from DPDS by Student's t test (P ≤ 0.005).
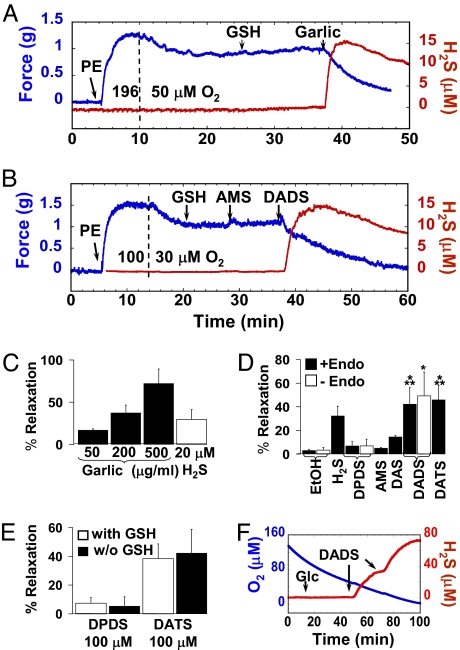
Increased garlic consumption in some populations is associated with lower incidence of hypertension (1). To test the vasorelaxant effect of garlic-induced H2S production, phenylephrine (PE)-precontracted aorta rings were suspended in a 37°C organ bath containing 1 mM GSH under physiological O2 conditions and provided with 50, 200, and 500 μg/ml garlic. These additions resulted in concentration-dependent simultaneous vasorelaxation and H2S production (Fig. 3 A and C). To establish the chemical efficacy of H2S production on vasoactivity, specific garlic-derived organic polysulfides were added to aorta ring preparations, again under physiological O2 conditions. PE-precontracted aorta rings showed maximum relaxation with DATS and DADS and minimum relaxation with DPDS and AMS (Fig. 3 B and D), effects that paralleled their H2S yields (Fig. 2), suggesting a link between bioactivity and production of this signal molecule. In the presence of authentic H2S donors such as the lipophilic garlic polysulfides, H2S is formed either on the cell surface or in the cytoplasm, most likely leading to a substantially higher local and relatively sustained H2S dose that is more potent in producing vascular relaxation than are direct bolus additions of H2S.
Fig. 3.
Garlic- and single garlic-derived organic polysulfide-induced vasoactivity and H2S production of PE-precontracted rat aorta rings at physiological O2 levels. Representative simultaneous traces of vasoactivity (blue line) and H2S production (red line) in a 15-ml organ bath of Krebs–Henseleit buffer (pH 7.35) at 37°C containing 5 μM indomethacin and 100 μM L-NAME, with 100 nM PE and 1 mM GSH additions at arrows with alterations of O2 from 196 to 50 μM at the dashed line and 500 μg/ml garlic addition at the arrow (A) and alterations of O2 from 100 to 30 μM at the dashed line and addition of 100 μM each AMS and DADS at the arrows (B). (C and D) Percentage vasorelaxation of aorta rings supported by 50, 200, and 500 μg/ml garlic (black bars) compared with 20 μM H2S (white bar) (C; each bar represents the mean ± SD of four to six experiments) and 100 μM each DPDS, AMS, DAS, DADS, or DATS, as well as ethanol vehicle and 20 μM H2S (D; each bar represents mean ± SD of three to nine experiments). Endothelium-intact aortic segments (black bars) are compared with endothelium-denuded segments (white bars). Levels are statistically different compared with DPDS (*, P ≤ 0.001; n = 6–9) and AMS (**, P ≤ 0.01; n = 3) by Student's t test. (E) Percent relaxation by 100 μM DPDS or DATS with or without 1 mM GSH; each bar represents mean ± SD of four to six experiments. (F) Representative polarographic traces of DADS-induced H2S production by entire aorta (eight segments, ≈30 mg fresh weight; red line) and simultaneous O2 concentration (blue line) in 10 mM PBS with 50 μM DTPA, with 10 mM glucose (Glc) and 100 μM DADS additions at the arrows, in the 2-ml closed multisensor respirometer chamber.
Mechanically denuded aortic segments exhibiting KCl-mediated contraction but no acetylcholine-mediated relaxation remained responsive to the relaxation effects of DADS (Fig. 3D), demonstrating that a functional endothelium was not required for this response. The addition of GSH to the precontracted aorta rings was required to measure H2S production as shown in Fig. 3 A and B, but experiments conducted with and without GSH do not show a difference in vasorelaxation (Fig. 3E). Furthermore, H2S production by reaction of garlic-derived organic polysulfides with GSH in the absence of RBCs is most likely supplemented by H2S production by aorta directly (Fig. 3F).
Discussion
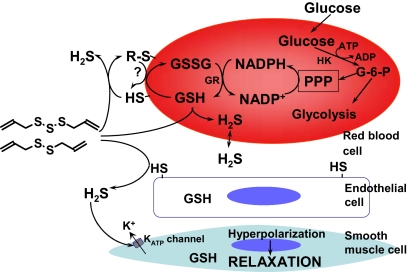
Garlic has been used for centuries in the treatment of diverse ailments in many cultures (1). Here we demonstrate that garlic and garlic-derived organic polysulfides induce H2S production in a thiol-dependent manner and that this signal molecule mediates the vasoactivity of garlic (Fig. 4). Organic polysulfides such as DATS and DADS act as H2S donors when they react with biological thiols including GSH, and glucose is necessary to maintain the reduced GSH pool, likely via the pentose phosphate pathway-mediated NADPH production that supports GSH reductase activity. We observed that exofacial membrane protein thiols play an important role in H2S production from garlic-derived organic polysulfides, suggesting the participation of a transplasma membrane reductase system (16), perhaps driven by NADPH and GSH, that acts to maintain exofacial thiols in the reduced state, thereby sustaining H2S production. In addition, we observed that these compounds can cross the membrane, as previously reported for allicin (17), to react with intracellular GSH because blocking the exofacial membrane thiols did not completely abrogate H2S production. The data presented here suggest that garlic-induced H2S production can occur in any thiol-containing cell and that H2S thus produced diffuses through plasma membranes causing vascular smooth muscle cell relaxation, likely via activation of KATP channels (8). Other membrane channels may also be affected by garlic. For example, garlic-derived compounds activate TRP channels in sensory neurons and human embryonic kidney cells (18, 19).
Fig. 4.
Proposed model of garlic-induced H2S production and H2S function in the vascular system. Garlic-derived organic polysulfides with allyl moieties and more than two sulfur atoms (see Fig. 5) react with exofacial membrane thiols and cross the cell membrane to react with GSH to produce H2S. Glucose is the main energy source of RBCs, supporting glycolysis and pentose phosphate pathway (PPP) reduction of NADP+ to NADPH, a cofactor of GSH reductase (GR), which maintains the intracellular GSH pool. GSH may also participate in transmembrane electron transfer to reduce exofacial thiols (16). H2S production then leads to vasorelaxation via vascular smooth muscle cell KATP-linked hyperpolarization (8).
Reports on garlic-mediated (20–22) and H2S-mediated (8) vascular smooth muscle relaxation indicate that both are based in part on NO signaling pathways, but the main action of both garlic (20) and H2S (8) is likely the opening of vascular smooth muscle cell membrane KATP channels, leading to depolarization and blood vessel dilation. Garlic-mediated vasorelaxation has previously been studied under the traditional high 95% O2 (900 μM) (20, 21) where spontaneous and biological H2S oxidation would be accelerated. High O2 levels also likely increase the activity of NO synthase (23), thereby increasing the observed contribution of the NO signaling pathways. Interestingly, under high O2 conditions, the effects of endothelium removal or Nω-nitro-l-arginine-methyl ester (L-NAME) addition to eliminate NO production are minor if the concentration of garlic extract (or allicin) is increased (21). H2S-mediated vasorelaxation decreases at higher O2 levels in part as a result of H2S oxidation but also because sulfide oxidation products mediate vasoconstriction (10). In contrast, at lower, more physiological O2 levels, a decrease in NO synthase activity and a relative increase in H2S persistence may alter the observed efficacy of these signaling pathways. We have found that H2S, acting as a reductant and nucleophile, reacts with S-nitrosothiol species to release NO, providing another mechanism by which H2S could augment vasorelaxation (24).
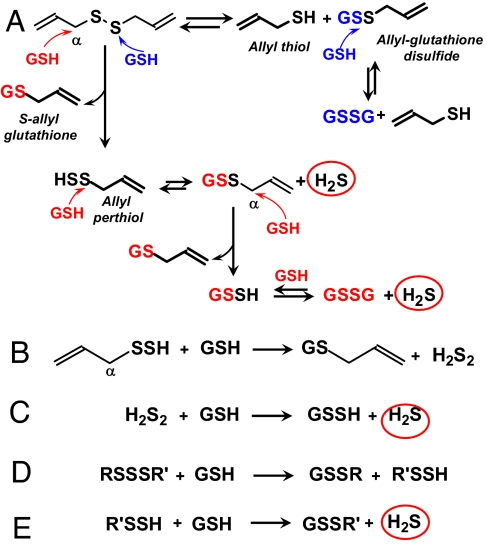
We observed that the chemical conversion of garlic-derived organic polysulfides to H2S is facilitated by allyl substituents and by increasing numbers of tethering sulfur atoms (see Fig. 2 for chemical structures). A similar structure–activity correlation has been observed for the cancer-preventative effects of garlic-derived organic polysulfides (25, 26). The facile formation of H2S from the reaction of DADS with GSH indicates that this reaction proceeds at least in part by a different mechanism from the more common thiol/disulfide exchange because the exchange reaction does not produce H2S (Fig. 5A, blue). We propose that, in a reaction competing with thiol/disulfide exchange, DADS undergoes nucleophilic substitution at the α carbon, yielding S-allyl-glutathione (27) and allyl perthiol, a key intermediate in the formation of H2S (28–31) (Fig. 5A, red). The resulting allyl perthiol then undergoes nucleophilic substitution at the S-atom in a manner analogous to thiol/disulfide exchange, yielding allyl-GSSG and H2S. The allyl-glutathione disulfide is an additional target for nucleophilic substitution at the α carbon, leading to further H2S production. The allyl perthiol may also undergo nucleophilic substitution at the α carbon yielding S-allyl-glutathione and H2S2 that in turn reacts with GSH to produce H2S (Fig. 5, reactions B and C). Organic disulfides substituted with only one allyl group may also produce H2S, albeit in lower yields. In contrast, organic disulfides with propyl substituents such as DPDS show only trace H2S production, confirming Streitwieser (32), who estimates that the rate of nucleophilic substitution at the α carbon in an allylic residue is ≈100 times faster than with an N-propyl residue. Organic trisulfides and probably higher polysulfides may undergo nucleophilic substitution at an S-atom, yielding a perthiol or higher hydropolysulfides that then react further with GSH to form H2S (Fig. 5, reactions D and E). Elemental sulfur also reacts with GSH to yield H2S in a similar manner (data not shown). Protein thiols and other biological thiols may compete with GSH for organosulfur compounds in certain microenvironments such as plasma membranes, resulting in H2S formation as well as protein covalent modifications.
Fig. 5.
H2S production from organic polysulfides by thiol reactions. (A) Proposed mechanism of H2S production by reaction of DADS and GSH via α carbon nucleophilic attack (red); H2S is not produced by thiol/disulfide exchange (blue). (B) Nucleophilic substitution of allyl perthiol at the α carbon followed by (C) thiol/disulfide exchange. (D and E) Thiol/disulfide exchange of organic trisulfides and higher polysulfides. See text below for description.
Few plants other than garlic contain allyl-substituted sulfur compounds, and garlic is the only one of these with a dietary use. We propose that H2S production from these garlic-derived organic polysulfides provides the basis for the long-term beneficial effects obtained from the habitual consumption of garlic. Although numerous clinical studies have demonstrated the beneficial effects of garlic on cardiovascular disease progression, there exist a sufficient number of studies that show little or no beneficial effects. Critical reviews attempting to address these contradictory results often site differences in subject health status, trial duration, and unknown active constituents in various garlic preparations as nonuniform factors contributing to inconsistent outcomes (33). If boosting endogenous H2S production in blood and tissues represents an essential benefit of ingesting garlic, then differences in this important variable may well influence the outcome and should be addressed in future clinical trials. In addition, our results suggest that the capacity to produce H2S can be used to standardize garlic dietary supplements.
Materials and Methods
Materials.
GSH, IAA, IAM, PBS, sodium sulfide, l-cysteine, N-acetylcysteine, homocysteine, glucose, m-phosphoric acid, L-NAME, DADS, DAS, DPDS, and AMS were obtained from Sigma (St. Louis, MO). DATS was purchased from LTK Labs (St. Paul, MN).
RBCs.
Collection of venous blood was made in heparin-containing tubes from healthy volunteers after obtaining informed consent, according to University of Alabama at Birmingham Institutional Review Board-approved procedures. Freshly collected blood was centrifuged at 1,000 × g for 10 min, and plasma and upper buffy coat were removed by aspiration. RBCs were washed by centrifugation three times with 10 mM PBS containing 50 μM DTPA (used to chelate metals) (pH 7.35) and finally resuspended to a hematocrit of 20% or 40% (vol/vol) using 10 mM PBS with 50 μM DTPA. To limit spontaneous oxidation of any H2S produced during respirometer experiments, RBCs in 10 mM PBS and 50 μM DTPA were first deoxygenated in a gently swirling argon-perfused tonometer to avoid hemolysis that could occur by bubbling. Initial experimental use of 40% (vol/vol) RBCs resulted in enough H2S production to allow the use of 20% (vol/vol) in subsequent experiments. Freshly collected RBCs required a preparation time of ≈4 h. Buffer sampled after respirometry experiments indicated <1% hemolysis.
Garlic.
Garlic bulbs were obtained from local supermarkets. Fresh garlic cloves were weighed (1–3 g), then pressed to obtain the juice, which was kept on ice. Before use, the juice was diluted 1:10 with water to obtain a stock of 100 mg/ml, then used within 2 h of preparation.
Respirometer Sensor Calibration.
The PHSS is sensitive and specific and allows real-time measurement of dissolved H2S concentration in physiological solution (12). PHSS performance and calibration were evaluated in a temperature-controlled multisensor oxygraph respirometer (Oroboros, Innsbruck, Austria) chamber containing 2 ml of stirred (500 rpm) 10 mM PBS with 50 μM DTPA (pH 7.35) at 37°C. The PHSS polarizing voltage was set at 150 mV with a free radical analyzer (Apollo 4000; WPI, Sarasota, FL), which also recorded the PHSS signal. The PHSS was calibrated before each experiment with freshly prepared anoxic sodium sulfide stock solution (0–200 μM), as described previously (see figure 5 in ref. 12), using the same buffer and conditions as the experiment. The respirometer polarographic oxygen sensor was calibrated in air-equilibrated (196 μM O2) 10 mM PBS with 50 μM DTPA; argon was used to deoxygenate the buffer in the respirometer before RBC addition.
H2S Production Measurement by Multisensor Respirometry.
H2S production by RBCs and thiols was demonstrated by injecting garlic juice or garlic-derived organic polysulfides (DAS, DADS, PDS, AMS, and DATS) into the respirometer chamber with PHSS and polarographic oxygen sensor containing 2 ml of 10 mM PBS with 50 μM DTPA for controls and thiols, or 20% (vol/vol) RBCs previously deoxygenated to 0–2 μM O2. For aorta H2S production, clean isolated rat aorta segments (≈30 mg wet weight) were placed in the respirometer chamber with 10 mM PBS, 50 μM DTPA, and 10 mM glucose (pH 7.35) at 37°C. H2S production was initiated by injection of 100 μM DADS at physiological O2 concentrations (50 and 25 μM O2). Data were analyzed with respirometer DatLab software (Oroboros) coupled with Apollo 4000 software (WPI).
GSH/GSSG Determination by HPLC-ECD.
GSH and GSSG were analyzed by using an HPLC procedure similar to the method of Melnyk et al. (34) for aminothiols but adapted to coulometric electrode array detection and optimized for GSH and GSSG. Briefly, freshly collected and prepared RBCs in anoxic 10 mM PBS with 50 μM DTPA (0.5 ml, 40% vol/vol) were placed in microtubes, treated with substrates, and incubated at room temperature for 20–30 min under an argon blanket, then centrifuged at 1,000 × g for 5 min at 4°C. RBCs were washed three times with 2 volumes of 10 mM PBS with 50 μM DTPA and resuspended again to 40% (vol/vol). RBCs (0.5 ml, 40% vol/vol) were mixed with equal volume cold 10% m-phosphoric acid with 2 mM EDTA and vigorously shaken for 1 min, then centrifuged at 16,000 × g for 2 min at 4°C. The supernatant was stored at −80°C and processed within 1 month. For HPLC analysis, samples were diluted 1:10 with 5% m-phosphoric acid and filtered. The sample (20 μl) was then separated on a Phenomenex Luna reversed-phase column 5μ C18(2) 250 × 4.60 mm provided with a Phenomenex guard column (ODS, 4 mm length × 3.0 mm inner diameter) using a mobile phase flow of 0.6 ml/min. An isocratic mixture of 50 mM phosphate buffer (pH 3.1) containing 100 μM sodium 1-octanesulfonate and acetonitrile (98.25:1.75) was used on a completely integrated HPLC system (Shimadzu LC-2010CHT). An ESA CoulArray Model 5600A was connected in tandem. Data were analyzed by using the ESA CoulArray for Windows 32 software. GSH and GSSG concentrations in control and treated RBCs, determined ≈4–6 h after blood was collected, were measured simultaneously for comparative purposes. Because RBC preparation and treatment were carried out with argon-sparged buffers, the RBCs would have experienced periodic hypoxic conditions, perhaps resulting in GSSG export and a lowered intracellular GSH pool (35). RBCs in vivo would likely have higher total GSH as well as GSH/GSSG ratios (15), which should enhance RBC H2S production.
Isometric Aorta Tension Measurements.
Sprague–Dawley rats (200–300 g) were housed in University of Alabama at Birmingham animal care facilities according to Institutional Animal Care and Use Committee procedures on a 12-h light/dark cycle with food and water available ad libitum. Isolated aorta was cleaned of adventitious tissue, and aorta rings were cut to 3 mm long. Some aorta rings were denuded of endothelium by gently rolling each ring on a small dowel, the efficacy of which was tested with KCl, PE, and acetylcholine. Aorta rings were mounted in an organ bath containing 15 ml of Krebs–Henseleit buffer (pH 7.35), with one end connected to a force transducer (Isolated Tissue Bath System; Radnoti, Monrovia, CA). The vessel bath chamber housing a PHSS and polarographic oxygen sensor was bubbled with a mass flow-controlled (Series 100; Sierra Instruments, Monterey, CA) mixture of N2 and air, each containing 5% CO2, to maintain a specific O2 concentration from 30 to 50 μM (36). Aorta rings with 5 μM indomethacin and 100 μM L-NAME were precontracted with 100 nM PE. Data were analyzed with AcqKnowledge software (BIOPAC Systems, Goleta, CA). Preliminary experiments with 10% (vol/vol) RBCs in the vessel bath showed garlic-induced vasorelaxation and H2S production (data not shown); however, bubbling the bath solution caused excessive foaming and eventual hemolysis, which precluded the use of RBCs. Accordingly, subsequent experiments were designed by using garlic or garlic-derived organic polysulfides with GSH at a concentration comparable to a 20% (vol/vol) RBC solution.
Acknowledgments
We acknowledge Michael Fallon, Junlan Zhang, Jo Morrison, and Asaf Stein for greatly appreciated help with the pilot in vivo blood pressure experiments. This work was supported by American Heart Association Grants 0625292B (to G.A.B.), 0455296B (to D.W.K.), and 0655312B (to R.P.P.) and National Institutes of Health (NIH) Grants HL58031 (to V.M.D.-U) and 08RGM073049A (to D.W.K.). T.S.I. is supported by NIH Cardiovascular Training Fellowship T32 HL007918 (to V.M.D.-U.).
Abbreviations
- H2S
hydrogen sulfide
- GSH
glutathione
- GSSG
GSH disulfide
- DATS
diallyl trisulfide
- DADS
diallyl disulfide
- DAS
diallyl sulfide
- DPDS
dipropyl disulfide
- AMS
allyl methyl sulfide
- DTPA
diethylenetriaminepentaacetic acid
- L-NAME
Nω-nitro-l-arginine-methyl ester
- IAA
iodoacetic acid
- IAM
iodoacetamide
- PHSS
polarographic H2S sensor
- PE
phenylephrine.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 17907.
References
- 1.Banerjee SK, Maulik SK. Nutr J. 2002;1:4. doi: 10.1186/1475-2891-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amagase H. J Nutr. 2006;136:716S–725S. doi: 10.1093/jn/136.3.716S. [DOI] [PubMed] [Google Scholar]
- 3.Freeman F. J Agric Food Chem. 1995;43:2332–2338. [Google Scholar]
- 4.Wang R. FASEB J. 2002;16:1792–1798. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- 5.Pryor WA, Houk KN, Foote CS, Fukuto JM, Ignarro LJ, Squadrito GL, Davies KJ. Am J Physiol. 2006;291:R491–R511. doi: 10.1152/ajpregu.00614.2005. [DOI] [PubMed] [Google Scholar]
- 6.Cotton FA, Wilkinson G. Advanced Inorganic Chemistry. New York: Wiley; 1988. [Google Scholar]
- 7.Myers RJ. J Chem Ed. 1986;63:687–690. [Google Scholar]
- 8.Zhao W, Zhang J, Lu Y, Wang R. EMBO J. 2001;20:6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sivarajah A, McDonald MC, Thiemermann C. Shock. 2006;26:154–161. doi: 10.1097/01.shk.0000225722.56681.64. [DOI] [PubMed] [Google Scholar]
- 10.Koenitzer JR, Isbell TS, Patel HD, Benavides GA, Dickinson DA, Patel RP, Darley-Usmar VM, Lancaster JR, Jr, Doeller JE, Kraus DW. Am J Physiol. 2007;292:H1953–H1960. doi: 10.1152/ajpheart.01193.2006. [DOI] [PubMed] [Google Scholar]
- 11.Searcy DG, Lee SH. J Exp Zool. 1998;282:310–322. doi: 10.1002/(sici)1097-010x(19981015)282:3<310::aid-jez4>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 12.Doeller JE, Isbell TS, Benavides G, Koenitzer J, Patel H, Patel RP, Lancaster JR, Jr, Darley-Usmar VM, Kraus DW. Anal Biochem. 2005;341:40–51. doi: 10.1016/j.ab.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 13.Beutler E. Red Cell Metabolism. New York: Churchill Livingstone; 1986. [Google Scholar]
- 14.Cereser C, Guichard J, Drai J, Bannier E, Garcia I, Boget S, Parvaz P, Revol A. J Chromatogr B. 2001;752:123–132. doi: 10.1016/s0378-4347(00)00534-x. [DOI] [PubMed] [Google Scholar]
- 15.Giustarini D, Dalle-Donne I, Colombo R, Milzani A, Rossi R. Free Radical Biol Med. 2003;35:1365–1372. doi: 10.1016/j.freeradbiomed.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 16.Kennett EC, Kuchel PW. IUBMB Life. 2003;55:375–385. doi: 10.1080/15216540310001592843. [DOI] [PubMed] [Google Scholar]
- 17.Miron T, Rabinkov A, Mirelman D, Wilchek M, Weiner L. Biochim Biophys Acta. 2000;1463:20–30. doi: 10.1016/s0005-2736(99)00174-1. [DOI] [PubMed] [Google Scholar]
- 18.Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Hogestatt ED, Julius D, Jordt SE, Zygmunt PM. Proc Natl Acad Sci USA. 2005;102:12248–12252. doi: 10.1073/pnas.0505356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hinman A, Chuang HH, Bautista DM, Julius D. Proc Natl Acad Sci USA. 2006;103:19564–19568. doi: 10.1073/pnas.0609598103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashraf MZ, Hussain ME, Fahim M. J Ethnopharmacol. 2004;90:5–9. doi: 10.1016/j.jep.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 21.Ku DD, Abdel-Razek TT, Dai J, Kim-Park S, Fallon MB, Abrams GA. Clin Exp Pharmacol Physiol. 2002;29:84–91. doi: 10.1046/j.1440-1681.2002.03596.x. [DOI] [PubMed] [Google Scholar]
- 22.Al-Qattan KK, Thomson M, Al-Mutawa'a S, Al-Hajeri D, Drobiova H, Ali M. J Nutr. 2006;136:774S–776S. doi: 10.1093/jn/136.3.774S. [DOI] [PubMed] [Google Scholar]
- 23.Abu-Soud HM, Rousseau DL, Stuehr DJ. J Biol Chem. 1996;271:32515–32518. doi: 10.1074/jbc.271.51.32515. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez J, Maloney RE, Rassaf T, Bryan NS, Feelisch M. Proc Natl Acad Sci USA. 2003;100:336–341. doi: 10.1073/pnas.0234600100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bianchini F, Vainio H. Environ Health Perspect. 2001;109:893–902. doi: 10.1289/ehp.01109893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Munday R, Munday JS, Munday CM. Free Radical Biol Med. 2003;34:1200–1211. doi: 10.1016/s0891-5849(03)00144-8. [DOI] [PubMed] [Google Scholar]
- 27.Germain E, Chevalier J, Siess MH, Teyssier C. Xenobiotica. 2003;33:1185–1199. doi: 10.1080/00498250310001636840. [DOI] [PubMed] [Google Scholar]
- 28.Steudel R, Albertsen A. J Chromatogr. 1992;606:260–263. [Google Scholar]
- 29.Rohwerder T, Sand W. Microbiology. 2003;149:1699–1709. doi: 10.1099/mic.0.26212-0. [DOI] [PubMed] [Google Scholar]
- 30.Chatterji T, Gates KS. Bioorg Med Chem Lett. 2003;13:1349–1352. doi: 10.1016/s0960-894x(03)00103-3. [DOI] [PubMed] [Google Scholar]
- 31.Munchberg U, Anwar A, Mecklenburg S, Jacob C. Org Biomol Chem. 2007;5:1505–1518. doi: 10.1039/b703832a. [DOI] [PubMed] [Google Scholar]
- 32.Streitwieser A., Jr . Solvolytic Displacement Reactions. New York: McGraw–Hill; 1962. [Google Scholar]
- 33.Rahman K, Lowe GM. J Nutr. 2006;136:736S–740S. doi: 10.1093/jn/136.3.736S. [DOI] [PubMed] [Google Scholar]
- 34.Melnyk S, Pogribna M, Pogribny I, Hine RJ, James SJ. J Nutr Biochem. 1999;10:490–497. doi: 10.1016/s0955-2863(99)00033-9. [DOI] [PubMed] [Google Scholar]
- 35.Sharma R, Awasthi S, Zimniak P, Awasthi YC. Acta Biochim Pol. 2000;47:751–762. [PubMed] [Google Scholar]
- 36.Isbell TS, Koenitzer JR, Crawford JH, White CR, Kraus DW, Patel RP. Methods Enzymol. 2005;396:553–568. doi: 10.1016/S0076-6879(05)96047-3. [DOI] [PubMed] [Google Scholar]