Abstract
Billions of songbirds migrate several thousand kilometers from breeding to wintering grounds and are challenged with crossing ecological barriers and facing displacement by winds along the route. A satisfactory explanation of long-distance animal navigation is still lacking, partly because of limitations on field-based study. The navigational tasks faced by adults and juveniles differ fundamentally, because only adults migrate toward wintering grounds known from the previous year. Here, we show by radio tracking from small aircraft that only adult, and not juvenile, long-distance migrating white-crowned sparrows rapidly recognize and correct for a continent-wide displacement of 3,700 km from the west coast of North America to previously unvisited areas on the east coast. These results show that the learned navigational map used by adult long-distance migratory songbirds extends at least on a continental scale. The juveniles with less experience rely on their innate program to find their distant wintering areas and continue to migrate in the innate direction without correcting for displacement.
Keywords: behavior, bird migration, displacement, orientation, white-crowned sparrow
Birds are well known for their exceptional navigational abilities that can guide migrating birds from breeding to wintering grounds separated by thousands of miles. The nature of the migratory orientation program enabling individual birds to find their species-specific wintering grounds (1) and how this program interacts with external factors, such as ecological barriers and wind displacement, is still not clear (2). In many migratory species, individuals return year after year to precisely the same nesting or wintering sites (3). Their ability to do this is presumed to be based on a learned navigational map (4). In some species, juvenile birds travel alone (5), departing the breeding grounds during their first migration in the fall (6) without the guidance of experienced adults (7). They apparently rely on an innate orientation program for guidance toward their species-specific wintering areas (1), which can apparently steer them very precisely over thousands of kilometers (8). In the absence of guidance by experienced conspecifics, this program is considered of critical importance for juvenile birds to survive the migration and ultimately breed (9).
Displacement experiments that transport birds from one locality to a distant location to record the orientation response to this novel site provide a powerful tool to investigate navigational mechanisms. In a now classic experiment by Perdeck (10), >11,000 starlings, Sturnus vulgaris, were displaced by airplane from The Netherlands to Switzerland during 1955–1957. Whereas displaced juvenile starlings were recovered in a southwesterly direction (equivalent to the normal migratory direction of the species) toward Spain, adult starlings were recovered in a northwesterly direction toward their known wintering sites in Northwest Europe. This was interpreted as showing that juvenile starlings find their species-specific wintering grounds by flying in an inherited direction, whereas adults navigate toward their previously experienced wintering grounds. The 58 adults and 171 juveniles recovered in the same season allowed relatively little information about the accuracy and relative success of the migratory navigation mechanisms involved, because individual tracks cannot be reconstructed, and ring recoveries may suffer from severe biases (e.g., spatial variation in recovery probability; ref. 11).
Although Perdeck's study (10) forms the basis for understanding orientation and navigation of migratory birds, it is unclear whether we can generalize to other species from studying starlings, which are social, short-distance migrants. Very few other displacement experiments involving free-flying juvenile migrants have been performed. Band recoveries of displaced juvenile teals, Anas crecca, and starlings failed to show signs of compensation (12, 13), and those of sparrowhawks, Accipiter nisus, (14) showed only a weak tendency, if any. The orientation behavior as observed by satellite telemetry of displaced juvenile white storks, Ciconia ciconia, a highly social, long-distance migrant, is difficult to interpret regarding the presence or absence of compensation (15). In contrast, the recoveries of experienced adults generally showed compensation for displacements in starlings and white-crowned sparrows (10, 16). However, more recently, a displacement study by Åkesson et al. (17) of caged individual white-crowned sparrows suggested that even juvenile migrants may be able to correct for displacements, and a review of previous displacement experiments with juvenile migrants tested in cages came to a similar conclusion (18).
Here, we extend our knowledge of avian navigational capacities by conducting a continent-wide displacement experiment during the fall migration of the long-distance migrating race of the white-crowned sparrow, Zonotrichia leucophrys gambelii (19). These sparrows face much more demanding navigational challenges during long-distance migrations than the species studied in previous displacement experiments involving free-flying juvenile migrants. Although individuals congregate in large numbers at stopover sites during migration, actual (nocturnal) migratory flight is thought to be undertaken individually (20). Homing toward previously visited wintering grounds in adult birds has been demonstrated from distances similar to the displacement distance in this study by using band recoveries (16). In the first study, Mewaldt translocated white-crowned sparrows wintering in San Jose, CA, to the gulf coast (Louisiana), and in a second year to the east coast (Maryland). In both years, observed banded individuals returned to San Jose in the winter after each displacement. When translocating birds even further, to Korea, no birds returned (21). The single band recovery from spring of a bird apparently en route from the east coast toward the normal breeding grounds indicated that the birds did not return to the normal wintering grounds until after breeding, but otherwise information on when and how the animals returned is lacking.
We alleviate the main problem of earlier field experiments, where the behavior after displacement was inferred from later band recoveries and extend our observational abilities during migratory flight by radio-tracking individual migrants. This methodological advance is critical for observing decisions during the early phase of the birds' navigation shortly after displacement. Although it is possible to test migratory orientation in cages, a recent review documented a number of effects that could potentially confound the orientation response observed in “Emlen funnel” experiments compared with free-living birds (22). Furthermore, conducting experiments in wild, unrestrained birds is necessary to test the results from captive experiments that suggest that even migratory juvenile migrants may be able to correct for displacements (17, 18). Tracking the movements of animals for distances of more than a few tens of kilometers generally require radio-tracking from the air or space (23). We used a small aircraft (24) to track the movements of Gambel's white-crowned sparrows after displacement from Sunnyside, WA, to Princeton, NJ (Fig. 1). This method allowed us to record the flight tracks up to 122 km after release as well as to investigate individual behavior in detail.
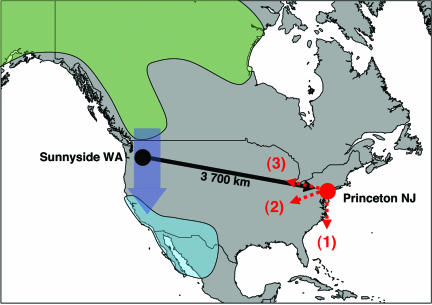
Fig. 1.
The displacement of white-crowned sparrows, Zonotrichia leucophrys gambelii, from Sunnyside, WA, to Princeton, NJ. Possible migration routes after release at Princeton are shown as normal migration direction (1), toward wintering area (2), and back toward capture site (3). Breeding area (green), wintering area (cyan), and normal migration route (blue) are indicated.
Results
The recorded tracks of adult (n = 15) and juvenile (n = 15) birds after displacement are clearly different (Fig. 2). On average, adult birds moved west-southwest toward their expected wintering grounds in the southwest U.S. and northwest Mexico (Fig. 3). By contrast, juvenile birds continued in their expected southerly migration direction (25) (Fig. 3). In this analysis, we used the last observed stopover position of a bird as a measure of its migration direction and included only birds that moved >25 km radial distance from the release site. We detected a significant difference in the track directions between adult and juvenile birds (Watson–Williams F test: n = 8 (adults), 9 (juveniles), F = 52.01, df = 1, 15, P < 0.001; Fig. 3). Relaxing the radial distance criterion and including the final positions of all birds that moved >10 km from the release site yielded similar results (Watson–Williams F test: n = 14, 11, F = 37.15, df = 1, 23, P < 0.001).
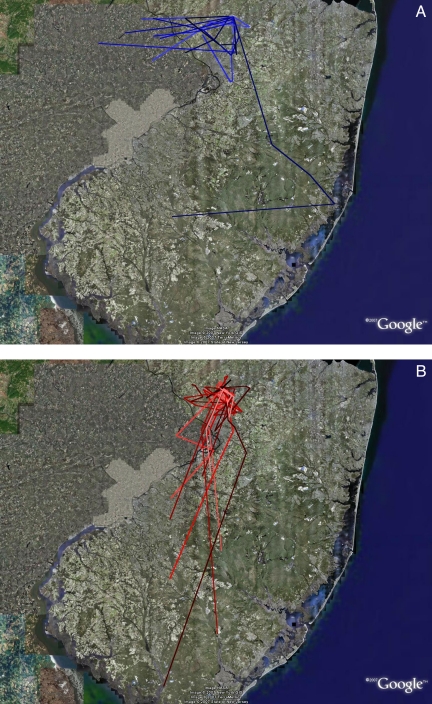
Fig. 2.
Tracks of adult (blue) and juvenile (red) birds released at Princeton after displacement from Sunnyside, WA. Adults and juveniles were released at sites 7.3 km apart. Images were created by using Google Earth mapping service.
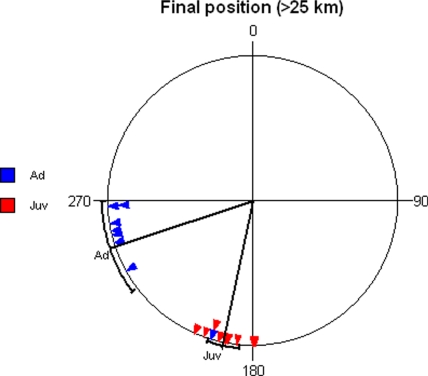
Fig. 3.
Direction from the release site to the last observed position. Only positions >25 km from the release site are included. Adults are shown in blue, and juveniles are shown in red. For adults, the mean vector is α = 252 ± 18° and r = 0.931 (Z = 6.94, n = 8, P < 0.001, Rayleigh test), and for juveniles, it is α = 192 ± 6° and r = 0.99 (Z = 8.82, n = 9, P < 0.001, Rayleigh test). Mean directions and 95% confidence intervals are shown for each group, respectively. The single adult with a southerly orientation probably drifted initially in strong northwesterly winds.
One adult bird appeared to fly in a southerly direction similar to that chosen by most juveniles (Fig. 3). However, this bird was the only individual that flew in strong (42 km h−1) winds. Despite its westerly heading (26), the northwesterly winds blew it toward the southeast. During its subsequent flight under calm wind conditions, this individual continued on a westerly path.
The average track direction of adult birds that moved >25 km differed significantly from the direction toward the capture site (280° in Fig. 1; P < 0.01, confidence interval test) but not from the direction toward the centre of the wintering grounds at 115° W, 30° N (252°; P > 0.05, confidence interval test), and was also significantly different from the normal migration direction (180°; P < 0.01, confidence interval test). The average track direction of juvenile birds differed from both the direction toward the capture site (280°; P < 0.01, confidence interval test) as well as from the direction toward the expected wintering ground (252°; P < 0.05, confidence interval test). The juveniles' average direction also differed significantly from due south (P < 0.01, confidence interval test).
The birds made rapid decisions about their migratory orientation after displacement. On average, individuals took <3 days to move >5 km from the release site. The final positions (>25 km radial distance) included in the study were reached an average of 7 days after release, and no bird was followed for >11 days.
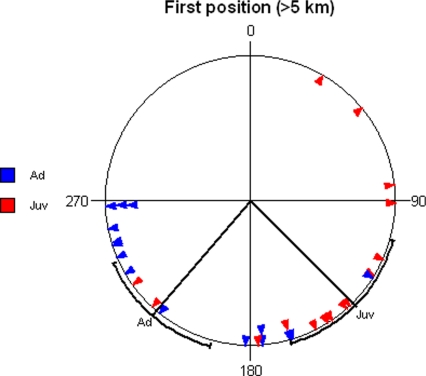
Radio-tracking data indicated that all birds took off individually. A larger spread was observed in directions of the first position of an individual found grounded >5 km from the release site than of their last position (adults: r = 0.70 versus r = 0.93; juveniles: r = 0.631 versus r = 0.99, Figs. 3 and 4). There was a significant difference between adult and juvenile birds in the directions of the first position >5 km from the release site (Watson–Williams F test: n = 15, 17, F = 17.83, df = 1, 28, P < 0.001; Fig. 4). For adults, the average direction (Fig. 4) did not significantly differ from the one later observed (Watson–Williams F test: n = 8, 15, F = 2.99, df = 1, 21, P = 0.10; Figs. 3 and 4). In juveniles, this direction (Fig. 4) differed from the one observed later (Watson–Williams F test: n = 9, 17, F = 6.73, df = 1, 24, P = 0.016; Figs. 3 and 4). Because the birds left the release sites individually and later changed their direction, we conclude that each bird's final direction is independent of those of the other birds.
Fig. 4.
Direction from release site to the first observed position >5 km from the release site. Adults are shown in blue, and juveniles are shown in red. For adults, the mean vector is α = 221 ± 25° and r = 0.698 (Z = 7.31, n = 15, P < 0.001, Rayleigh test), and for juveniles, it is α = 135 ± 29° and r = 0.631 (Z = 5.96, n = 15, P = 0.002, Rayleigh test). Mean directions and 95% confidence intervals are shown for each group, respectively.
Discussion
The experience-dependent reaction to displacement lends support to Perdeck's paradigm of migratory bird orientation (10). A navigation system presumed to be based on experience has also been shown in caged adult migrants (27, 28). However, previous experiments that tested migratory orientation in cages suggested that juveniles are able to compensate for the displacement. Because adult birds are able to compensate, juvenile birds are supposedly in the process of constructing a navigational map along the migratory route (18). The difference between our study and previous ones could be due to the use of orientation cages, where birds remain within a cage while attempting to depart for migration (22), or alternatively, it may be that our juvenile birds were displaced outside the maximum range of their map that is under construction.
Our study is the first to document age-specific reorientation movements after a continent-wide displacement and within the first hours upon release. It seems highly unlikely that the adult birds used path integration during the displacement to reorient. A path integration system becomes very imprecise over the long distances our birds were displaced.
The results provide insights into the nature of navigation during long-distance migration. On the basis of one migratory journey from Alaska to southwest North America, white-crowned sparrows obtain information that allows them to reach their wintering ground from an area that their normal migratory route does not encompass. Gaining and retaining such information is presumably adaptive because it would allow them to reach their wintering grounds after natural displacements. Juveniles, on the other hand, continue in the species-specific migratory direction after displacement. This suggests that, during migration, homing to a known location is triggered only by reaching that final destination and, possibly, after spending time in it. Because the juvenile birds were caught en route, neither stopovers nor partial travel on the southerly migratory route in the first year seems to trigger homing back to the migratory route. Furthermore, our experiment indicates that the navigational map of adult white-crowned sparrows encompasses at least the continental U.S. and allows them to correct for vast displacements very rapidly (within days, at least), hinting that migratory birds may possess a global navigational map. Even though adult white-crowned sparrows return to a specific winter home range, at this large scale the “map” may just provide a bird with a sign on a gradient, e.g., letting the bird know whether it is east or west of its goal, as observed previously by Åkesson et al. (17).
Currently, magnetic cues seem the most likely candidates for the basis of a map stretching this far (29). However, the small difference in geomagnetic intensity across longitudes in North America makes magnetic intensity an unlikely candidate for distinguishing between the east and the west coast, and celestial or olfactory cues cannot be ruled out (30).
In the past, studies of navigation in passerine birds have generally been restricted to the laboratory because of limitations on field-based study (30). We have demonstrated that it is possible to study the navigation behavior of small migratory birds in the field and provide insights into their behavior. These results demonstrate that the ability to track small animals continuously is essential to gain an understanding of the behavior of free-living migrants. Ultimately, a complete understanding of the mechanisms used by adults and juvenile passerine birds will require a global tracking system for small animals (23).
Materials and Methods
Experimental Animals.
The white-crowned sparrows Zonotrichia leucophrys gambelii used for the experiment were captured in mist-nets on the afternoon of 14 September and the morning of 15 September 2006 at a historic stopover site in central Washington State (Sunnyside, WA; 46.321° N, 120.005° W). The birds were transported by car to Seattle, WA, where they were housed overnight in 3 × 3 × 2-m aviaries. On 16 September, the birds were transported by commercial airplane to Newark, NJ, in the pet area. This compartment is controlled for temperature and pressure maintaining cabin levels and has no windows but some continuous dim lights. Immediately upon arrival, the birds were transported by car to Princeton, NJ, where they were housed in groups of three in laboratory bird cages until released. Individuals of the same age class were kept together in the cages. Throughout their stay in captivity, the birds had ad libitum access to food and water, except when being transported by car or airplane, when only fruit and seeds were available. All birds were healthy and appeared in good migration condition at release, i.e., all except one bird who possessed very little s.c. fat (fat score of all birds at release ranged between 1 and 4, except one with a fat score of 0).
New Jersey is within the species' full range (including other subspecies), which includes most of the U.S. Overall, the expected flight routes after release are also within the species' distribution limits, so we expected the translocated birds to find suitable habitat for survival regardless of which alternative migration route they chose.
Release Procedure.
The birds were released at Princeton on 17, 20, and 21 September. On each day, five adults and five juveniles were released. To control for any potentially confounding influence from individuals of the other age class, adult and juveniles were released in similar habitat at two locations that were ≈7 km apart. This distance is small compared with the scale at which the birds' migratory movements were followed, and, thus, local effects on the birds' orientation could be excluded. Juvenile birds were released at the Princeton University Stony Ford field station (40.354° N, 74.720° W) and adult birds at Princeton Airport (40.398° N, 74.657° W). All birds were present at noon at the release site on the day of release, allowing the birds to get accustomed to the new environment before any possible initiation of migration at night.
Tracking.
Before release, a 0.5-g radio transmitter attached to a small piece of cotton was glued between the birds' shoulders with eyelash adhesive to skin from which the feathers had been removed. It is highly unlikely that birds with radiotransmitters are not able to use their magnetic compass. Radio transmitters produce a very weak magnetic field in the very short period they emit a signal (≈16 msec/sec). The radio field effect emitted by our transmitters was measured as <1,000 nT 1 cm from the transmitter and had no discernable effect on the earth's magnetic field 2 cm from the transmitter (measured by using a hand-held sampler including a 2-axis magnetometer with a resolution of 100 nT). A 2-cm distance corresponds approximately to the distance from the radio transmitter to a sparrow's head in a live, alert bird (but the sparrow's head could be closer to the transmitter during sleeping). Furthermore, earlier studies have shown clear orientation (including magnetic) in birds fitted with radio transmitters (31).
After release, the birds' movements were monitored by ground crews using tracking vehicles. When birds moved away from their release sites (usually during the night), they were radio-tracked from a small aircraft. In several cases, individual take-offs were missed because of the large number of birds being tracked; these birds were later located during daytime tracking flights from the aircraft. To ease fast scanning for transmitters, only 10 different radio frequencies were used for the 30 transmitters. For each frequency, we used three easily distinguished pulse rates. All transmitters emitted signals in the range of 164–165 MHz, and frequencies within that range were randomly allocated among adults and juveniles. All birds released on the same day had transmitters with different frequencies but the same pulse rate.
Flight paths were reconstructed from GPS waypoints recorded from birds located to the nearest 200 m. The birds were located from small airplanes (Cessna 152 or 170) by using a two-antenna setup on the aircraft. The antennas were attached in horizontal polarization to the wing struts and pointed down at an angle of 60° perpendicular to the airplane's flight path. We listened to signals on both antennas simultaneously using two AOR AR8200 receivers that scanned through the 10 respective channels, switching between channels every 3 seconds. The audio outputs of both receivers were fed into a mono input of an electronically noise-reduced aviation headset. When a signal was detected, the scanning was halted, and we determined which antenna received the strongest signal by subsequently turning down the volume on each receiver. The pilot then circled into the direction of the strongest signal until the signal strengths from both receivers were equal and appeared strongest. From test trials with stationary transmitters, we determined that the location precision by using this method is <200 m radius.
Approval.
The experiments were approved by Princeton University's Institutional Animal Care and Use Committee and by the New Jersey and Washington State Fish and Wildlife Services.
Acknowledgments
We are grateful to Jason Davis, Alex Coverdill, and Gang Wang for help with field work in Washington and to Rachel Muheim for initial discussions on the topic. R.A.H. was supported by Marie Curie Outgoing International Fellowship MOIF-CT-2005-021508, administered by the University of Leeds. M.W. acknowledges support by the National Geographic Society and the National Science Foundation, Small Grants for Exploratory Research, Integrative Organismal Biology (IOB) 0528881 and the MIGRATE Research Coordination Network, IOB 0541740.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Sutherland WJ. Nature. 1988;334:471–472. [Google Scholar]
- 2.Alerstam T. Science. 2006;291:300–303. doi: 10.1126/science.291.5502.300. [DOI] [PubMed] [Google Scholar]
- 3.Berthold P. Bird Migration: A General Survey. New York: Oxford Univ Press; 2001. [Google Scholar]
- 4.Wallraff HG. In: Orientation in Birds. Berthold P, editor. Basel: Birkhäuser; 1991. pp. 128–165. [Google Scholar]
- 5.Larkin RP. In: Avian Navigation. Papi F, Wallraff HG, editors. Berlin: Springer; 1982. pp. 28–37. [Google Scholar]
- 6.Pulido F. Bioscience. 2007;57(2):165. [Google Scholar]
- 7.Couzin ID, Krause J, Franks NR, Levin S. Nature. 2005;433:513–516. doi: 10.1038/nature03236. [DOI] [PubMed] [Google Scholar]
- 8.Thorup K, Rabøl J. J Avian Biol. 2001;32:111–119. [Google Scholar]
- 9.Alerstam T, Hedenström A. J Avian Biol. 1998;29:343–369. [Google Scholar]
- 10.Perdeck AC. Ardea. 1958;46:1–37. [Google Scholar]
- 11.Crissey WF. J Wildl Manag. 1955;19:75–83. [Google Scholar]
- 12.Perdeck AC. Ardea. 1967;55:194–202. [Google Scholar]
- 13.Wolff WJ. Ardea. 1970;58:131–141. [Google Scholar]
- 14.Drost R. Proc Int Ornithol Congr (Rouen 1938) 1938;9:502–521. [Google Scholar]
- 15.Chernetsov N, Berthold P, Querner U. J Exp Biol. 2004;207:937–943. doi: 10.1242/jeb.00853. [DOI] [PubMed] [Google Scholar]
- 16.Mewaldt LR. Science. 1960;46:941–942. doi: 10.1126/science.146.3646.941. [DOI] [PubMed] [Google Scholar]
- 17.Åkesson S, Morin J, Muheim R, Ottosson U. Curr Biol. 2005;15:1591–1597. doi: 10.1016/j.cub.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 18.Thorup K, Rabøl J. Behav Ecol Sociobiol. 2007;61:825–841. [Google Scholar]
- 19.Chilton G, Baker MC, Barrentine CD, Cunningham MA. In: The Birds of North America. No 183. Poole A, Gill F, editors. Philadelphia: Acad Nat Sci; 1995. and Am Ornithol Union, Washington, DC. [DOI] [Google Scholar]
- 20.DeWolfe BB, West GC, Peyton LJ. Condor. 1973;75:43–59. [Google Scholar]
- 21.Mewaldt LR, Cowley LT, Won P-O. Auk. 1973;90:857–861. [Google Scholar]
- 22.Muheim R, Moore FR, Phillips JB. J Exp Biol. 2006;209:2–17. doi: 10.1242/jeb.01960. [DOI] [PubMed] [Google Scholar]
- 23.Wikelski M, Kays R, Kasdin J, Thorup K, Smith JA, Cochran WW, Swenson GW., Jr J Exp Biol. 2007;210:181–186. doi: 10.1242/jeb.02629. [DOI] [PubMed] [Google Scholar]
- 24.Michener MC, Walcott C. J Exp Biol. 1967;47:99–131. doi: 10.1242/jeb.47.1.99. [DOI] [PubMed] [Google Scholar]
- 25.Cortopassi AJ, Mewaldt LR. Bird-Banding. 1965;36:141–165. [Google Scholar]
- 26.Cochran WW, Kjos CJ. Illinois Nat Hist Surv Bull. 1985;33:297–330. [Google Scholar]
- 27.Munro U, Munro JA, Phillips JB. Naturwissenschaften. 1997;84:26–28. [Google Scholar]
- 28.Munro U, Munro JA, Phillips JB, Wiltschko W. Austral J Zool. 1997;45:189–198. [Google Scholar]
- 29.Bingman VP, Cheng K. Ethol Ecol Evol. 2005;17:295–318. [Google Scholar]
- 30.Wallraff HG. Avian Navigation: Pigeon Homing as a Paradigm. Berlin: Springer; 2005. [Google Scholar]
- 31.Cochran WW, Mouritsen H, Wikelski M. Science. 2004;304:405–408. doi: 10.1126/science.1095844. [DOI] [PubMed] [Google Scholar]