Abstract
CPEB, a cytoplasmic polyadenylation element-binding protein, plays an important role in translational control of maternal mRNAs in early animal development. During Xenopus oocyte maturation, CPEB undergoes a Cdc2-mediated phosphorylation- and ubiquitin-dependent degradation that is required for proper entry into meiosis II. However, the precise mechanism of CPEB degradation, including the identity of the responsible E3 ubiquitin ligase, is not known. Here, we show that the SCFβ-TrCP E3 ubiquitin ligase complex targets CPEB for degradation during Xenopus oocyte maturation. β-TrCP, the F-box protein of SCFβ-TrCP, specifically binds to a sequence 190TSGFSS195 (termed here the TSG motif) of CPEB, thereby targeting CPEB for degradation. β-TrCP binding depends on phosphorylation of Thr-190, Ser-191, and Ser-195 in the TSG motif. Among these residues, Ser-191 is phosphorylated by the Polo-like kinase Plx1, which binds CPEB at a specific Thr-125 residue prephosphorylated by Cdc2. Finally, Cdc2-mediated phosphorylation of other multiple Ser residues, previously implicated in CPEB degradation, is required for both Thr-125 phosphorylation and β-TrCP binding, presumably causing conformational changes of CPEB. We propose that Cdc2 and Plx1 sequentially phosphorylate CPEB and target it for SCFβ-TrCP-dependent degradation in Xenopus oocytes. We suggest that many other proteins carrying the TSG-like motif may be targeted by SCFβ-TrCP.
Keywords: cdc2, phosphorylation, Plk1/Plx1, SCFβ-TrCP ubiquitin ligase, translation
In animals, full-grown oocytes contain a large amount of maternal mRNAs that are translationally dormant or masked (1). In vertebrates, translational activation of certain maternal mRNAs promotes oocyte maturation, during which (immature) oocytes undergo two consecutive M phases and arrest again at metaphase of meiosis II (2, 3). Temporal and selective mechanisms are involved in this activation of maternal mRNAs. Cytoplasmic polyadenylation of the 3′ untranslated region (3′UTR) of mRNA, which is faithfully correlated with translational activation of the mRNA (4), is required for oocyte maturation in many species (5). Cytoplasmic polyadenylation of maternal mRNAs generally requires two specific cis-acting elements, a cytoplasmic polyadenylation element (CPE) and a universal hexanucleotide poly(A) signal in the 3′UTR, both of which bind specific trans-acting proteins (1, 5).
CPEB, a highly conserved CPE-binding protein first identified in Xenopus oocytes (6), mediates cytoplasmic polyadenylation of many CPE-containing mRNAs (7). During Xenopus oocyte maturation, two different types of CPEB modification occur and play a role in differential mRNA translation or proper meiotic progression. Phosphorylation of CPEB on Ser-174, which occurs at an early stage of maturation and is mediated by Aurora-A or other kinases (8, 9), is required for early activation of a class of mRNAs such as that encoding Mos (8). On the other hand, a large fraction (70–90%) of CPEB proteins undergoes a ubiquitin/proteasome-dependent degradation at entry into meiosis I (10, 11). This degradation causes a change in the CPEB/CPE ratio and results in activation of another class of mRNAs, such as those encoding cyclin B1 and Erp1, thereby driving entry into meiosis II (11, 12). It is worth noting that CPEB is degraded in maturing oocytes in many other species as well (13–15).
The ubiquitin-dependent degradation of CPEB in maturing Xenopus oocytes requires both a PEST sequence [a Pro/Glu/Ser/Thr-rich sequence typical of short-lived proteins (16)] and a Cdc2-catalyzed phosphorylation of multiple Ser residues in CPEB protein (10, 11). The PEST sequence is conserved in CPEB proteins from many other species (5), and clam CPEB also undergoes a PEST- and Cdc2-phosphorylation-dependent degradation in oocytes (13). However, the precise mechanism of CPEB degradation, including the identity of the responsible E3 ubiquitin ligase, is not known in any species. Here, we identify SCFβ-TrCP as the responsible ubiquitin ligase and elucidate the molecular mechanism of CPEB degradation in Xenopus oocytes. We discuss our results in the context of recognition motifs by SCFβ-TrCP, the general mechanism of CPEB degradation, and the possible importance of this degradation in other biological processes.
Results
Involvement of SCFβ-TrCP in the Degradation of CPEB During Oocyte Maturation.
CPEB undergoes hyperphosphorylation and ubiquitin/proteasome-dependent degradation at the time of germinal vesicle breakdown [(GVBD) a hallmark for entry into meiosis I] during Xenopus oocyte maturation (10, 11). On the other hand, the SCF (Skp1/Cul1/F-box protein) ubiquitin ligase complexes target a variety of phosphoproteins for degradation in diverse cellular processes (17). These findings prompted us to investigate whether any SCF complex(es) could be involved in the degradation of CPEB during Xenopus oocyte maturation. Among many SCF complexes, we examined the possible role of SCFβ-TrCP in CPEB degradation, because this SCF complex plays a role in meiotic progression in (male) mice (18) and is functional during oocyte maturation in Xenopus (19). β-TrCP is the F-box protein of SCFβ-TrCP and generally recognizes phosphorylated motifs of target proteins (refs. 17 and 20; see also below). Therefore, first we overexpressed a dominant-negative mutant of β-TrCP (β-TrCPΔF) in immature Xenopus oocytes (21) and then monitored the levels of endogenous CPEB during progesterone-induced oocyte maturation. In either nonexpressing or control GST-expressing oocytes, CPEB was hyperphosphorylated (see the size shifts) and considerably degraded at GVBD (Fig. 1A), as reported in refs. 10 and 11. In β-TrCPΔF-expressing oocytes, however, the degradation of CPEB was nearly completely inhibited, and both its levels and phosphorylation remained high even after GVBD (Fig. 1A), suggesting that (endogenous) SCFβ-TrCP was involved in CPEB degradation during maturation. We then examined whether CPEB could physically interact with β-TrCP during maturation. When coexpressed in oocytes and then pulled down by using glutathione beads, GST-tagged CPEB, but not GST alone, was found to be associated with Myc3-tagged β-TrCP in maturing GVBD oocytes but not in immature oocytes (Fig. 1B). Furthermore, when immunoprecipitated from GVBD oocytes, Myc3-β-TrCP was found to be bound specifically to not only GST-CPEB but also endogenous CPEB (Fig. 1C). In addition, the amount of β-TrCP bound to CPEB was considerably increased when CPEB degradation was prevented by the proteasome inhibitor MG132 [supporting information (SI) Fig. 5]. Thus, these results suggest that SCFβ-TrCP is the ubiquitin ligase involved in the proteasome-dependent degradation of CPEB during Xenopus oocyte maturation.
Fig. 1.
Involvement of SCFβ-TrCP in the degradation of CPEB during maturation. (A) Immature oocytes (IMO) were left uninjected (No inject.) or injected with 23 ng of mRNA encoding either β-TrCPΔF or GST (as control), cultured overnight, and treated with progesterone. Samples were collected at the indicated stages and times (min after GVBD) and analyzed by immunoblotting (IB) with anti-Xenopus CPEB antibody. (B) Immature oocytes coinjected with 4.6 ng of mRNA encoding Myc3-β-TrCP and 4.6 ng of mRNA encoding either GST-CPEB or GST were cultured overnight and treated with progesterone. Fifteen oocytes at the indicated stages were subjected to GST-pulldown assays (GST-PD) and analyzed by immunoblotting with anti-GST and anti-Myc antibodies. (C) Immature oocytes injected with 4.6 ng of GST-CPEB mRNA together with (+) or without (−) 4.6 ng of Myc3-β-TrCP mRNA were cultured overnight and treated with progesterone. Thirty oocytes at the indicated stages were subjected to immunoprecipitation (IP) with anti-Myc antibody and then analyzed by immunoblotting with the indicated antibodies. Exo, exogenous GST-CPEB; Endo, endogenous CPEB.
Identification of a β-TrCP-Binding Site in CPEB.
In preliminary experiments, β-TrCP was found to bind the N-terminal half of CPEB during maturation (data not shown). This N-terminal region contains the PEST sequence, which is required for the degradation of CPEB during maturation (10) and is evolutionarily well conserved (ref. 11; Fig. 2A). Interestingly, we noticed that the PEST sequence of Xenopus CPEB, consisting of ≈30 aa, harbors a highly conserved short sequence 190TSGFSS195 (hereafter called the TSG motif) (Fig. 2A), which resembles the conventional, doubly phosphorylated DSG motif (DpSGΦXpS, where Φ represents a hydrophobic amino acid and X represents any amino acid) that is recognized by β-TrCP (20). To test whether the TSG motif could function as a β-TrCP-binding site of CPEB, first we expressed in oocytes CPEB mutants with substituted alanine at the individual residues of the TSG motif and monitored their stabilities during oocyte maturation. These analyses revealed that most of the mutants (T190A, S191A, G192A, F193A, and S195A) were stable even after GVBD, whereas the S194A mutant was unstable, similar to wild-type (WT) CPEB (Fig. 2B). We then asked whether or not the respective mutants (tagged with GST) could bind β-TrCP by coexpressing each of the mutants and β-TrCP (tagged with Myc3), and then by performing GST-pulldown assays. Compared with WT CPEB, the five stable mutants, but not the unstable S194A mutant, showed strongly reduced interactions with β-TrCP at GVBD (Fig. 2C). These results, showing a good, structural, and functional correspondence of the TSG motif to the conventional DSG motif, suggest that the TSG motif is a functional β-TrCP-binding site in CPEB.
Fig. 2.
Identification of the TSG motif as a β-TrCP-binding site in CPEB. (A) Schematic representation of Xenopus CPEB (XeCPEB) protein and the evolutionary conservation of the TSG motif. At the bottom, the conventional, doubly phosphorylated DSG motif is shown. RRM, RNA-recognition motif; ZnF, zinc finger. (B) Immature oocytes (IMO) were injected with 1 ng of mRNA encoding GST-tagged WT CPEB or indicated mutants, cultured overnight, treated with protesterone, and, at the indicated times, analyzed by immunoblotting with anti-GST antibody. Thirty percent GVBD and 50% GVBD indicate the times that 30% and 50%, respectively, of 30 injected oocytes underwent GVBD. (C) Binding of β-TrCP to the indicated TSG-motif mutants was analyzed as in Fig. 1B. (D) Nonphosphorylated (Non-P) or phosphorylated (2P and 3P) peptides, coupled to beads, were incubated with oocyte extracts expressing Myc3-β-TrCP, subjected to peptide-pulldown assays, and immunoblotted for Myc3-β-TrCP.
Because dual phosphorylation of the DSG motif is essential for β-TrCP binding (20), we tested whether phosphorylation at the corresponding S191 and S195 residues (and at T190) of the TSG motif was required for β-TrCP binding. For this, we incubated bead-bound synthetic peptides having either doubly (pS191/pS195; 2P), triply (pT190/pS191/pS195; 3P), or non (non-P)-phosphorylated TSG motifs with oocyte extracts expressing β-TrCP. Peptide-pulldown assays revealed that, whereas non-P peptides could not bind β-TrCP, both the 2P and 3P peptides could, the latter showing ≈3-fold stronger binding to β-TrCP than the former (Fig. 2D). These results suggest that phosphorylation of S191 and S195 is essential for β-TrCP binding, and that additional phosphorylation of T190 significantly enhances the binding (probably mimicking the acidic Asp residue of the DSG motif). Taken together, these results strongly suggest that the TSG motif functions as a β-TrCP-binding site in CPEB if phosphorylated at the relevant residues.
Plx1 Binds CPEB and Phosphorylates the TSG Motif.
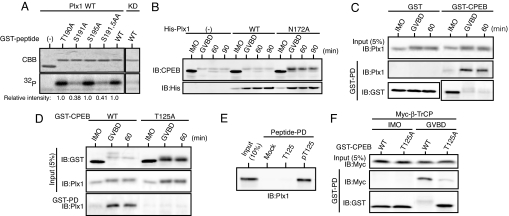
We next intended to identify the kinase(s) responsible for phosphorylation of the TSG motif in CPEB. Interestingly, inspection of the TSG motif and its surrounding sequence revealed that at least one of the three essential Ser/Thr residues in the TSG motif, or S191, lies in the Polo-like kinase Plk1 phosphorylation motif (D/E-X-S/T) (22). We therefore tested whether Plx1, the Xenopus homolog of mammalian Plk1, could directly phosphorylate the TSG motif on any Ser/Thr residue. We performed in vitro Plx1 kinase assays by using [γ-32P]ATP and GST-CPEB peptides (residues 181–200), which contained either the WT or the mutated TSG motifs (T190A, S191A, S195A, or S191/195A). Recombinant active Plx1, but not kinase-dead Plx1, was able to phosphorylate the WT peptides, and this phosphorylation was significantly (≈60%) reduced only when S191 was mutated to Ala (Fig. 3A). Furthermore, anti-phospho-S191 antibody could significantly recognize the Plx1-phosphorylated WT peptides (SI Fig. 6A; also see SI Fig. 6B). Thus, S191 (but not T190 or S195) was a direct phosphorylation site by Plx1 in vitro, consistent with it lying in the Plx1 consensus motif.
Fig. 3.
Phosphorylation of the TSG motif by Plx1 and identification of a Plx1-docking site in CPEB. (A) Direct phosphorylation of S191 by Plx1 in vitro. The indicated GST-fused CPEB peptides (residues 181–200) were incubated with either WT or kinase-dead (KD) Plx1 and [γ-32P]ATP, separated by SDS/PAGE, and stained with Coomassie brilliant blue (CBB) or autoradiographed (32P). Incorporation of 32P into the respective peptides was quantitated and is represented in relative intensity (numbers under gels). (B) Immature oocytes injected with 9.2 ng of mRNA encoding either His-tagged WT or N172A Plx1 were processed as in Fig. 1A and analyzed by immunoblotting with the indicated antibodies. Oocytes expressing N172A Plx1 underwent GVBD 2–3 h later than control and WT Plx1-expressing oocytes. (C) Physical interaction between CPEB and Plx1. Immature oocytes injected with 4.6 ng of mRNA encoding GST-CPEB or GST alone were cultured and treated with progesterone; 20 oocytes were then subjected to GST-pulldown assays to detect endogenous Plx1. (D) Stability and Plx1-binding of T125A CPEB were analyzed as in B and C. (E) Bead-bound synthetic peptides containing either nonphosphorylated T125 (T125) or phosphorylated T125 (pT125) were incubated with oocyte extracts and subjected to pulldown assays to detect endogenous Plx1. (F) Binding of β-TrCP to T125A CPEB was analyzed as in Fig. 2C.
Unfortunately, phosphorylation of S191 in endogenous CPEB could not appreciably be detected by the anti-phospho-S191 antibody (data not shown), probably because of the fairly low titer of the antibody and phosphorylation of the adjacent T190 residue (by an unknown kinase). However, a D189A mutant, which had a disrupted Plx1 consensus motif (A-X-S191), failed to be phosphorylated by Plx1 in vitro and was substantially stable in maturing oocytes, similar to the S191A mutant (SI Fig. 7). Therefore, Plx1 probably phosphorylated CPEB on S191 in vivo and thereby promoted its degradation. Indeed, consistent with the Plx1-promoted CPEB degradation, overexpression of a dominant-negative Plx1 mutant (N172A; see ref. 23) could significantly inhibit the degradation of (endogenous) CPEB during maturation (Fig. 3B). Furthermore, and importantly, ectopically expressed GST-CPEB was found to be very strongly associated with (endogenous) Plx1 in maturing oocytes, or ≈8-fold more strongly than in immature oocytes (after normalization of the CPEB levels) (Fig. 3C). Thus, these results suggest that Plx1 physically interacts with CPEB and phosphorylates its TSG motif on S191 during maturation.
Identification of a Plx-Docking Site in CPEB.
Given the presence of a phospho-Plk1-docking motif (S-pS/pT-P/X) in Plk1 target proteins (24), the interaction between Plx1 and CPEB might occur via a similar docking motif present in CPEB. In preliminary experiments, an N-terminal region (residues 100–150) of Xenopus CPEB was essential for CPEB to interact with Plx1 in maturing oocytes (data not shown). Interestingly, this region contains a 124STP sequence that matches the Plk1-docking motif (SpTP). We therefore addressed whether (phosphorylated) T125 would be involved in CPEB-Plx1 interactions. First, we examined the stability of a T125A mutant (tagged with GST) during maturation. This mutant was less phosphorylated and substantially more stable than WT CPEB during maturation (Fig. 3D, input), suggesting that T125 phosphorylation was required for both full phosphorylation and degradation of CPEB. We then tested for the interaction of the T125A mutant and (endogenous) Plx1 by GST-pulldown assays. Compared with WT CPEB, the T125A mutant could not bind Plx1 during maturation (Fig. 3D, GST-PD), suggesting that T125 phosphorylation was essential for the interaction of CPEB with Plx1. Indeed, synthetic peptides containing phosphorylated T125, but not those containing nonphosphorylated T125, could efficiently bind Plx1 in oocyte extracts (Fig. 3E). Finally, compared with WT CPEB, the T125A mutant showed a greatly reduced relative binding to β-TrCP at GVBD (Fig. 3F), consistent with its enhanced stability (Fig. 3 D and F). These results, together with the above results (Fig. 3 A–C and SI Fig. 7), strongly suggest that Plx1 binds CPEB at phosphorylated T125 and then phosphorylates the TSG motif on S191, thereby promoting β-TrCP binding and degradation of CPEB.
Phosphorylation of T125 by Cdc2.
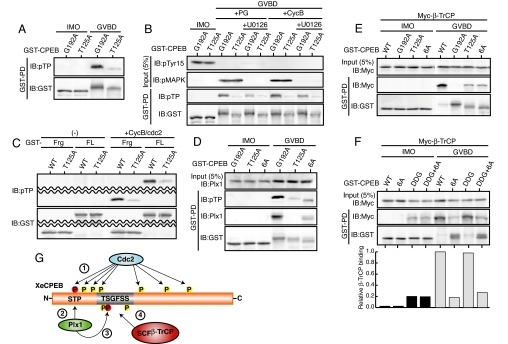
Given these results, prior phosphorylation of T125 would be a crucial step for CPEB degradation. We therefore intended to identify the kinase responsible for phosphorylation of T125. Before this, we tried to detect phosphorylation of T125 in vivo. For this, we used a universal anti-phospho-Thr-Pro antibody, because T125 is followed by a Pro residue (see above). After being pulled down from maturing but not immature oocytes, G192A, a stable TSG-motif mutant of CPEB (Fig. 2B), could be recognized by the anti-pTP antibody much more strongly than T125A (which was slightly less stable than G192A) (Fig. 4A). Thus, T125 (followed by Pro) was probably phosphorylated during maturation. Given this result, T125 might be phosphorylated by either Cdc2 or MAPK, which are both proline-directed kinases and are active at the time of GVBD (25). To determine which kinase was responsible for T125 phosphorylation, we expressed G192A or T125A (as control) in oocytes and then examined T125 phosphorylation during maturation in the presence of the Cdc2 inhibitor roscovitine or the MAPK inhibitor U0126. Whereas T125 phosphorylation did not occur in the presence of roscovitine (which totally inhibited maturation) (SI Fig. 8A), it did occur at GVBD even in the presence of U0126 (Fig. 4B), suggesting that Cdc2, but not MAPK, was involved in T125 phosphorylation. Indeed, Cdc2 activation by ectopic expression of cyclin B could induce GVBD and T125 phosphorylation even in the presence of U0126 (Fig. 4B), whereas MAPK activation by expression of Mos (25) failed to do so in the presence of roscovitine (SI Fig. 8A). Furthermore, WT CPEB was associated with β-TrCP and degraded normally even in the presence of U0126 but not roscovitine (SI Fig. 8B). Finally, both CPEB fragments and full-length CPEB proteins could be phosphorylated on T125 by Cdc2 kinase in vitro (Fig. 4C) [and Cdc2-phosphorylated full-length CPEB could be efficiently phosphorylated on S191 by Plx1 in vitro (SI Fig. 6B)]. Altogether, these results suggest that Cdc2, but not MAPK, phosphorylates CPEB on T125, thereby creating a docking site for Plx1.
Fig. 4.
Phosphorylation of T125 by Cdc2 and the role for other Cdc2-mediated phosphorylations in CPEB degradation. (A) Detection of T125 phosphorylation in vivo. Ten oocytes injected with 4.6 ng of mRNA encoding GST-CPEB (G192A or T125A) were cultured overnight, treated with progesterone, and subjected to GST-pulldown assays to detect phosphorylated T125 with anti-phospho-Thr-Pro (pTP) antibody. Note that the anti-pTP antibody could recognize G192A but not T125A CPEB at GVBD. (B) Immature oocytes injected with 4.6 ng of mRNA encoding the indicated GST-CPEB proteins were cultured overnight, left untreated or treated with U0126 for 1 h, treated with progesterone (PG) or injected with 9.2 ng of cyclin B1 mRNA (CycB) to induce maturation, and processed as in A. In “Input,” the inactivation status of Cdc2 and the activation status of MAPK were also analyzed by immunoblotting with anti-phospho-Tyr-15 (pTyr15) and anti-phospho-MAPK (pMAPK) antibodies, respectively. (C) Direct phosphorylation of T125 by Cdc2 in vitro. GST-fused fragments (Frg; residues 110–220) or full-length proteins (FL) of either WT or T125A CPEB were incubated with cyclin B1-Cdc2 complexes and then analyzed by immunoblotting with anti-pTP antibody. (D) T125 phosphorylation and Plx1-binding of 6A-CPEB were analyzed as in A and Fig. 3B. (E and F) β-TrCP binding to the indicated CPEB mutants was analyzed as in Fig. 2C. In F, the band intensities of β-TrCP and the respective mutants (gels) were quantitated, and their relative bindings are also shown (graph). (G) A model for the molecular mechanism of CPEB degradation during Xenopus oocyte maturation. Activated Cdc2 phosphorylates CPEB at both T125 and multiple Ser residues (marked with a circled 1), Plx1 binds to phosphorylated T125 (marked with a circled 2), Plx1 then phosphorylates S191 in the TSG motif (marked with a circled 3), and SCFβ-TrCP recognizes the (triply) phosphorylated TSG motif and targets CPEB for degradation (marked with a circled 4). Phosphorylation of multiple Ser residues by Cdc2 may cause conformational changes of CPEB, thereby facilitating β-TrCP binding as well as T125 phosphorylation.
Role for Other Cdc2-Mediated Phosphorylations in CPEB Degradation.
Previous work showed that CPEB degradation during maturation requires a Cdc2-mediated phosphorylation of six Ser residues (138, 144, 184, 210, 248, and 423) in the Ser-Pro motif (11). However, how these multiple phosphorylations are involved in the degradation of CPEB remains unclear. We therefore addressed the role for the multiple Ser phosphorylations in CPEB degradation. First, we confirmed that 6A-CPEB, in which all of the six Cdc2-phosphorylatable Ser residues were mutated to Ala (11), was hypophosphorylated and stable at GVBD; its stability was similar to that of T125A but somewhat lower than that of G192A (SI Fig. 9A; also see Fig. 4 D and E). Very interestingly, when T125 phosphorylation was examined, this phosphorylation as well as Plx1-binding of 6A-CPEB was shown to be significantly reduced, as compared with that of G192A, but not to the background levels as in T125A (Fig. 4D). Notably, however, β-TrCP-binding of 6A-CPEB was as low as that of T125A (Fig. 4E), rather consistent with their comparable stabilities (SI Fig. 9A and Fig. 4 D and E). These results suggest that the six Ser phosphorylations are required, at least in part, for not only T125 phosphorylation (which induces a Plx1-dependent phosphorylation of S191) but also some other event(s) involved in efficient β-TrCP binding. If so, one possibility would be that the multiple Ser phosphorylations are required for a proper phosphorylation of T190 and S195 (in addition to S191) in the TSG motif (Fig. 2), thereby being involved in efficient β-TrCP binding. To test this possibility, we prepared a CPEB mutant (DDG) in which T190, S191, and S195 in the TSG motif were all mutated to Asp [to mimic a phosphorylated DSG motif (21)] and then examined whether this mutant would be unstable irrespective of phosphorylations by Cdc2. Interestingly, the DDG mutant was (only) weakly bound to β-TrCP and rather stable in immature oocytes, whereas it was very strongly bound to β-TrCP (see the relative binding) and unstable during maturation, similar to WT CPEB (Fig. 4F and SI Fig. 9B). This finding suggests that maturation-associated modification, perhaps phosphorylation by Cdc2, is required for the DDG mutant (or the triply phosphorylated TSG motif) to efficiently bind β-TrCP. Consistent with this, a DDG/6A double mutant, which was also weakly bound to β-TrCP in immature oocytes, did not show any great increase in β-TrCP binding and was stable during maturation, similar to 6A-CPEB (Fig. 4F). Thus, these results suggest that the six Ser phosphorylations by Cdc2 are probably not simply required for phosphorylations of the TSG motif, but are rather required for yet another event(s) involved in efficient β-TrCP binding. Given their rather dispersed locations and their requirement for T125 phosphorylation by Cdc2 itself, the multiple Ser phosphorylations by Cdc2 might cause conformational changes of CPEB that allow efficient β-TrCP binding as well as T125 phosphorylation.
Discussion
In Xenopus oocytes, progression through meiotic maturation requires the degradation of CPEB (11, 12). Despite its importance, however, the precise mechanism of CPEB degradation is not known (7, 26). In this study, we have elucidated the molecular mechanism of CPEB degradation in Xenopus oocytes, which is summarized in Fig. 4G.
Our results show that the SCFβ-TrCP ubiquitin ligase is involved in the degradation of CPEB (Fig. 1). Moreover, the well conserved sequence 190TSGFSS195, termed here the TSG motif, serves as a β-TrCP-binding site in CPEB if phosphorylated on T190, S191, and S195 (Fig. 2). β-TrCP generally recognizes the conventional doubly phosphorylated DSG motif (DpSGΦXpS) (20), the nonphosphorylated DDG motif (DDGΦXD) (21), or their chimeric motifs of target proteins (27). The functional, triply phosphorylated TSG motif (pTpSGFSpS) of CPEB resembles the DSG and DDG motifs in both the primary sequence and the electric charge distributions. Therefore, these properties may allow the TSG motif to bind the WD40 repeats of β-TrCP through specific hydrogen bonds and electrostatic interactions (28). Interestingly, the TSG motif (consensus; TSGΦXS) is present in at least several hundred human proteins, including the transcription factor FoxP2 and the circadian clock protein Per1 (see SI Table 1); notably the motif of Per1 (TSGCSS) has been suggested to be a phosphodegron for β-TrCP (29). Thus, a significant number of proteins carrying the TSG motif [and a TSG-related SSG motif (SSGΦXS)] might be targeted by β-TrCP (SI Table 1).
Our data show that Plk1(Plx1) phosphorylates CPEB on S191 in the TSG motif and thereby promotes its β-TrCP binding and degradation (Fig. 3). They also show that Cdc2 phosphorylates CPEB on T121 (in the TP motif) and thereby creates a docking site for Plk1(Fig. 4 A–C). To date, Plk1 also phosphorylates three cell-cycle regulators, Wee1 (27), Emi1 (30), and Erp1 (31), on Ser residues in their DSG or DSG-like motifs, thereby targeting them for SCFβ-TrCP-depdendent degradation. Unlike the case with CPEB (and Wee1), however, the Plk1-docking site of Erp1 is created by CaMKII- but not Cdc2-mediated phosphorylation (31). This is, however, probably due to the somewhat loose consensus sequence of the Plk1-docking motif (S-pS/pT-P/X), in which the S/T-P sequence is the Cdc2 phosphorylation motif (24). In this context, the Plk1-docking motif of human CPEB might be phosphorylated by a kinase(s) other than Cdc2, because it does not end with a Pro residue (GenBank accession no. BC050629). In any case, this study demonstrates that Plk1 also phosphorylates and targets a translation-related protein, or CPEB, for SCFβ-TrCP-dependent degradation.
As reported in ref. 11, 6A-CPEB, in which all of the six Cdc2-phosphorylatable Ser residues were mutated to Ala, is stable during Xenopus oocyte maturation (Fig. 4 D and E). Interestingly, the six Ser phosphorylations by Cdc2 are required, at least in part, for both T125 phosphorylation and β-TrCP binding (Fig. 4 D and E). Further analysis suggests that the multiple Ser phosphorylations may cause conformational changes of CPEB, thereby facilitating β-TrCP binding as well as T125 phosphorylation (Fig. 4F). If so, and given the rather dispersed locations of the six Ser residues (11), their phosphorylations might act to expose the TSG motif as well as the T125 residue. In the case of Cdc25A, phosphorylation of nearby residues has been suggested to expose the DDG motif, thereby enhancing β-TrCP binding (21).
CPEB undergoes degradation during oocyte maturation in various species (13–15), its TSG motif and STP/X motif (for Plk1 docking) being evolutionarily well conserved (ref. 11; Fig. 2A). Thus, the mechanism of CPEB degradation uncovered here (Fig. 4G) may be fairly general. Furthermore, given the function of CPEB in diverse biological processes (7), CPEB degradation might also occur and play a role in some other biological processes besides oocyte maturation and embryonic division (11, 32). For example, in mice and rats, CPEB proteins are concentrated in the postsynaptic density of neurons to activate translation of α-CaMKII mRNA (33). Changing levels of synaptic activity can then alter protein composition in the postsynaptic density and induce synaptic remodeling in a Plk2- and ubiquitin-dependent manner (34). Therefore, it is possible that Plk2, a close relative of Plk1, targets the postsynaptic density-concentrated CPEB proteins for SCFβ-TrCP-dependent degradation for synaptic remodeling. Future studies are needed to elucidate the role for CPEB degradation in such important biological processes.
Materials and Methods
Preparation of Oocytes.
Full-grown Xenopus oocytes were prepared, microinjected, and cultured as described in ref. 35. Maturation was induced by treating oocytes with 5 μg/ml progesterone. In some experiments, oocytes were treated with 100 μM U0126 (v1121; Promega, Madison, WI) before 1 h of progesterone treatment.
cDNAs and in Vitro Transcription.
Antibodies and Immunoblotting.
Protein-Binding Assays.
For GST-pulldown or immunoprecipitation assays, oocytes expressing GST-fusion proteins or Myc3-tagged proteins were homogenized in a binding buffer (20 mM sodium phosphate, pH 8.0/80 mM β-glycerophosphate/0.5% Triton-X/1 mM EDTA/1 mM DTT/200 mM PMSF/2 mM pepstatin/1 mM NaF/1 mM Na3VO4) and centrifuged briefly. The supernatants, equivalent to 10–30 oocytes, were incubated with 10 μl of slurry of glutathione-Sepharose 4B beads (17-0756-01; GE Healthcare, Chalfont St. Giles, U.K.) for GST-pulldown or coincubated with 2 μl of anti-Myc antibody (ab9106; Abcam, Cambridge, MA) and 10 μl of slurry of protein G beads (20398; Pierce, Rockford, IL) for immunoprecipitation for 30 min at 4°C with constant mixing. The beads were washed three times with the binding buffer, eluted with 2× SDS sample buffer, and then subjected to immunoblotting with appropriate antibodies. For peptide-pulldown assays, the synthetic peptides used were CDSDTSGFSSGSD, CDSDTpSGFSpSGSD, and CDSDpTpSGFSpSGSD in Fig. 2D and CDSEAGGHSSTPT and CDSEAGGHSSpTPT in Fig. 3E. These peptides were covalently linked to SulfoLink coupling gel (20401; Pierce) at a concentration of 1 mg of peptide per 1 ml of gel, according to manufacture's instructions. Ten micrograms of the peptides bound to the gel were incubated with 30 μl of oocyte extracts (equivalent to 10 oocytes) for 30 min at 4°C, pulled down, and subjected to immunoblotting with appropriate antibodies.
In Vitro Kinase Assays.
Supplementary Material
Acknowledgments
We thank members of N.S.'s laboratory for discussions and K. Gotoh for typing the manuscript. This work was supported by a scientific grant from the CREST Research Project of Japan Science and Technology Agency (to N.S.).
Abbreviations
- CPE
cytoplasmic polyadenylation element
- GVBD
germinal vesicle breakdown.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0706952104/DC1.
References
- 1.Curtis D, Lehmann R, Zamore PD. Cell. 1995;81:171–178. doi: 10.1016/0092-8674(95)90325-9. [DOI] [PubMed] [Google Scholar]
- 2.Gray NK, Wickens M. Annu Rev Cell Dev Biol. 1998;14:399–458. doi: 10.1146/annurev.cellbio.14.1.399. [DOI] [PubMed] [Google Scholar]
- 3.Nebreda AR, Ferby I. Curr Opin Cell Biol. 2000;12:666–675. doi: 10.1016/s0955-0674(00)00150-2. [DOI] [PubMed] [Google Scholar]
- 4.Wormington M. Curr Opin Cell Biol. 1993;5:950–954. doi: 10.1016/0955-0674(93)90075-2. [DOI] [PubMed] [Google Scholar]
- 5.Mendez R, Richter JD. Nat Rev Mol Cell Biol. 2001;2:521–529. doi: 10.1038/35080081. [DOI] [PubMed] [Google Scholar]
- 6.Hake LE, Richter JD. Cell. 1994;79:617–627. doi: 10.1016/0092-8674(94)90547-9. [DOI] [PubMed] [Google Scholar]
- 7.Richter JD. Trends Biochem Sci. 2007;32:279–285. doi: 10.1016/j.tibs.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Mendez R, Hake LE, Andresson T, Littlepage LE, Ruderman JV, Richter JD. Nature. 2000;404:302–307. doi: 10.1038/35005126. [DOI] [PubMed] [Google Scholar]
- 9.Keady BT, Kuo P, Martínez SE, Yuan L, Hake LE. J Cell Sci. 2007;120:1093–1103. doi: 10.1242/jcs.03416. [DOI] [PubMed] [Google Scholar]
- 10.Reverte CG, Ahearn MD, Hake LE. Dev Biol. 2001;231:447–458. doi: 10.1006/dbio.2001.0153. [DOI] [PubMed] [Google Scholar]
- 11.Mendez R, Barnard D, Richter JD. EMBO J. 2002;21:1833–1844. doi: 10.1093/emboj/21.7.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tung JJ, Padmanabhan K, Hansen DV, Richter JD, Jackson PK. Cell Cycle. 2007;6:725–731. doi: 10.4161/cc.6.6.3936. [DOI] [PubMed] [Google Scholar]
- 13.Thom G, Minshall N, Git A, Argasinska J, Standart N. Biochem J. 2003;370:91–100. doi: 10.1042/BJ20021462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lapasset L, Pradet-Balade B, Lozano JC, Peaucellier G, Picard A. Dev Biol. 2005;285:200–210. doi: 10.1016/j.ydbio.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 15.Uzbekova S, Arlot-Bonnemains Y, Dupont J, Dalbiès-Tran R, Papillier P, Pennetier S, Thélie A, Perreau C, Mermillod P, Prigent C, et al. Biol Reprod. 2007 doi: 10.1095/biolreprod.107.061036. in press. [DOI] [PubMed] [Google Scholar]
- 16.Rechsteiner M, Rogers SW. Trends Biochem Sci. 1996;21:267–271. [PubMed] [Google Scholar]
- 17.Cardozo T, Pagano M. Nat Rev Mol Cell Biol. 2004;5:739–751. doi: 10.1038/nrm1471. [DOI] [PubMed] [Google Scholar]
- 18.Guardavaccaro D, Kudo Y, Boulaire J, Barchi M, Busino L, Donzelli M, Margottin-Goguet F, Jackson PK, Yamasaki L, Pagano M. Dev Cell. 2003;4:799–812. doi: 10.1016/s1534-5807(03)00154-0. [DOI] [PubMed] [Google Scholar]
- 19.Gutierrez GJ, Vögtlin A, Castro A, Ferby I, Salvagiotto G, Ronai Z, Lorca T, Nebreda AR. Nat Cell Biol. 2006;8:1084–1094. doi: 10.1038/ncb1472. [DOI] [PubMed] [Google Scholar]
- 20.Fuchs SY, Spiegelman VS, Kumar KGS. Oncogene. 2004;23:2028–2036. doi: 10.1038/sj.onc.1207389. [DOI] [PubMed] [Google Scholar]
- 21.Kanemori Y, Uto K, Sagata N. Proc Natl Acad Sci USA. 2005;102:6279–6284. doi: 10.1073/pnas.0501873102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barr FA, Silljé HHW, Nigg EA. Nat Rev Mol Cell Biol. 2004;5:429–440. doi: 10.1038/nrm1401. [DOI] [PubMed] [Google Scholar]
- 23.Liu J, Lewellyn AL, Chen LG, Maller JL. J Biol Chem. 2004;279:21367–21373. doi: 10.1074/jbc.M400482200. [DOI] [PubMed] [Google Scholar]
- 24.Elia AEH, Cantley LC, Yaffe MB. Science. 2003;299:1228–1231. doi: 10.1126/science.1079079. [DOI] [PubMed] [Google Scholar]
- 25.Sagata N. Bioessays. 1997;19:13–21. doi: 10.1002/bies.950190105. [DOI] [PubMed] [Google Scholar]
- 26.DeRenzo C, Seydoux G. Trends Cell Biol. 2004;14:420–426. doi: 10.1016/j.tcb.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Watanabe N, Arai H, Iwasaki J, Shiina M, Ogata K, Hunter T, Osada H. Proc Natl Acad Sci USA. 2005;102:11663–11668. doi: 10.1073/pnas.0500410102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu G, Xu G, Schulman BA, Jeffrey PD, Harper JW, Pavletich NP. Mol Cell. 2003;11:1445–1456. doi: 10.1016/s1097-2765(03)00234-x. [DOI] [PubMed] [Google Scholar]
- 29.Shirogane T, Jin J, Ang XL, Harper JW. J Biol Chem. 2005;280:26863–26872. doi: 10.1074/jbc.M502862200. [DOI] [PubMed] [Google Scholar]
- 30.Moshe Y, Boulaire J, Pagano M, Hershko A. Proc Natl Acad Sci USA. 2004;101:7937–7942. doi: 10.1073/pnas.0402442101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rauh NR, Schmidt A, Bormann J, Nigg EA, Mayer TU. Nature. 2005;437:1048–1052. doi: 10.1038/nature04093. [DOI] [PubMed] [Google Scholar]
- 32.Groisman I, Huang Yi, Mendez R, Cao Q, Theurkauf W, Richter JD. Cell. 2000;103:435–447. doi: 10.1016/s0092-8674(00)00135-5. [DOI] [PubMed] [Google Scholar]
- 33.Huang Yi, Carson JH, Barbarese E, Richter JD. Genes Dev. 2003;17:638–653. doi: 10.1101/gad.1053003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yi JJ, Ehlers MD. Pharmacol Rev. 2007;59:14–39. doi: 10.1124/pr.59.1.4. [DOI] [PubMed] [Google Scholar]
- 35.Inoue D, Sagata N. EMBO J. 2005;24:1057–1067. doi: 10.1038/sj.emboj.7600567. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.