Abstract
We have previously identified several members of the Wnt/β-catenin pathway that are differentially expressed in a mouse model with deficient coronary vessel formation. Systemic ablation of β-catenin expression affects mouse development at gastrulation with failure of both mesoderm development and axis formation. To circumvent this early embryonic lethality and study the specific role of β-catenin in coronary arteriogenesis, we have generated conditional β-catenin-deletion mutant animals in the proepicardium by interbreeding with a Cre-expressing mouse that targets coronary progenitor cells in the proepicardium and its derivatives. Ablation of β-catenin in the proepicardium results in lethality between embryonic day 15 and birth. Mutant mice display impaired coronary artery formation, whereas the venous system and microvasculature are normal. Analysis of proepicardial β-catenin mutant cells in the context of an epicardial tracer mouse reveals that the formation of the proepicardium, the migration of proepicardial cells to the heart, and the formation of the primitive epicardium are unaffected. However, subsequent processes of epicardial development are dramatically impaired in epicardial-β-catenin mutant mice, including failed expansion of the subepicardial space, blunted invasion of the myocardium, and impaired differentiation of epicardium-derived mesenchymal cells into coronary smooth muscle cells. Our data demonstrate a functional role of the epicardial β-catenin pathway in coronary arteriogenesis.
Keywords: cardiovascular development, smooth muscle, proepicardium, Wnt signaling
The epicardium is the outermost cell layer of the postlooped heart. It consists of a single layer of flattened mesothelial cells that are in direct contact with the pericardial fluid. Cells that migrate from the epicardium give rise to the cellular elements of the coronary blood vessels (reviewed in ref. 1).
The embryonic epicardium originates from a primarily extracardiac primordium, the proepicardum, which is located at the septum transversum near the venous pole of the heart (2, 3). In mouse embryos, proepicardial cells reach the heart predominantly in the form of free-floating vesicles that traverse the pericardial cavity, adhere to the initially naked myocardial surface, and subsequently form the epicardial covering of the heart (reviewed in ref. 1). The recruitment of coronary vessel progenitor cells from the proepicardium and embryonic epicardium involves several steps of epithelial-mesenchymal transition (EMT). As a result of proepicardial EMT, the extracellular matrix of free-floating proepicardial vesicles becomes populated with mesenchymal cells (4–8). Subsequently, the primitive epicardium gives rise to the subepicardial mesenchyme, also by means of EMT.
Recent data suggest that the primitive epicardium and epicardium-derived cells (EPDCs) modulate the maturation of other cardiac components, including the embryonic myocardium and the cardiac conduction system (9–14). We have previously shown that the embryonic epicardium is a key signaling tissue responsible for the transmission of the morphogenic signal derived from retinoic acid and identified several components of the Wnt/β-catenin signaling pathway that are down-regulated upon retinoid signaling deficiency, in particular, β-catenin and its activator Wnt9b (15). However, it remains to be shown whether Wnt/β-catenin signaling plays a specific role in the development of coronary progenitor cells.
β-Catenin is an important signaling molecule throughout development and organogenesis, because it is the final effector of the canonical Wnt ligands (16, 17). Wnts are secreted, cysteine-rich glycoproteins that are highly conserved among species and bind to frizzled (Fz) receptors. In the absence of a receptor-bound canonical Wnt, cytosolic β-catenin is serine- and threonine-phosphorylated, rapidly ubiquitinated, and degraded. Binding of Wnts to Fz proteins in the presence of the coreceptor low-density lipoprotein receptor-related protein 5 or 6 results in activation of Dishevelled. Dishevelled inhibits the glycogen synthase kinase 3β-containing phosphorylation complex, thereby promoting accumulation of cytosolic β-catenin, which then translocates to the nucleus, and activates the transcription of numerous genes implicated in proliferation, differentiation, and other cellular processes (reviewed in ref. 18).
The development of numerous organ systems depends on Wnt signaling (reviewed in ref. 19). In the heart, Wnt signaling plays a pivotal role in cardiomyocyte specification, and both activation and inhibition of Wnt signaling affect the formation of the cardiomyocyte compartment (20–27), suggesting that the muscular component of the heart depends on the cellular context in which Wnt stimulation occurs (27).
To explore the role of Wnt/β-catenin in the development of coronary progenitor cells and its implication on cardiac morphogenesis, we have generated a conditional β-catenin mutant mouse lacking β-catenin expression in the proepicardium and its derivatives.
Results
Inactivation of the β-Catenin Gene by G5-Cre-Mediated Deletion Leads to Embryonic Lethality.
We generated a conditional knockout (KO) mouse lacking β-catenin expression in the proepicardium (epiBC-KO) by interbreeding a floxed β-catenin mouse (28) with the proepicardium-expressed Gata5-Cre (G5-Cre) mouse (15). Double heterozygous mice for the β-catenin floxed allele and G5-Cre (BCf/+:G5cre/+) were born at the expected Mendelian ratio. In contrast, no mice homozygous for the β-catenin floxed allele and G5-Cre heterozygous (BCf/f:G5cre/+) were found at birth. We subsequently determined that lethality of epiBC-KO mice occurs between embryonic stage 15.5 (E15.5) and birth [supporting information (SI) Table 1]).
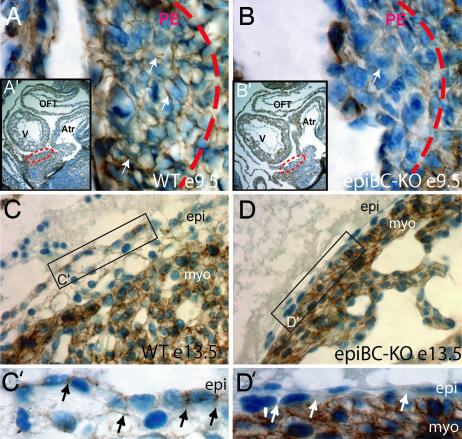
We tested the ability of Cre to ablate β-catenin in the G5-Cre expressing cells by direct examination of the β-catenin protein content by using immunohistochemistry with a β-catenin-specific antibody. At day 9.5, β-catenin is already efficiently removed in the proepicardium, with nearly 100% of proepicardial cells negative for β-catenin expression (Fig. 1 A and B). In the hearts of older embryos (E13.5), β-catenin is completely ablated in the epicardium with no detectable modification of β-catenin expression in the myocardium (Fig. 1 C–D′), thus demonstrating that G5-Cre efficiently removes β-catenin expression from the proepicardium and its derivatives without substantially affecting myocardial cells.
Fig. 1.
Ablation of β-catenin protein in proepicardial and epicardial cells. Immunohistochemical staining of β-catenin protein in sections from embryonic hearts. (A–B′) WT E9.5 (A and A′) and epicardial β-catenin mutants E9.5 (epiBC-KO) (B and B′) show the specific ablation of β-catenin in the proepicardial mutant cells, compared with the WT control littermate. Dotted circles in A′ and B′ indicate the localization of the proepicardium in the embryo E9.5. A and B are magnifications of the proepicardia in WT (A) and mutant (B). (C and D) In the epicardium of older embryos (E13.5) β-catenin appears in the WT epicardial cells (C), whereas its expression is ablated in the mutant epicardial cells (D). (C′ and D′) Zoom image that visualizes details of epicardial β-catenin expression. β-Catenin signal is detected by peroxide staining (brown) mainly in the cell membrane of WT embryos and is absent in the mutant epicardium (arrows). Nuclei are counterstained with hematoxylin staining (blue). V, ventricle; Atr, atrium; OFT, outflow track; PE, proepicardium; epi, epicardium; myo, cardiac myocytes. (Magnifications: A and B, ×630; A′ and B′, ×100; C and D, ×400; C′ and D′, ×630.)
Impaired Cardiac Growth in Epicardium-Restricted β-Catenin Mutant Embryos.
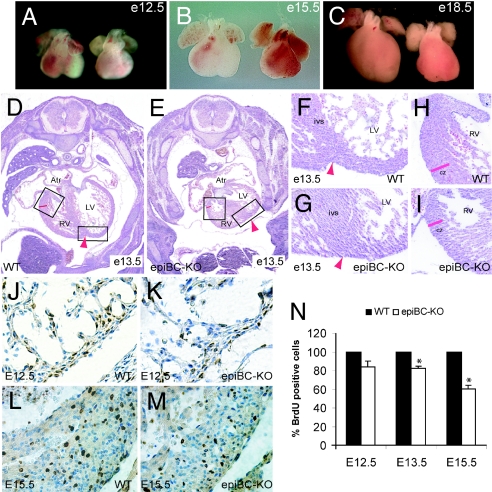
Despite their normal overall embryonic growth (data not shown), epiBC-KO mice display a pronounced reduction in cardiac size (Fig. 2 A–C) that clearly manifest after E13.5. Other defects include rotation of the heart axis toward the left hemi-thorax (Fig. 2 D and E), thin compact-zone myocardium (Fig. 2 H and I), and impaired formation of the interventricular sulcus caused by reduced expansion (ballooning) of the ventricles (Fig. 2 F and G, arrowheads). The thin myocardium defect correlates with a decrease in myocardial cell proliferation, which has been verified by BrdUra incorporation analysis (Fig. 2 J–N). Quantification of myocardial BrdUra incorporation indicates a trend reduction (15.7% reduction) of BrdUra-positive cells in E12.5 mutant hearts. At E13.5, reduction in BrdUra incorporation reaches statistical significance with an 18% decrease at E13.5 and an additional 39.7% reduction at E15.5 as compared with their WT littermate controls (Fig. 2N). Similar data have been gathered upon analysis of phosphorylated histone-3 immunohistochemistry in the epiBC-KO hearts (SI Fig. 6), thus indicating that the ablation of β-catenin in the epicardium causes a deficiency in the proliferative capacity of the myocardium. No increased apoptosis was observed in epiBC-KO hearts (SI Fig. 7).
Fig. 2.
Cardiac growth defects in epicardium-restricted β-catenin mutant mice. (A–C) Gross morphological comparison of cardiac size between E12.5 (A), E15.5 (B), and E18.5 (C) WT hearts (left hearts) and epiBC-KO hearts (right hearts). (D–I) H&E staining of E13.5 WT mice (D, F, and H) and epiBC-KO mice (E, G, and I). As indicated by the boxes, F–I are magnifications of images in D and E. (J–M) BrdUra immunostaining of paraffin sections from E12.5 (J and K), and E15.5 (L and M) hearts. BrdUra staining is brown, and nuclear counterstaining with hematoxylin is blue. Atr, atrium; RV, right ventricle; LV, left ventricle; ivs, interventricular septum; cz, compact zone myocardium; epi, epicardium. Arrowheads point to the interventricular sulcus. (N) Quantification of BrdUra-positive nuclei in cardiac samples at different ages of development shows hypoproliferation of epiBC-KO hearts after E13.5. Data are expressed as percentage of the mean ± SE relative to control and compared by using two-tailed Student's t analysis. Significant differences were defined as P < 0.05. (Magnifications: A–C, ×20; D and E, ×100; F–I, ×200; J–M, ×400.)
Epicardial β-Catenin Expression Is Essential for Subepicardial Layer Formation and Proper Epicardial EMT.
To analyze the fate of epicardial cells in epiBC-KO mice, we interbred the epiBC mouse with a transgenic mouse line in which epicardial cells are genetically labeled with β-galactosidase (Wilms' tumor1-LacZ, LacZWT1) (29). Backcross of BCf/+:G5cre/+:LacZWT1 with BCf/f yield epicardial-LacZ+ cells deficient in β-catenin (BCf/f: G5cre/+:LacZWT1; named epiBC-KOLacZ) and epicardial-LacZ+ cells expressing β-catenin (BCf/f: LacZWT1; named wt LacZ).
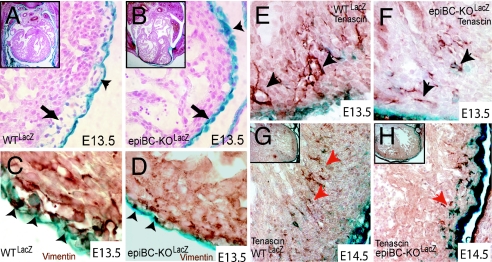
In both wtLacZ and in epiBC-KOLacZ mice, the outer layer of the myocardium becomes enveloped with a continuous epithelial sheet of epicardial cells (Fig. 3 A and B, blue), indicating that migration from the proepicardium is largely unaffected by proepicardial mutation of β-catenin. The epicardium of wtLacZ mouse is connected to the myocardium by a subepicardial tissue layer (Fig. 3A, arrow) that is rich in extracellular-matrix proteins and that contains mesenchymal cells of epicardial origin (1). In contrast, the subepicardial space is absent in epiBC-KOLacZ mice, as shown by the direct physical contiguity between the epicardium and the myocardial layers (Fig. 3B, arrow). Also, nascent subepicardial vascularization is absent in epiBC-KOLacZ hearts (Fig. 3A, arrowhead).
Fig. 3.
β-Galactosidase staining of proepicardial derivatives using Wilms' tumor transgenic mouse shows deficient expansion of the subepicardial space and impaired EMT in the epiBC-KO mice. (A and B) Proepicardial derivatives (blue) in E13.5 wt LacZ (A) and E13.5 epiBC-KOLacZ counterstained with nuclear fast red (B). Observe the lack of subepicardial space (arrows) and lack of subepicardial vascularization (arrowheads) in the mutant hearts. (C and D) In vivo EMT as measured by coexpression of Wilm's tumor and vimentin in the epicardium. Epithelial cells activated to EMT coexpress both epithelial (Wilm's tumor, blue) and mesenchymal markers (vimentin, brown). Coexpression (arrowheads) is not detected in epiBC-KO epicardium. (E–H) For migration analysis, tenascin/LacZ costaining was performed in hearts of E13.5 WTLacZ (E), E13.5 epiBC-KOLacZ (F), E14.5 WTLacZ (G), and E14.5 epiBC-KOLacZ (H) mice. Red arrows point to migrating cells. (Magnifications: A and B, ×200; E–H, ×400; C and D, ×630; Insets A, B, G, and H, ×100.)
Epicardial cells that transform to subepicardial mesenchymal cells express the marker vimentin (30). Costaining of EPDCs LacZ+/vimentin demonstrates a dramatic reduction of vimentin+ cells in the mutant subepicardium (Fig. 3 C and D). In contrast to wtLacZ, coexpression of LacZ (Fig. 3 C and D, blue) and vimentin (Fig. 3 C and D, brown) is rarely observed within the epicardium of epiBC-KOLacZ (Fig. 3 C and D, arrowheads). Using a combination of LacZWT1 and the cell motility marker tenascin (31), we monitored the evolution of EPDCs during their invasion of the myocardium. At E13.5, WT EPDCs undergoing migration (LacZ+/tenascin+) are detected in the interventricular septum as clusters arranged subepicardially and migrating inward to the myocardium (Fig. 3E). WT EPDCs, at E14.5, are observed in the free myocardial wall in parallel-arranged clusters located in a medial position of the compact zone myocardium (Fig. 3G), whereas LacZ expression is down-regulated, indicating differentiation of EPDCs (29). In contrast, epiBC-KOLacZ hearts show sparse LacZ-tenascin costaining that remains subepicardial without down-regulation of LacZ staining (Fig. 3H). Direct quantification of tenascin/LacZ cells indicates a reduction of migrating EPDCs in the interventricular septum and compact zone of 68.8% and 53.3%, respectively (SI Fig. 8) in the epiBC-KO hearts. These data indicate that EPDCs mutant for β-catenin have a reduced capacity to invade the myocardium.
Impaired Coronary Artery Formation in Epicardium-Restricted β-Catenin Mutant Hearts.
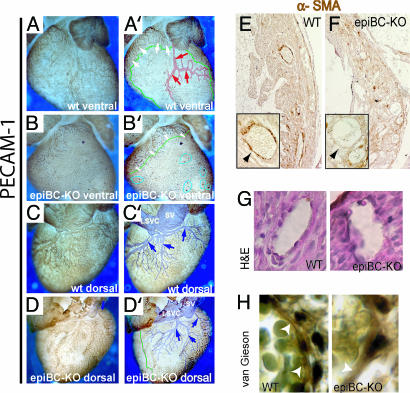
Despite a well preserved microvasculature, the main arterial vessels that run within the myocardial layer from the base toward the apex of the ventricle (see Fig. 4 A and A′, red arrows) are completely absent in the epiBC-KO hearts (five epiBC-KO and five WT mice analyzed, 100% penetrance of the defect), as shown by platelet/endothelial cell adhesion molecule-1 (PECAM-1) staining (Fig. 4 B and B′). In addition, the aorta of epiBC-KO mice is not connected to the coronary arteries but instead is connected to a primitive vascular plexus via two capillary structures that are located at the sides where the two coronary arteries normally take their origin (SI Figs. 9 and 10).
Fig. 4.
Epicardium-specific β-catenin mutation leads to coronary artery defects as shown by whole-mount PECAM-1 immunostaining and α-SMA. (A–B′) Ventral view of the intact whole-mount PECAM-1 staining of WT (A and A′) and epiBC-KO (B and B′) mice shows the absence of coronary arterial vasculature (red arrows in computerized image A′) in the epiBC-KO mice at E18.5. (C–D′) Dorsal views depict coronary veins vasculature in WT (C and C′) and epiBC-KO (D and D′) embryos. Note that the coronary veins are well developed in epiBC-KO mice (blue in computerized image D′), although some nonvascular areas and numerous blood-filled cysts are detected in the subepicardial space (B′, blue circles). (E and F) Paraffin sections from E18.5 hearts were immunostained with α-SMA antibody in WT (E) and mutant (F) mice to analyze the smooth muscle component of the coronary vessels (arrows). (G and H) H&E stain (G) or Van Gieson stain (H) were used for histological analysis of the smooth muscle layer on the vessels. White arrowheads point to elastic fibers. (Magnifications: A–D′, ×20; E and F, ×200; G, H, and Insets E and F, ×630.)
The coronary veins are less severely affected compared with the coronary arteries of the epiBC-KO mice. In normal E18.5 mouse hearts, three veins, their main branches, and a network of small veins are found within the subepicardial tissue layer of the dorsal and lateral walls of the two ventricles (Fig. 4 C and C′, blue arrows). In the E18.5 epiBC-KO mice recovered alive, a well developed network of coronary veins is found within the subepicardial layer of the dorsal wall of the ventricles (Fig. 4 D and D′). The subepicardial layer of the ventral and left-lateral aspects of the free ventricular wall, however, remained free of a venous plexus (encircled by green line in Fig. 4B′).
The normal remodeling of the arterial portions of the coronary vascular plexus starts at the aorta from where it proceeds in a proximo-distal direction toward the ventricular apex. We do not detected alteration in the vascular plexus of young embryos (SI Fig. 11). The developing coronary arteries, thereby, become invested by parietal supporting cells that form the tunica media of smooth muscle cells (SMCs) (32). Remarkably, no parietal supporting cells are observed in the epiBC-KO mice, as indicated by α-smooth muscle actin immunostaining (Fig. 4 E and F, arrows) or H&E (Fig. 4G). Accordingly, we observe a reduction in elastic fibers, as shown by Van Gieson staining (Fig. 4H). These data suggest that epicardium-derived progenitor cells deficient for β-catenin have impaired differentiation into coronary SMCs.
β-Catenin Plays a Role in EPDCs' Differentiation to Smooth Muscle Lineage.
In the previous section we described that β-catenin mutant EPDCs have impaired the potential in vivo to express mesenchymal markers, decreased migration, and failed to form the arterial tunica media. To further test the mechanism of β-catenin action in EPDCs, we tested in vitro the ability of EPDCs to undergo EMT and smooth muscle differentiation.
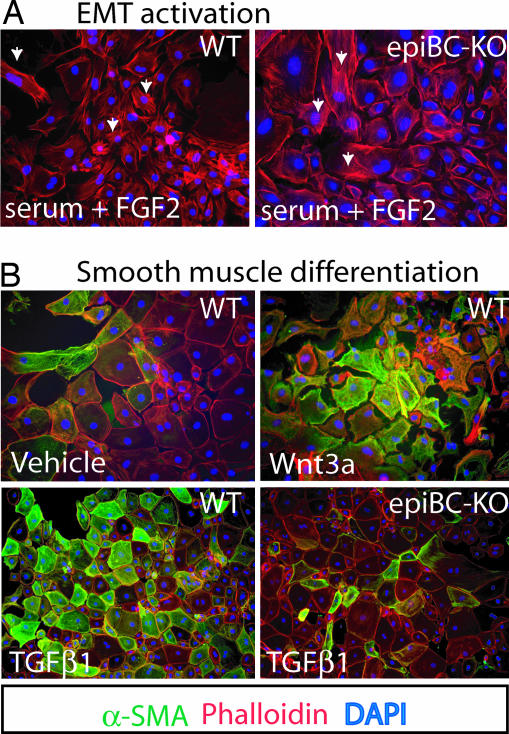
Epicardial cultures dissected from E12.5 hearts were used to analyze the capacity to undergo EMT of the epiBC-KO epicardium cells. In a set of three independent experiments, WT EPDCs treated with EMT-inducing media (FBS and FGF2) lose their epithelial nature by reduction of cell-to-cell contact and adopt a motile morphology (mesenchymal transformation) (Fig. 5A Left). Phalloidin staining shows redistribution of F-actin in the WT cells. A similar mesenchymal phenotype was found in explanted EPDCs isolated from epiBC-KO mice (Fig. 5A Right and SI Fig. 8 for quantitative analysis).
Fig. 5.
EMT and SMC differentiation of epicardium cultured cells. (A) EMT activation upon treatment with FBS and FGF2 in epicardium explanted cells from WT (Left) or epiBC-KO (Right) E12.5 embryo hearts. (B) SMC differentiation of WT epicardium cultured cells upon stimulation with vehicle (Upper Left), Wnt3A (Upper Right), or TGF-β1 (Lower Left). Stimulation with TGF-β1 was also tested in epicardium cells from epiBC-KO (Lower Right). Phalloidin (red) shows cell morphology, DAPI (blue) indicates nuclear staining, and α-SMA expression by immunostaining is green. (Magnifications: ×200.)
During cardiac development, part of the EPDCs that migrate into subepicardial space and through the myocardium differentiate into SMCs. Differentiation conditions can be recapitulated in vitro upon addition of specific factors, including TGF-β1 (33). As shown in Fig. 5B, treatment with TGF-β1 induces the expression of the SMC marker α-smooth muscle actin (SMA) in WT EPDCs after 8 days in culture. In contrast, β-catenin mutant EPDCs fail to up-regulate α-SMA in all fields analyzed. Conversely, the activation of Wnt canonical pathway in EPDCs by treatment with Wnt3a induces differentiation to SMC as demonstrated by the up-regulation of α-SMA (Fig. 5B), and as smMHC and MLCK (SI Fig. 12), smooth muscle lineage markers (34). Three independent experiments in duplicate were analyzed.
Discussion
β-Catenin in the Epicardium.
During the initial phase of formation of the epicardium, the epicardial mesothelial cells are directly attached to the myocardium. Subsequently, a space filled with extracellular matrix forms between the primitive epicardium and the myocardium. The formation of the subepicardial space is probably caused by a combination of local down-regulation of cell adhesion molecules and enhanced secretion of extracellular matrix proteins (reviewed in ref. 35). Using a double epiBC-KOLacZ transgenic mouse model, we show that proepicardial cells lacking β-catenin colonize the heart and establish the primitive epicardium. This finding suggests that β-catenin does not play essential roles in the formation of the proepicardium and primitive epicardium. However, epicardial cells devoid of β-catenin expression fail to trigger the normal expansion of the subepicardial space. They, furthermore, fail to down-regulate LacZ expression from the Wilms' tumor 1 transgene, suggesting that active β-catenin expression in the embryonic epicardium is required to trigger transcriptional modifications necessary for epicardial development.
Mutations that alter epicardial EMT impair coronary vessel formation (36, 37). We demonstrate that, in contrast to WTLacZ, coexpression of LacZ (Wilms' tumor, epicardial marker) and vimentin (mesenchymal cells) is rarely observed in epiBC-KOLacZ, indicating that the loss of β-catenin compromises the epicardial EMT normally required in vivo for the proper development of the subepicardial space. Furthermore, in mouse embryonic heart, tenascin is transiently expressed at restricted sites closely associated with cell motility during tissue remodeling, and it labels the cells invading the ventricular myocardium (31). We show a significant reduction of LacZ/tenascin costaining in epiBC-KO hearts, thus indicating impairment of EPDCs to migrate through the myocardium. Importantly, although in vivo EMT and cell migration are affected, they are not totally obliterated, demonstrating some degree of EMT activation in β-catenin mutant cells. In addition, our in vitro studies show that both WT and epiBC-KO epicardium-explanted monolayers have a similar capacity to reorganize F-actin fibers, suggesting that, at least in these in vitro conditions, epicardial β-catenin is not essential for EMT stimulation. The apparent discrepancy between in vitro and in vivo data may result from differences in the cellular environment. EpiBC-KO hearts lack a subepicardial space required for in vivo EMT (38), in contrast to the permissive EMT environment provided in the in vitro studies. Other alternative hypothesis can explain a reduced number of epicardial cells within the myocardial wall, including increased cell death, lower cell proliferation, and defective cell migration. In this regard, we show here that, whereas apoptosis is unchanged in epiBC-KO hearts, cell proliferation and cell migration are consistently decreased upon epicardial β-catenin ablation. This result is consistent with the described role of Wnt9a in the promotion of cell proliferation during cardiac cushion mesenchyme development (39).
We report here a failure of remodeling of the primitive coronary plexus into coronary arteries, although some degree of perfusion from the aorta into the vasculature occurs (Fig. 4). The mature coronary vascular system arises from the remodeling of a primitive endothelial vascular plexus (40). The primitive coronary vascular plexus initially has no connections to the aorta and the sinus venosus and remains unaffected in epiBC-KO hearts (SI Fig. 11), further supporting a specific defect in the vascular smooth muscle lineage. Perfusion of the primitive coronary vascular plexus then triggers the remodeling process during which two of the connections to the aorta are transformed into the proximal stems of the two coronary arteries and the adjacent portions of the coronary vascular plexus are transformed into the distal stems and branches of the two coronary arteries (40). In the epiBC-KO mice, the aorta is not connected to coronary arteries but is connected to a primitive vascular plexus via two small vascular tubes that are located at the sides where the two coronary arteries normally take their origin. This finding suggests that in the epiBC-KO mice, the ingrowth of the coronary plexus-derived endothelial sprouts into the aortic sinuses took place but the remodeling of the coronary vascular plexus into the coronary arteries did not occur, probably because of the absence of parietal supporting cells as demonstrated by the lack of a vascular media layer. It is important to note that although the main alteration of the epiBC-KO is observed at the arterial pole, some venous structures are affected as well, with nonvascularized tissue, preferentially in the ventral and left lateral walls of the ventricles. Whether arterially derived signals are involved in the proper development of the venous pole is currently unknown.
Impaired Development of EPDCs and Myocardial Growth Defects.
Several data suggest that the growth of the embryonic and fetal myocardium depends on proper development of the epicardium and EPDCS. Microsurgical ablation of the proepicardium results in deficient formation of the epicardium, deficient coronary vasculature, and a severe growth defect of the compact layer myocardium called “thin myocardium syndrome” (41). Genetic ablation of several epicardial molecules not only affects the development of the epicardium and EPDCs but also causes remarkable growth defects of the compact layer myocardium (13, 41, 42). In the present study, we have found that epicardium-specific deficiency in β-catenin expression does not only disturb the development of EPDCs but additionally leads to hypoplasia of the compact layer myocardium and reduced expansion (ballooning) of the ventricles. This result is explained by the significant reduction of myocardium proliferation in epiBC-KO mice. We demonstrate here that β-catenin is specifically mutated in the epicardium by using the G5-Cre mice, and that β-catenin protein in the myocardium is not modified by the mutation, thus supporting the notion of very different requirements for β-catenin in the myocardium and the epicardial compartments. At the present time, we do not know whether the myocardial hypoplasia of epiBC-KO mice results from nutritional defects secondary to defective formation of the coronary arteries, although the growth defects before vascular remodeling suggest that epicardial β-catenin mutation, at least in part, impairs the release of growth-promoting signals normally generated by EPDCs.
Role of Wnts in Epicardial Development.
Several canonical Wnt ligands and Fz receptors are expressed in a spatially and temporally regulated manner consistent with a function during early heart development. At gastrula stages of mouse embryos, Wnt2a is expressed in the heart-forming fields and it is later restricted to the pericardium (43). Within or lateral to the primitive streak, Wnt3a, Wnt5a, Wnt5b, and Wnt8c are expressed (44–48). At E10.5, murine Wnt8 is detected in the myocardium and four additional members of the Wnt family (Wnt-2b, Wnt5a, Wnt7a and Wnt9a) are expressed in the tubular heart of chicken embryos (reviewed in ref. 49). In addition to the diversity of Wnt ligands, many different Wnt receptors of the Fz family have been detected in the mesoderm of the heart-forming fields, cardiac neural crest cells, and the adult heart, including mouse Fz-2, Fz-4, Fz-9, Xenopus Fz-7, Fz-8, Fz-10a, Fz-10b, or human Fz-1, Fz-2, Fz-7, Fz-8, Fz-9 (reviewed in ref. 49). This large number of different ligands and receptors suggests that Wnt proteins influence diverse aspects of cardiogenesis.
It is important to note that β-catenin is part of the E-cadherin/β-catenin complex that links this complex to the cytoskeleton and plays a role in epithelial polarity (50). β-catenin is a bifunctional protein involved in both cell–cell adhesion through its participation in the adherens junctions and the regulation of gene transcription downstream of Wnt-secreted molecules. Both of these processes are potentially important in epicardial development and may be responsible for the defective coronary vasculature observed in the epiBC-KO mouse. Our previous observations in which lithium chloride (glycogen synthase kinase β inhibitor) treatment enhances vascular tube formation (15) and differentiation of epicardial-derived SMCs (data not shown) suggest a direct involvement of the Wnt-dependent transcriptional pathway in epicardial development. Two recent reports support this hypothesis. Missense mutation of low-density lipoprotein receptor-related protein 6 in humans, which encodes a coreceptor of Wnt results in early coronary artery disease and multiple cardiovascular risk factors (51). In addition, a TCF/Lef-LacZ reporter of β-catenin transcriptional activity has identified both the proepicardium and the epicardium as active targets of Wnt-mediated transcriptional activity (27). Here, we have demonstrated progenitor differentiation into the smooth muscle lineage is largely impaired in the epiBC-KO epicardium. Conversely, stimulation with Wnt enhances epicardial smooth muscle differentiation, further supporting a model in which the actions of β-catenin on coronary remodeling are, at least in part, directly mediated by canonical Wnt stimulation. The source of Wnt that activate coronary differentiation is currently unclear.
Methods
Generation of Epicardium-Restricted β-Catenin Mutants.
Epicardium-restricted β-catenin mutant mice (epiBC-KO) were generated upon crossing the G5-Cre line (15) with the β-catenin floxed mice (BC line) (28). For breeding purposes, BCf/f mice or mice heterozygous for the floxed β-catenin (BCf/+) were crossed with BCf/+ mice that are heterozygous for the epicardial Cre transgene (Cre/+). Primers for genotyping were: G5cre, G5cre1, 5′-ATC TTC CAG CAG GCG CAC CAT TGC CCC TGT-3′, G5cre2, 5′-TGA CGG TGG GAG AAT GTT AAT CCA TAT TGG-3′; BCfl, RM41, 5′-AAG GTA GAG TGA TGA AAG TTG TT-3′, RM42, 5′-CAC CAT GTC CTC TGT CTA TTC-3′. RM41/RM42 results in the amplification of a WT band of 221 bp and a floxed β-catenin band of 324 bp. The presence of the Cre gene is determined by the amplification of a 450-bp band by using Cre1/Cre2 primers. Embryos were obtained from time pregnancies, with plug date defined as E0.5.
Generation of Epicardium-Restricted β-Catenin Mutants in the Context of Wilms' Tumor LacZ.
Epicardium-restricted β-catenin mutant in the context of Wilms' tumor1-LacZ mice, epiBC-KOLacZ, were generated upon crossing epiBC line with the line expressing β-galactosidase under Wilms' tumor 1 promotor (LacZWT1) (29). For breeding purposes, BCf/+: G5cre/+: LacZWT1 mice were crossed with BCf/f mice, to generate BCf/f: G5cre/+: LacZWT1 (epiBC-KOLacZ) mice. Primers for genotyping were: LacZ1, 5′-CGG GTT GTT ACT CGC TCA CAT-3′, LacZ2, 5′-ATG CAG AGG ATG ATG CTC GTG-3′. Primers used for G5cre and BC floxed allele are described above.
Histology, Immunohistochemistry, and β-Galactosidase Staining.
Dissected mouse embryos were fixed in 4% paraformaldehyde overnight, washed with PBS, dehydrated and embedded in paraffin wax, or cryopreserved in 30% sucrose and embedded in OCT. Sections of 10-μm thickness were stained with H&E for histological analysis. For immunostaining, paraffin sections were processed for heat-induced epitope retrieval by using citrate buffer at pH 6.0 and heated for 45 s in the microwave. Antibodies used were as follows: 1:5,000 diluted β-catenin (Abcam, Cambridge, MA), 1:200 diluted vimentin (Sigma, St. Louis, MO), 1:200 diluted tenascin (Chemicon, Temecula, CA), undiluted α-SMA-HRP (DAKO, Glostrup, Denmark), and mouse monoclonal α-smooth muscle actin (Sigma). Elastin fibers were labeled with Van Gieson stain. To visualize the epicardial cells in the epiBC-KOLacZ mouse, embryos were fixed in 2% paraformaldehyde and washed with PBS. β-Galactosidase staining was performed on whole mount with X-Gal solution at 37°C until color developed. Nuclear red fast (Vector, Burlingame, CA) was used as a counterstain.
BrdU Labeling Analysis.
Pregnant, WT, and epiBC mice were i.p.-injected with BrdU (50 mg/kg) 1 h before death. Detection of BrdUra-positive cells was performed by immunohistochemistry with the BrdU Staining Kit from Zymed Laboratories (Carlsbad, CA). Results were obtained after scoring the percentage of BrdU-positive nuclei/total nuclei in five different areas of five histological sections per embryo in a total of three embryos per stage. Three independent WT embryos were compared with three independent mutants.
Whole-Mount PECAM-1 Immunohistochemistry.
Hearts were harvested from WT and epiBC-KO embryos and fixed in 4% paraformaldehyde overnight, dehydrated by methanol series, and stored overnight in 100% methanol at −20°C. Whole-mount immunostaining was performed as described (13, 52). The primary antibody used was rat anti-mouse PECAM-1 (1:200; R&D, Minneapolis, MN). The secondary biotinylated goat anti-rat IgG antibody (1:200; Vector) was followed by Vectastain ABC-peroxidase reagent and diaminobenzidine visualization (Vector). After hearts were photographed and analyzed, they were embedded in paraffin and sectioned. Paraffin sections were then stained with eosin and mounted, and histological sections were analyzed.
Arterial/Vein Morphological Identification.
In the prenatal and postnatal hearts of rat and mice, the coronary arteries have a completely intramyocardial course, whereas the coronary veins have a subepicardial course (53, 54). Both types of coronary vessels have typical branching patterns and are connected either to the aorta (coronary arteries) or the sinus venosus (coronary veins). In PECAM-stained whole-mount hearts, coronary arteries thus were identified on the basis of (i) their intramyocardial course, which was indicated by the fact that these vessels were superficially crossed by myocardial capillaries, and (ii) their branching pattern. Coronary veins were identified on the basis of (i) their subepicardial course, which was indicated by the fact that these vessels run superficially from the myocardial capillaries, and (ii) their branching patterns and connections to the sinus venosus (left superior vena cava/coronary sinus in the mature heart).
Isolation and Culture of Embryonic Epicarial Cells.
Hearts from E12.5 were dissected, and ventricular chambers were placed epicardial side down on culture dishes coated with 1% gelatin. The hearts were cultured in 1:1 mixture of DMEM and medium 199 containing 100 units/ml penicillin, 100 μg/ml streptomycin, 10% FBS, and 2 ng/ml basic fibroblast growth factor (B&D, Franklin Lakes, NJ) as described (33). After 48 h, epicardial cell monolayers were formed and the explanted hearts were removed. For EMT activation experiments, the epicardial explants were cultured for 6 days and the media were changed every 2 days. Phalloidin staining was used to identify motile phenotype. To study SMCs differentiation, after removal of the hearts, the culture medium was replaced with DMEM-PS without growth factors or with recombinant human TGF-β1 (PeproTech, Rocky Hill, NJ) or Wnt3a (B&D) at a final concentration of 50 and 10 ng/ml, respectively. The cells received fresh medium every 3 days and were processed for α-smooth muscle immunostaining at 8 days.
Supplementary Material
Acknowledgments
M.Z. was the recipient of an award from the Spanish Ministerio de Educación y Ciencia and is currently a trainee of the California Institute for Regenerative Medicine. This work was supported by National Institutes of Health Grant HL065484 (to P.R.-L.). Work in J.M.'s laboratory is supported by Deutsche Forschungsgemeinschaft Grant MA 2377/4-1/4.
Abbreviations
- EMT
epithelial-mesenchymal transition
- EPDC
epicardium-derived cell
- Fz
frizzled
- KO
knockout
- G5-Cre
Gata5-Cre
- E(n)
embryonic stage n
- SMC
smooth muscle cell
- PECAM-1
platelet/endothelial cell adhesion molecule-1
- SMA
smooth muscle actin.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0702415104/DC1.
References
- 1.Manner J, Perez-Pomares JM, Macias D, Munoz-Chapuli R. Cells Tissues Organs. 2001;169:89–103. doi: 10.1159/000047867. [DOI] [PubMed] [Google Scholar]
- 2.Viragh S, Challice CE. Anat Rec. 1981;201:157–168. doi: 10.1002/ar.1092010117. [DOI] [PubMed] [Google Scholar]
- 3.Komiyama M, Ito K, Shimada Y. Anat Embryol. 1987;176:183–189. doi: 10.1007/BF00310051. [DOI] [PubMed] [Google Scholar]
- 4.Perez-Pomares JM, Carmona R, Gonzalez-Iriarte M, Atencia G, Wessels A, Munoz-Chapuli R. Int J Dev Biol. 2002;46:1005–1013. [PubMed] [Google Scholar]
- 5.Perez-Pomares JM, Phelps A, Sedmerova M, Wessels A. Dev Dyn. 2003;227:56–68. doi: 10.1002/dvdy.10284. [DOI] [PubMed] [Google Scholar]
- 6.Perez-Pomares JM, Phelps A, Sedmerova M, Carmona R, Gonzalez-Iriarte M, Munoz-Chapuli R, Wessels A. Dev Biol. 2002;247:307–326. doi: 10.1006/dbio.2002.0706. [DOI] [PubMed] [Google Scholar]
- 7.Perez-Pomares JM, Macias D, Garcia-Garrido L, Munoz-Chapuli R. Dev Biol. 1998;200:57–68. doi: 10.1006/dbio.1998.8949. [DOI] [PubMed] [Google Scholar]
- 8.Perez-Pomares JM, Macias D, Garcia-Garrido L, Munoz-Chapuli R. Dev Dyn. 1997;210:96–105. doi: 10.1002/(SICI)1097-0177(199710)210:2<96::AID-AJA3>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 9.Chen TH, Chang TC, Kang JO, Choudhary B, Makita T, Tran CM, Burch JB, Eid H, Sucov HM. Dev Biol. 2002;250:198–207. doi: 10.1006/dbio.2002.0796. [DOI] [PubMed] [Google Scholar]
- 10.Gittenberger-de Groot AC, Blom NM, Aoyama N, Sucov H, Wenink AC, Poelmann RE. Novartis Found Symp. 2003;250:125–34. doi: 10.1002/0470868066.ch8. discussion 134–141 and 276–279. [DOI] [PubMed] [Google Scholar]
- 11.Jenkins SJ, Hutson DR, Kubalak SW. Dev Dyn. 2005;233:1091–1101. doi: 10.1002/dvdy.20393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwee L, Baldwin HS, Shen HM, Stewart CL, Buck C, Buck CA, Labow MA. Development (Cambridge, UK) 1995;121:489–503. doi: 10.1242/dev.121.2.489. [DOI] [PubMed] [Google Scholar]
- 13.Lavine KJ, Yu K, White AC, Zhang X, Smith C, Partanen J, Ornitz DM. Dev Cell. 2005;8:85–95. doi: 10.1016/j.devcel.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Morabito CJ, Dettman RW, Kattan J, Collier JM, Bristow J. Dev Biol. 2001;234:204–215. doi: 10.1006/dbio.2001.0254. [DOI] [PubMed] [Google Scholar]
- 15.Merki E, Zamora M, Raya A, Kawakami Y, Wang J, Zhang X, Burch J, Kubalak SW, Kaliman P, Belmonte JC, et al. Proc Natl Acad Sci USA. 2005;102:18455–18460. doi: 10.1073/pnas.0504343102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCrea PD, Turck CW, Gumbiner B. Science. 1991;254:1359–1361. doi: 10.1126/science.1962194. [DOI] [PubMed] [Google Scholar]
- 17.Butz S, Stappert J, Weissig H, Kemler R. Science. 1992;257:1142–1144. doi: 10.1126/science.257.5073.1142-a. [DOI] [PubMed] [Google Scholar]
- 18.Miller JR. Genome Biol. 2002;3:3001.1–3001.15. doi: 10.1186/gb-2001-3-1-reviews3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uusitalo M, Heikkila M, Vainio S. Exp Cell Res. 1999;253:336–348. doi: 10.1006/excr.1999.4710. [DOI] [PubMed] [Google Scholar]
- 20.Park M, Wu X, Golden K, Axelrod JD, Bodmer R. Dev Biol. 1996;177:104–116. doi: 10.1006/dbio.1996.0149. [DOI] [PubMed] [Google Scholar]
- 21.Schneider VA, Mercola M. Genes Dev. 2001;15:304–315. doi: 10.1101/gad.855601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marvin MJ, Di Rocco G, Gardiner A, Bush SM, Lassar AB. Genes Dev. 2001;15:316–327. doi: 10.1101/gad.855501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tzahor E, Lassar AB. Genes Dev. 2001;15:255–260. doi: 10.1101/gad.871501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lickert H, Kutsch S, Kanzler B, Tamai Y, Taketo MM, Kemler R. Dev Cell. 2002;3:171–181. doi: 10.1016/s1534-5807(02)00206-x. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura T, Sano M, Songyang Z, Schneider MD. Proc Natl Acad Sci USA. 2003;100:5834–5839. doi: 10.1073/pnas.0935626100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naito AT, Shiojima I, Akazawa H, Hidaka K, Morisaki T, Kikuchi A, Komuro I. Proc Natl Acad Sci USA. 2006;103:19812–19817. doi: 10.1073/pnas.0605768103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin L, Cui L, Zhou W, Dufort D, Zhang X, Cai CL, Bu L, Yang L, Martin J, Kemler R, et al. Proc Natl Acad Sci USA. 2007;104:9313–9318. doi: 10.1073/pnas.0700923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R. Development (Cambridge, UK) 2001;128:1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- 29.Moore AW, McInnes L, Kreidberg J, Hastie ND, Schedl A. Development (Cambridge, UK) 1999;126:1845–1857. doi: 10.1242/dev.126.9.1845. [DOI] [PubMed] [Google Scholar]
- 30.Dettman RW, Denetclaw W, Jr, Ordahl CP, Bristow J. Dev Biol. 1998;193:169–181. doi: 10.1006/dbio.1997.8801. [DOI] [PubMed] [Google Scholar]
- 31.Imanaka-Yoshida K, Matsumoto K, Hara M, Sakakura T, Yoshida T. Differentiation. 2003;71:291–298. doi: 10.1046/j.1432-0436.2003.7104506.x. [DOI] [PubMed] [Google Scholar]
- 32.Kattan J, Dettman RW, Bristow J. Dev Dyn. 2004;230:34–43. doi: 10.1002/dvdy.20022. [DOI] [PubMed] [Google Scholar]
- 33.van Tuyn J, Atsma DE, Winter EM, van der Velde-van Dijke I, Pijnappels DA, Bax NA, Knaan-Shanzer S, Gittenberger-de Groot AC, Poelmann RE, van der Laarse A, et al. Stem Cells. 2007;25:271–278. doi: 10.1634/stemcells.2006-0366. [DOI] [PubMed] [Google Scholar]
- 34.Owens GK, Kumar MS, Wamhoff BR. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 35.Munoz-Chapuli R, Perez-Pomares JM, Macias D, Garcia-Garrido L, Carmona R, Gonzalez-Iriarte M. Ital J Anat Embryol. 2001;106:187–196. [PubMed] [Google Scholar]
- 36.Morabito CJ, Kattan J, Bristow J. Curr Opin Cardiol. 2002;17:235–241. doi: 10.1097/00001573-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 37.Mikawa T, Fischman DA. Proc Natl Acad Sci USA. 1992;89:9504–9508. doi: 10.1073/pnas.89.20.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wessels A, Perez-Pomares JM. Anat Rec. 2004;276:43–57. doi: 10.1002/ar.a.10129. [DOI] [PubMed] [Google Scholar]
- 39.Person AD, Garriock RJ, Krieg PA, Runyan RB, Klewer SE. Dev Biol. 2005;278:35–48. doi: 10.1016/j.ydbio.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 40.Poelmann RE, Gittenberger-de Groot AC, Mentink MM, Bokenkamp R, Hogers B. Circ Res. 1993;73:559–568. doi: 10.1161/01.res.73.3.559. [DOI] [PubMed] [Google Scholar]
- 41.Pennisi DJ, Ballard VL, Mikawa T. Dev Dyn. 2003;228:161–172. doi: 10.1002/dvdy.10360. [DOI] [PubMed] [Google Scholar]
- 42.Tevosian SG, Deconinck AE, Tanaka M, Schinke M, Litovsky SH, Izumo S, Fujiwara Y, Orkin SH. Cell. 2000;101:729–739. doi: 10.1016/s0092-8674(00)80885-5. [DOI] [PubMed] [Google Scholar]
- 43.Monkley SJ, Delaney SJ, Pennisi DJ, Christiansen JH, Wainwright BJ. Development (Cambridge, UK) 1996;122:3343–3353. doi: 10.1242/dev.122.11.3343. [DOI] [PubMed] [Google Scholar]
- 44.Jaspard B, Couffinhal T, Dufourcq P, Moreau C, Duplaa C. Mech Dev. 2000;90:263–267. doi: 10.1016/s0925-4773(99)00236-1. [DOI] [PubMed] [Google Scholar]
- 45.Hume CR, Dodd J. Development (Cambridge, UK) 1993;119:1147–1160. doi: 10.1242/dev.119.4.1147. [DOI] [PubMed] [Google Scholar]
- 46.Takada S, Stark KL, Shea MJ, Vassileva G, McMahon JA, McMahon AP. Genes Dev. 1994;8:174–189. doi: 10.1101/gad.8.2.174. [DOI] [PubMed] [Google Scholar]
- 47.Liu P, Wakamiya M, Shea MJ, Albrecht U, Behringer RR, Bradley A. Nat Genet. 1999;22:361–365. doi: 10.1038/11932. [DOI] [PubMed] [Google Scholar]
- 48.Dealy CN, Roth A, Ferrari D, Brown AM, Kosher RA. Mech Dev. 1993;43:175–186. doi: 10.1016/0925-4773(93)90034-u. [DOI] [PubMed] [Google Scholar]
- 49.Brade T, Manner J, Kuhl M. Cardiovasc Res. 2006;72:198–209. doi: 10.1016/j.cardiores.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 50.Aberle H, Schwartz H, Hoschuetzky H, Kemler R. J Biol Chem. 1996;271:1520–1526. doi: 10.1074/jbc.271.3.1520. [DOI] [PubMed] [Google Scholar]
- 51.Mani A, Radhakrishnan J, Wang H, Mani MA, Nelson-Williams C, Carew KS, Mane S, Najmabadi H, Wu D, Lifton RP. Science. 2007;315:1278–1282. doi: 10.1126/science.1136370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lavine KJ, White AC, Park C, Smith CS, Choi K, Long F, Hui CC, Ornitz DM. Genes Dev. 2006;20:1651–1666. doi: 10.1101/gad.1411406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ratajska A, Fiejka E. Anat Embryol (Berl) 1999;200:533–540. doi: 10.1007/s004290050301. [DOI] [PubMed] [Google Scholar]
- 54.Ciszek B, Skubiszewska D, Ratajska A. J Anat. 2007;211:53–56. doi: 10.1111/j.1469-7580.2007.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.