Abstract
The Escherichia coli enzyme succinate:ubiquinone oxidoreductase [(succinate dehydrogenase (SdhCDAB)] couples succinate oxidation to ubiquinone reduction and is structurally and functionally equivalent to mitochondrial complex II, an essential component of the aerobic respiratory chain and tricarboxylic acid cycle. All such enzymes contain a heme within their membrane anchor domain with a highly contentious, but as-yet-undetermined, function. Here, we report the generation of a complex II that lacks heme, which is confirmed by both optical and EPR spectroscopy. Despite the absence of heme, this mutant still assembles properly and retains physiological activity. However, the mutants lacking heme are highly sensitive to the presence of detergent. In addition, the heme does not appear to be involved in reactive oxygen species suppression. Our results indicate that redox cycling of the heme in complex II is not essential for the enzyme's ubiquinol reductase activity.
Keywords: complex II, electron paramagnetic resonance, electron transfer, fumarate reductase, quinone
The Escherichia coli complex II homolog, succinate:ubiquinone oxidoreductase (SQR) [succinate dehydrogenase (SdhCDAB)], is remarkably similar to the mitochondrial enzyme, both in structure and function (1–3). As a vital component of the tricarboxylic acid cycle, it links the oxidation of succinate to the reduction of ubiquinone. The structures of the eukaryotic and bacterial homologues are quite superimposable, and key residues throughout all four subunits are conserved between bacteria and eukaryotes. Importantly, sequence conservation of residues between E. coli SQR and human complex II extends to many residues implicated in human paragangliomas and pheochromocytomas (4, 5).
E. coli SQR has a b-type heme within the membrane domain that is essentially identical to that found in the eukaryotic enzyme. The role of this heme has been the focus of great debate. Although it is redox-active (6), it is not known whether it is essential for enzyme catalysis. This ambiguity stems from the fact that the heme does not lie on the electron-transfer relay connecting succinate oxidation with the site of ubiquinone binding (the Q-site) within the membrane-intrinsic domain of the enzyme. Succinate oxidation at a cytosolic-active site, flavin adenine dinucleotide (FAD), releases 2H+ and two electrons that must be transferred sequentially through a [2Fe-2S] cluster, a [4Fe-4S] cluster, and a [3Fe-4S] cluster, ultimately reducing ubiquinone at the Q-site. The single heme b lies in the intersubunit space coordinated by the SdhCD membrane anchor polypeptides (Fig. 1A). Because the Q-site is positioned between the heme and the [3Fe-4S] cluster, it is not clear whether the heme undergoes obligatory redox chemistry during enzyme turnover. Inspection of the structure (Fig. 1A), however, reveals that the heme lies 11.4 Å from the [3Fe-4S] cluster and 6.5 Å from the ubiquinone that cocrystallized with the enzyme (1). These distances are short enough for rapid electron transfer among the [3Fe-4S] cluster, the bound ubiquinone, and the heme. Interestingly, the E. coli genome encodes a quinol:fumarate oxidoreductase (QFR; FrdABCD), a paralog of SQR that shares much of its catalytic and structural properties (7, 8), but that lacks heme. Thus, determining the role of the heme of SQR would be an important step in gaining a complete understanding of its structure and function.
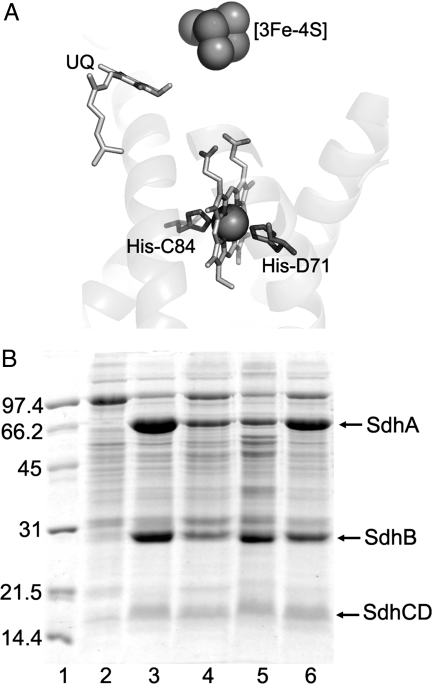
Fig. 1.
Expression of wild-type and E. coli SQR mutants. (A) Close-up view of the membrane domain of SdhCDAB. The two His axial heme ligands are indicated. (B) SDS/PAGE analysis of SQR-enriched membranes. Lane 1, low-molecular-mass markers (Bio-Rad); lane 2, DW35 membranes; lane 3, DW35/SdhCDAB membranes; lane 4, DW35/SdhCDH71YAB membranes; lane 5, SASX41B/SdhCDH71YAB membranes; lane 6, DW35/SdhCH84YDAB membranes. Finally, 45 μg of protein were loaded in each lane.
Previous experiments to create a heme-free SQR by expressing the enzyme in a hemA E. coli-deletion strain, which is unable to synthesize heme, failed as a result of improper enzyme assembly, prompting the hypothesis that the heme is necessary for proper subunit formation and integration (9). In addition, further attempts to remove the heme by site-directed mutagenesis proved to be ineffective (10, 11). Here we successfully created a heme-free mutant of E. coli SQR, which retains catalytic activity and many wild-type properties.
Results
Mutations of Heme Ligands Result in Fully Assembled, Heme-Free SQR.
The x-ray structure (1) of the E. coli SQR reveals that the heme b, as in the mammalian enzyme, is coordinated by two conserved His residues, one from each of the two membrane-bound subunits (Fig. 1A). A previous mutation of one of the heme ligands, SdhCH84LDAB, does not result in a loss of the heme possibly because of the close proximity of SdhC His-30, which is able to function as a surrogate ligand. Also, the SdhCDH71QAB mutation results in the conversion of the low-spin hexacoordinated heme into a high-spin pentacoordinated heme, as observed by EPR (11). We generated SdhCDH71YAB and SdhCH84YDAB mutants, each of which we hypothesized would eliminate the heme and introduce a hydrogen bond bridging the Tyr side chain and the imidazole of the corresponding histidine on the opposite subunit.
E. coli cells containing overexpressed SdhCDH71YAB or SdhCH84YDAB SQR were dark brown in color, whereas cells containing overexpressed wild-type SQR are brown with a slightly red hue. SDS/PAGE indicates that subunit assembly is unaffected by our mutations to the heme ligands (Fig. 1B). After cell lysis, all four subunits of the SdhCDH71YAB and SdhCH84YDAB mutants were clearly visible on SDS/PAGE. The concentration of SQR in membrane samples can be assayed by estimating the amount of acid-labile flavin (12). Membranes containing overexpressed wild-type, SdhCDH71YAB, and SdhCH84YDAB SQR contain 3.1, 1.0, and 2.4 nmol of SQR per mg of membrane protein, respectively. Based on the amounts of SQR assembled into the cytoplasmic membrane and our SDS/PAGE analyses (Fig. 1B), it is clear that these mutations do not significantly alter the structural characteristics of the SdhCD membrane domain, although mutating the SdhD His-71 ligand has a more pronounced effect on the stability of the enzyme. Although the loss of the heme cofactor leads to increased instability of the enzyme, both mutant membrane subunits are still capable of properly anchoring the soluble SdhAB dimer to the membrane.
UV-VIS and EPR Spectroscopies Confirm the Loss of the Heme Cofactor.
Dithionite-reduced minus “as is” air-oxidized absorbance spectra of membranes enriched in mutant SQR indicate a substantial lack of heme absorbance (Fig. 2A). Spectra of membranes enriched in wild-type SQR exhibit a strong Soret absorbance at 427 nm, a broad β-absorbance with maxima at 530 nm, and considerable α-absorbance at 559 nm. In the DW35 host membranes, the observed absorbances arise from the hemes of the bo3- and bd-oxidase (13), and there is a noticeable shift in the absorbance maxima in these membranes. The heme absorption spectra of both the SdhCDH71YAB and SdhCH84YDAB mutants are greatly diminished in the amplitude of the Soret absorbance as well as α-absorbance, compared with the wild-type SQR. In fact, the amplitudes of these absorbances are lower than those found in spectra of the DW35 host strain. The likely explanation for this finding is that the contribution from the spectrum of the heme from the oxidase is diluted out of the membranes by the overexpression of the heme-free SQR. Further confirmation of the loss of the b heme through measurement of the protoheme IX content was determined by the pyridine hemochromogen difference spectra (Table 1). Both SQR Tyr mutants contained only background amounts of b heme produced by the bo3- and bd-oxidase present in the host strain DW35.
Fig. 2.
Biophysical analysis of SQR-enriched DW35 membranes. (A) Dithionite reduced minus air-oxidized absorbance spectra. (B) [Fe-S] cluster EPR spectra. (C) Low-spin heme EPR spectra. (D) High-spin heme EPR spectra. Spectra in B are normalized for FAD content, and spectra in C and D are normalized to a protein concentration of 30 mg·ml−1.
Table 1.
Physiological activity and Km determinations of ubiquinol reduction and menaquinol oxidation in membranes enriched in SQR
| Host/enzyme | Succinate/Q0 turnover, s−1 | Km Q0, mM | Q1/PES | Km Q1, μM | Plumbagin/fumarate turnover, s−1 | Plumbagin Km, mM | Heme b protein, nmol/mg |
|---|---|---|---|---|---|---|---|
| DW35/SdhCDAB | 37 | 0.20 | 1.01 | 2.5 | 30 | 0.13 | 4.1 |
| DW35/SdhCH84YDAB | 22 | 0.71 | 0.7 | 8 | 16 | 0.10 | 0.5 |
| DW35/SdhCDH71YAB | 18 | 0.31 | 0.94 | 10 | 16 | 0.11 | 0.6 |
| DW35/pBR322* | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.6 |
| SASX41B/SdhCDH71YAB | 18 | 0.27 | n.d. | n.d. | n.d. | n.d. | n.d. |
*DW35/pBR322 is the host strain, which does not contain either SdhCDAB or FrdABCD so that it shows no activity for either succinate–ubiquinone reductase activity or menaquinol–fumarate oxidase activity. It has normal levels for bo- and bd-oxidase. n.d., not determined.
EPR spectroscopy was used to verify the cofactor composition in the mutant enzymes (Fig. 2B). For the EPR experiments, membranes enriched in SQR were fully oxidized with 0.8 mM dichlorophenolindophenol (DCPIP) to conditions where only the [3Fe-4S] cluster and heme are EPR-visible. The signal arising from the [3Fe-4S] cluster in the SdhB subunit appears as a peak at g = 2.02 (Fig. 2B), with a broad trough immediately upfield. This signal is easily detected in membranes enriched in wild-type SQR, but is lacking in the DW35 host membranes. EPR spectra of membranes containing SdhDH71Y or SdhCH84Y SQR exhibited a [3Fe-4S] cluster spectrum identical to that of wild-type membranes. The amount of enzyme assembled in the mutants' membranes reflected by the [3Fe-4S] cluster and FAD levels was, however, reduced by approximately one third, compared with wild-type enzyme. These results affirm the conclusion that enzyme assembly has been largely unaffected by the mutation.
The heme in E. coli SQR is EPR-detectable and has a characteristically high anisotropic low-spin spectrum with a gz of 3.66 (Fig. 2C) (14). This signal is obviously absent in membranes enriched in the mutant SQR, as well as in membranes of the DW35 host strain. The signal at g = 3.36 is found in all of the membrane preparations and can be assigned to the heme b558 of cytochrome bd (15).
Another EPR signal detectable in DW35 membranes is contributed by the pentacoordinated high-spin hemes of bo3- (16) and bd-oxidase (15). Previous studies of an SdhCDH71QAB mutant indicated that a high-spin pentacoordinate heme can be incorporated into SQR (11), and it was necessary to eliminate this possibility for our SdhCH84YDAB and SdhCDH71YAB mutants. A sharp peak is observed at g = 6.01 in DW35 membranes, corresponding to the presence of high-spin hemes (Fig. 2D). In wild-type SQR-enriched membranes, this signal is diminished because the cytochrome oxidase concentration is diluted by SQR overexpression. A similar pattern was recorded in both of the mutants. Any conversion from low- to high-spin heme is easily detected by EPR (11), but here such a conversion is clearly absent. Taken together the optical and EPR data unambiguously indicate that both of our mutations have eliminated the heme from its regular position within the membrane domain.
Evidence of a competent Q-site is provided by the presence of a ubisemiquinone radical observed by EPR. Wild-type SQR cycles through a semiquinone intermediate (Em,7 = +100 mV) at the Q-site, which is detectable by potentiometric titration of the g = 2.005 signal in SQR-containing membranes (6). As the redox titration of SdhCDH71YAB membranes indicates (Fig. 3), both the FAD and ubisemiquinone radicals, which are found in the wild-type SQR, are preserved in the mutant SQR at the appropriate midpoint potentials. Titrations of SdhCH84YDAB-enriched membranes are similar (data not shown). The KSTAB of the ubisemiquinone radical in wild-type SQR has a value of ≈1 (6), and this value is unperturbed in either of the mutants.
Fig. 3.
Potentiometric titration of the g = 2.005 EPR signal in SdhCDH71YAB-enriched DW35 membranes. Peak troughs are plotted against Eh and were normalized to 100% signal intensity. Both the flavosemiquinone (open squares) and ubisemiquinone (open diamonds) are visible.
Heme-Free SQR Variants Retain Physiological Activity.
The physiological activities of the mutant SQR were characterized by a spectrophotometric assay (Table 1). When using Q0 as a substrate, the turnover rates for the heme-free SQR decreased to ≈50–60% of that of the wild type, but the Km for Q0 in the mutants was within the range of that found with wild-type SQR. By using Q1 as a substrate, the mutant enzymes show an even better retention of activity compared with wild type (Table 1). The succinate:Q1-reductase activity, when described as a ratio to the nonphysiological succinate:phenazine ethosulfate (PES) activity, is unity in the wild-type enzyme, whereas there is only a 6% and 30% loss in activity in the SdhCDH71YAB and SdhCH84YDAB samples, respectively. The Km values for Q1 were only slightly elevated. During catalysis of the reverse reaction by using reduced plumbagin as a menaquinol analog, the heme-free mutants lost ≈50% of wild-type turnover, similar to the effect observed on succinate:Q0 reductase activity, which suggests that the absence of heme affects both processes similarly. The similarities in Km for both ubiquinone and menaquinol in the wild-type and mutant enzymes suggest that the loss of the heme does not result in major changes in the structural components of the Q-site.
The in vivo functionality of the enzyme was determined by monitoring aerobic growth of E. coli DW35 harboring plasmids expressing SQR in succinate-supplemented minimal medium. Either the SdhCH84YDAB or SdhCDH71YAB mutant was competent in sustaining growth under these conditions because it had a similar doubling time (≈2 h) as the wild-type SQR-expressing cells (data not shown).
Heme-Free SQR Enzymes Are Highly Sensitive to Detergent.
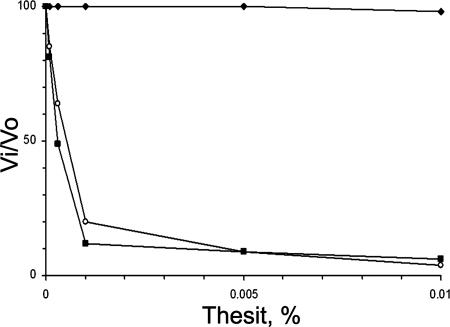
Although the lack of heme cofactor does not appear to have serious effects on catalytic activity in the native membrane, the same cannot be said for the enzyme in the presence of detergent. Normally, wild-type SQR can tolerate detergent extraction and ion exchange chromatography while remaining highly active (1). However, in the case of the SdhCH84YDAB or SdhCDH71YAB mutant, incubation of these SQR-enriched membranes with low Thesit (C12E9) concentrations, below the critical micellar concentration of 0.1 mM, results in a dramatic loss of succinate:Q1-reductase activity (IC50 of 0.0005% or ≈20 μM) (Fig. 4). Moreover, after the addition of other detergents commonly used for extraction (Triton X-100 or dodecylmaltoside) at concentrations of 0.01%, the wild-type SQR is unaffected, whereas both heme-free SQR lose nearly all physiological activity (data not shown). The sensitivity of the mutant enzymes to low levels of detergent (Fig. 4) is consistent with some disruption of the structural integrity of the enzyme because there also is a time-dependent loss of succinate oxidase activity in the PES reaction (data not shown) as well as the Q1-reductase activity (Fig. 4). This finding is consistent with the known sensitivity of the SdhAB enzyme to air or temperature when removed from the SdhCD subunits (11). Standard purification of detergent-solubilized mutant SQR (17) was attempted by anion exchange chromatography, but was unsuccessful. In contrast to wild type, the mutant SQR eluted with a significant depletion of SdhCD (data not shown), indicative of dissociation of SdhAB from the membrane anchor. Unfortunately, the detergent sensitivity of the mutant SQR precludes any isolation of the intact complex. Indeed, the effect of detergent confirms the role of the heme in providing structural stability for the enzyme.
Fig. 4.
Effect of Thesit on succinate:Q1-reductase activity. Membranes were incubated for 5 min at 30°C in the reaction medium with indicated Thesit concentrations before initiation of the reaction. Filled diamonds, SdhCDAB; filled squares, SdhCDH71YAB; open circles, SdhCH84YDAB.
Loss of the Heme Does Not Affect Reactive Oxygen Species (ROS) Production.
It has been proposed that the heme acts as an electron sink during enzyme turnover to prevent prolonged exposure of the ubisemiquinone radical to oxidants in the cellular environment (1). In support of this hypothesis, the E. coli paralog, QFR, which lacks heme, generates 20-fold more ROS than SQR during aerobic succinate oxidation (18). Therefore, we assayed the production of superoxide radicals by our heme-free SQR mutants during catalytic turnover (Fig. 5). The rate of ROS production by both the SdhCH84YDAB and SdhCDH71YAB mutant were reduced by 60%, compared with the wild-type enzyme. This finding correlates well with the physiological rate of succinate/Q0 turnover, which also was halved in the mutants. As such, it does not appear that the heme is acting to suppress ROS production in the wild-type SQR.
Fig. 5.
Analysis of superoxide radical production rates in DW35 membranes enriched in wild-type or mutant SQR.
Expression of the Heme-Free Mutants in a Heme-Deficient E. coli Strain.
It may be possible that a minute amount of heme, below our detection limits, may have been inserted into SdhCDH71YAB, and this small subset of heme-containing enzyme was responsible for some of the wild-type characteristics observed herein. To ensure that this notion was not the case, the SdhCDH71YAB was expressed in the E. coli strain, SASX41B, which is incapable of heme biosynthesis in the absence of the heme precursor, 5-aminolevulinic acid. Wild-type or heme-containing mutants of SQR do not assemble in this strain (11). Conversely, expression of the SdhCDH71YAB mutant was capable in the SASX41B strain (Fig. 1A), and enzyme assembly did not appear to be affected. However, when expressed in the SASX41B strain, the SdhCDH71YAB enzyme seemed to have an anomalous ratio between the SdhA and SdhB subunits, which was not observed in the DW35 strain. Additionally, the SdhCDH71YAB from both E. coli strains had similar kinetic properties with respect to the ubiquinone activity (Table 1).
Discussion
Oyedotun et al. (19) reported that mutation of the axial ligands of the heme in Saccharomyces cerevisiae complex II had minimal consequences on enzyme activity and quinone function. The results of these studies, however, showed that some fumarate-oxidizable heme was still retained in all of their mutants. In contrast, the two mutant SQR we have presented here unmistakably lack the heme as verified by convincing spectroscopic and EPR techniques. Considering that a large cofactor was removed from the interior of the protein, the removal of the heme from the E. coli SQR did not have a substantial effect on the enzyme's function. In addition to the heme ligands, the heme in the native enzyme must undoubtedly contact the protein component of the enzyme at multiple points within the membrane core. It will be interesting to see whether the loss of the heme has left a void in the intersubunit space or the membrane domain has shifted to compensate. The presence of a functional Q-site and the stability of the ubisemiquinone would suggest that the protein backbone has not been greatly impacted. Because the heme propionates are positioned ≈7 Å from the Q-site, changes in the protein structure around the heme should manifest as disruptions in Q-binding, and this effect is not observed in the membrane-bound enzyme.
The instability of the heme-free SQR in the presence of detergents emphasizes a structural role of the heme in the enzyme. Also, the disparity in the stoichiometry of SdhA and SdhB in the SdhCDH71YAB mutant when expressed in the heme-deficient E. coli strain SASX41B suggests that the enzyme may be more susceptible to proteolysis when the enzyme assembles in an environment completely devoid of heme. In DW35 cells, the heme may transiently associate with the hydrophobic SdhCD dimer in the mutant SQR during the initial stages of assembly and membrane integration before it is eventually lost, thereby stabilizing the membrane-intrinsic components before assembly of the final complex. In the absence of heme in the SASX41B cells, abnormal assembly or a loose association between subunits may increase the sensitivity of SdhA to proteolytic cleavage, and thus we may be observing some partially assembled enzyme within the membrane. This finding indicates a structural role for the heme not only in the holoenzyme, but also during the early phases of assembly, consistent with previous notions (20). The presence of a significant quantity of intact, fully functional SdhCDH71YAB enzyme, even in the SASX41B cells, suggests that insertion of the heme into the membrane domain of the wild-type protein may not necessarily precede formation of the fully folded apoenzyme, but the presence of heme may enhance the efficacy of folding.
We have shown that the heme is not an essential component of the catalytic mechanism, which is extraordinary given that the heme cofactor is conserved from prokaryotes to eukaryotes. However, E. coli QFR can carry out the same reaction in the absence of the heme. In addition, the heme b in mitochondrial complex II has been shown to be resistant to reduction by succinate (21), which is likely a consequence of its low midpoint potential (Em,7 = −185 mV in the mammalian, compared with Em,7 = +35 mV in the bacterial enzyme). In light of our results, it is entirely plausible that mammalian complex II may not rely on electron transfer through the heme at all.
Despite our observation that heme-free SQR is catalytically functional both in vitro and in vivo, the heme is clearly redox-active in the wild-type enzyme (6). The heme does not seem to have a role in reducing ROS production either. The lack of heme cofactor does not have an impact on the midpoint potential of nearby redox centers ([3Fe-4S] or quinone) (data not shown), and this notion can be rejected as the cause for the modest drop in activity observed here. It has been previously determined that the rate of electron transfer between the Q-site and the heme can be considered very fast (22). In a model where the electrons from the [3Fe-4S] cluster reduce the bound ubiquinone directly, the heme may act to lower the thermodynamic barrier of electron transfer from the [3Fe-4S] by delocalizing the negative charge on the ubisemiquinone, thus increasing the transfer rate of the second electron, which is likely coupled to the protonation of the quinone. It is difficult to surmise whether such a rate increase could result in the magnitude of changes observed herein because the kinetics of quinone reduction occur on a millisecond timescale, whereas electron transfer rates through the enzyme occur on a microsecond timescale (23). However, when electron transfer rates are lowered by modulating the midpoint potential of the [Fe-S] clusters, a significant effect is seen on the quinine-reduction kinetics (24).
In summary, the results reported here propose a function for the heme in E. coli SQR in maintaining a high rate of catalysis, in addition to its structural purpose, while excluding its absolute requirement in the catalytic mechanism, as well as its role in ROS suppression.
Materials and Methods
Bacterial Strains and Plasmids.
E. coli strains DW35 (ΔfrdABCD, sdhC::kan) (25) and SASX41B (HfrP02A hemA41 metB1 relA1) (26) were used for overexpression of SdhCDAB from the plasmid pFAS (pFRDsdhC+D+A+B+) as described in ref. 8. E. coli TG1 cells (K12, Δ(lac-proAB), supE, thi, hsd Δ5 F′[traD36 proA+B+lacIq lacZ ΔM15]) (Amersham Biosciences) were used for all cloning protocols. Site-directed mutagenesis methods were performed as described in ref. 6.
Cell Growth and Preparation of Membrane Vesicles.
DW35 cells expressing the appropriate enzymes were grown in batch cultures in Terrific Broth under microaerobic conditions and harvested as described in ref. 6. SASX41B cells were grown anaerobically in glycerol-fumarate minimal medium (27) in 19-liter carboys (from a 250-ml Luria-Bertani broth innoculum supplemented with 50 μg·ml−1 5-aminolevulinic acid) (28) at 37°C for 30 h. Further, 100 μg·ml−1 ampicillin was routinely used in all cell cultures. Additionally, 100 μg·ml−1 streptomycin and 50 μg·ml−1 kanamycin were added to all DW35 cultures. Growth studies were performed in Klett flasks containing minimal medium supplemented with 20 mM succinate (6).
PMSF was added to a final concentration of 0.2 mM before cell lysis by French Press. Membranes were isolated by differential centrifugation (6). Activation of the SQR enzyme required a 20-min incubation at 30°C with 1 mM malonate, and 100 mM Mops/5 mM EDTA was used ubiquitously as the buffer system. A modified Lowry assay (29) was used to determine the protein concentration in the membrane vesicles.
SDS/PAGE.
Protein samples were run on a 12.5% polyacrylamide gel. After a 5-min incubation at 70°C, 45 μg of protein were loaded onto each lane. Low-molecular-mass markers were obtained from Bio-Rad and Invitrogen. All gels were stained with Coomassie brilliant blue.
Enzyme Activity, b Heme Content, and ROS Generation.
Both the succinate:Q0 and plumbagin (5-hydroxy-methyl-napthalene-1,4-dione):fumarate assays have been described before (6). SQR-enriched membranes were added to an oxygen-depleted cuvette containing the appropriate substrates to initiate the reaction. The Q1-reductase and PES assays also have been described (30). All membranes were incubated with 1 mM malonate to remove the oxaloacetate inhibitor bound to the active-site FAD.
The measurement of superoxide production was performed with a standard cytochrome c reduction assay (24).
The protoheme IX content of cytochrome b was determined by the pyridine hemochromogen difference spectra (dithionite-reduced minus ferricyanide-oxidized) (ε558–540 = 23.98 mM−1·cm−1) as described in ref. 31.
Purification of SQR.
Membranes were solubilized with 1% Thesit (C12E9; Anatrace), and the extract was bound to a DEAE fast-flow anion exchange resin as described in ref. 17, with the exception that the initial buffer was 10 mM K-Pi (pH 7.5). The anion exchange resin containing the bound enzyme was collected on a small filter, washed with 75 mM NaCl, and eluted with 300 mM NaCl.
UV-VIS and EPR Spectroscopy.
Reduced minus oxidized spectra of the membranes were performed at a protein concentration of 0.5 mg·ml−1. Excess dithionite was added to the cuvette to reduce the sample before obtaining a sample spectra. A Hewlett Packard 8453 UV-VIS diode array spectrophotometer was used to perform all spectral readings.
To generate oxidized samples for EPR analysis, 170 μl of membranes was placed into a standard EPR tube, to which DCPIP was added to a final concentration of 0.8 mM before freezing. Potentiometric titrations were performed as reported (6). [Fe-S] cluster and heme spectra were recorded by using a Bruker Elexsys E500 EPR spectrophotometer equipped with an Oxford Instruments ESR900 flowing helium cryostat. All EPR experiments were performed at pH 7.0. EPR conditions were 9 K, 20 mW at 9.38 GHz, 10 Gpp. Studies done on the flavin and semiquinone radicals were performed by using a Bruker ESP300E spectrometer equipped with a Bruker liquid nitrogen evaporating cryostat at 150 K. EPR conditions were 150 K, 20 mW at 9.47 mW, 2 Gpp.
Acknowledgments
This work was supported by Canadian Institutes of Health Research Grant 74738, National Institutes of Health Grant GM61606, the Department of Veterans Affairs, and the Canada Foundation for Innovation. J.H.W. holds a Canada Research Chair in Membrane Biochemistry. Q.M.T. holds a Canadian Institutes of Health Research Canada Graduate Scholarship and an Alberta Heritage Foundation for Medical Research Studentship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Yankovskaya V, Horsefield R, Tornroth S, Luna-Chavez C, Miyoshi H, Leger C, Byrne B, Cecchini G, Iwata S. Science. 2003;299:700–704. doi: 10.1126/science.1079605. [DOI] [PubMed] [Google Scholar]
- 2.Huang LS, Sun G, Cobessi D, Wang AC, Shen JT, Tung EY, Anderson VE, Berry EA. J Biol Chem. 2006;281:5965–5972. doi: 10.1074/jbc.M511270200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun F, Huo X, Zhai Y, Wang A, Xu J, Su D, Bartlam M, Rao Z. Cell. 2005;121:1043–1057. doi: 10.1016/j.cell.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 4.Baysal BE, Ferrell RE, Willett-Brozick JE, Lawrence EC, Myssiorek D, Bosch A, van der Mey A, Taschner PE, Rubinstein WS, Myers EN, et al. Science. 2000;287:848–851. doi: 10.1126/science.287.5454.848. [DOI] [PubMed] [Google Scholar]
- 5.Niemann S, Muller U. Nat Genet. 2000;26:268–270. doi: 10.1038/81551. [DOI] [PubMed] [Google Scholar]
- 6.Tran QM, Rothery RA, Maklashina E, Cecchini G, Weiner JH. J Biol Chem. 2006;281:32310–32317. doi: 10.1074/jbc.M607476200. [DOI] [PubMed] [Google Scholar]
- 7.Iverson TM, Luna-Chavez C, Croal LR, Cecchini G, Rees DC. J Biol Chem. 2002;277:16124–16130. doi: 10.1074/jbc.M200815200. [DOI] [PubMed] [Google Scholar]
- 8.Maklashina E, Berthold DA, Cecchini G. J Bacteriol. 1998;180:5989–5996. doi: 10.1128/jb.180.22.5989-5996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakamura K, Yamaki M, Sarada M, Nakayama S, Vibat CR, Gennis RB, Nakayashiki T, Inokuchi H, Kojima S, Kita K. J Biol Chem. 1996;271:521–527. doi: 10.1074/jbc.271.1.521. [DOI] [PubMed] [Google Scholar]
- 10.Vibat CR, Cecchini G, Nakamura K, Kita K, Gennis RB. Biochemistry. 1998;37:4148–4159. doi: 10.1021/bi9716635. [DOI] [PubMed] [Google Scholar]
- 11.Maklashina E, Rothery RA, Weiner JH, Cecchini G. J Biol Chem. 2001;276:18968–18976. doi: 10.1074/jbc.M011270200. [DOI] [PubMed] [Google Scholar]
- 12.Singer TP, Salach J, Hemmerich P, Ehrenberg A. Methods Enzymol. 1971;18:416–427. [Google Scholar]
- 13.Salerno JC, Bolgiano B, Poole RK, Gennis RB, Ingledew WJ. J Biol Chem. 1990;265:4364–4368. [PubMed] [Google Scholar]
- 14.Yang X, Yu L, Yu CA. J Biol Chem. 1997;272:9683–9689. doi: 10.1074/jbc.272.15.9683. [DOI] [PubMed] [Google Scholar]
- 15.Rothery RA, Ingledew WJ. Biochem J. 1989;261:437–443. doi: 10.1042/bj2610437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cotter PA, Chepuri V, Gennis RB, Gunsalus RP. J Bacteriol. 1990;172:6333–6338. doi: 10.1128/jb.172.11.6333-6338.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horsefield R, Yankovskaya V, Tornroth S, Luna-Chavez C, Stambouli E, Barber J, Byrne B, Cecchini G, Iwata S. Acta Crystallogr D. 2003;59:600–602. doi: 10.1107/s0907444903002075. [DOI] [PubMed] [Google Scholar]
- 18.Messner KR, Imlay JA. J Biol Chem. 2002;277:42563–42571. doi: 10.1074/jbc.M204958200. [DOI] [PubMed] [Google Scholar]
- 19.Oyedotun KS, Yau PF, Lemire BD. J Biol Chem. 2004;279:9432–9439. doi: 10.1074/jbc.M311877200. [DOI] [PubMed] [Google Scholar]
- 20.Nihei C, Nakayashiki T, Nakamura K, Inokuchi H, Gennis RB, Kojima S, Kita K. Mol Genet Genomics. 2001;265:394–404. doi: 10.1007/s004380100444. [DOI] [PubMed] [Google Scholar]
- 21.Yu L, Xu JX, Haley PE, Yu CA. J Biol Chem. 1987;262:1137–1143. [PubMed] [Google Scholar]
- 22.Anderson RF, Hille R, Shinde SS, Cecchini G. J Biol Chem. 2005;280:33331–33337. doi: 10.1074/jbc.M506002200. [DOI] [PubMed] [Google Scholar]
- 23.Moser CC, Farid TA, Chobot SE, Dutton PL. Biochim Biophys Acta. 2006;1757:1096–1109. doi: 10.1016/j.bbabio.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 24.Cheng VW, Ma E, Zhao Z, Rothery RA, Weiner JH. J Biol Chem. 2006;281:27662–27668. doi: 10.1074/jbc.M604900200. [DOI] [PubMed] [Google Scholar]
- 25.Westenberg DJ, Gunsalus RP, Ackrell BA, Sices H, Cecchini G. J Biol Chem. 1993;268:815–822. [PubMed] [Google Scholar]
- 26.Sasarman A, Surdeanu M, Szegli G, Horodniceanu T, Greceanu V, Dumitrescu A. J Bacteriol. 1968;96:570–572. doi: 10.1128/jb.96.2.570-572.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Condon C, Weiner JH. Mol Microbiol. 1988;2:43–52. doi: 10.1111/j.1365-2958.1988.tb00005.x. [DOI] [PubMed] [Google Scholar]
- 28.Sasarman A, Surdeanu M, Horodniceanu T. J Bacteriol. 1968;96:1882–1884. doi: 10.1128/jb.96.5.1882-1884.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Markwell MA, Haas SM, Bieber LL, Tolbert NE. Anal Biochem. 1978;87:206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- 30.Maklashina E, Cecchini G. Arch Biochem Biophys. 1999;369:223–232. doi: 10.1006/abbi.1999.1359. [DOI] [PubMed] [Google Scholar]
- 31.Berry EA, Trumpower BL. Anal Biochem. 1987;161:1–15. doi: 10.1016/0003-2697(87)90643-9. [DOI] [PubMed] [Google Scholar]