Abstract
Accelerating rates of species extinction have prompted a growing number of researchers to manipulate the richness of various groups of organisms and examine how this aspect of diversity impacts ecological processes that control the functioning of ecosystems. We summarize the results of 44 experiments that have manipulated the richness of plants to examine how plant diversity affects the production of biomass. We show that mixtures of species produce an average of 1.7 times more biomass than species monocultures and are more productive than the average monoculture in 79% of all experiments. However, in only 12% of all experiments do diverse polycultures achieve greater biomass than their single most productive species. Previously, a positive net effect of diversity that is no greater than the most productive species has been interpreted as evidence for selection effects, which occur when diversity maximizes the chance that highly productive species will be included in and ultimately dominate the biomass of polycultures. Contrary to this, we show that although productive species do indeed contribute to diversity effects, these contributions are equaled or exceeded by species complementarity, where biomass is augmented by biological processes that involve multiple species. Importantly, both the net effect of diversity and the probability of polycultures being more productive than their most productive species increases through time, because the magnitude of complementarity increases as experiments are run longer. Our results suggest that experiments to date have, if anything, underestimated the impacts of species extinction on the productivity of ecosystems.
Keywords: biodiversity, ecosystem function, extinction, productivity, sampling effect
Over the past two decades, there has been increasing interest in understanding how species diversity affects the functioning of ecosystems (1–3). Research in this area has often been justified on grounds that (i) loss of biological diversity ranks among the most pronounced changes to the global environment (4), and (ii) reductions in diversity and corresponding changes in species composition could alter fluxes of energy and matter that underlie important services that ecosystems provide to humanity (e.g., production of food, pest/disease control, water purification, etc.; see refs. 5 and 6). Seminal experiments in this field focused on characterizing how plant species richness affects primary production (e.g., refs. 7 and 8), and generally showed that reducing the number of herbaceous plant species leads to less efficient use of soil nutrients and lower production of biomass (9–11). These experiments not only articulated the hypothesis that species loss can affect important ecological processes, they spawned a decade of experiments in which researchers manipulated the diversity of a wide variety of organisms to see how this impacts the functioning of many different ecosystems (12).
Within the past year, several formal metaanalyses have attempted to synthesize the results of >150 diversity-function experiments that have been performed to date (13–15). These metaanalyses have shown that, when averaged across all species used in an experiment, species loss tends to reduce the efficiency by which communities capture biologically essential resources and convert those into new biomass. This pattern has proven to be strikingly consistent across studies of bacterial, fungal, plant, and animal assemblages inhabiting terrestrial, freshwater, and marine ecosystems. But Cardinale et al. (14) also showed that resource capture and biomass production by the communities used in experiments begin to saturate at low levels of richness and that diverse communities seldom capture more resources or produce more biomass than their most productive species.
The fact that the most diverse communities produce no more biomass than their most productive species has led some to argue that the results of experiments are best explained by so-called “selection effects” (SE) (sometimes referred to as selection-probability or sampling effects; see refs. 14 and 16–18). Selection effects are species-specific impacts on biomass that are thought to occur when the most productive species are more likely to be included in and come to dominate the biomass of species rich polycultures. This interpretation has been controversial, in part, because it contradicts a common assumption that biodiversity will impact the production of biomass through species complementarity. Complementarity occurs when species exhibit various forms of niche partitioning that allow them to capture resources in ways that are complementary in space or time (2), or when interspecific interactions enhance the capture of resources by species when they are together (19, 20). Cardinale et al. (14) interpreted the results of their metaanalysis as evidence that complementarity may be weak or rare compared with selection effects; however, two things limited this conclusion. First, the data needed to discriminate among these alternative contributions to diversity effects had rarely been presented in studies; thus, the relative contributions of single vs. multiple species to biomass production have been interpreted indirectly from existing patterns rather than from any direct tests. Second, many, if not most, of the experiments reviewed by recent metaanalyses have been short-term, run for only a small number of growing seasons or a few generations of the focal organisms (9). Of those experiments that have run for multiple generations or growing seasons, most find that diversity effects and their underlying mechanisms change through time as species interactions structure experimental communities (21–26). This raises the possibility that past interpretations of diversity-function relationship have been heavily influenced by studies that have yet to allow population dynamics.
Here, we present results of a new metaanalysis that show how the effects of plant species richness on biomass production change through time and how these patterns are influenced by processes involving individual species (selection effects) verses multiple species [complementarity effects (CE)]. We gathered information on the total biomass of plants in the most diverse polyculture and the biomass of each species in monoculture for 44 independent experiments in which the authors directly manipulated the richness of three or more species as an independent variable (see Methods). For each date on which biomass was measured, we characterized the effect of plant richness on total plant biomass, using two log ratios that measure different aspects of the diversity effect. LRnet gives the net effect of plant richness on biomass as the proportional difference between the mean value of biomass in the most diverse polyculture and the mean value of all species grown in monoculture. In contrast, LRtrans tests whether diverse polycultures produce any more biomass than the single most productive species (called “transgressive” overyielding). This metric is calculated as the proportional difference between the mean biomass of the most diverse polyculture and the mean biomass of the species that achieves the highest biomass in monoculture. We first analyze each log ratio as a function of the duration of the experiments to examine how diversity effects change through time. Then, for a subset of studies with sufficient data, we use the statistical method of Loreau and Hector (27) to partition the net effect of diversity into two distinct components—one due to selection effects, and a second due to complementarity effects. This allows us to examine how changes in the net diversity effect through time are driven by changes in the contributions of single vs. multiple species to biomass production.
Results
Summary of Diversity Effects.
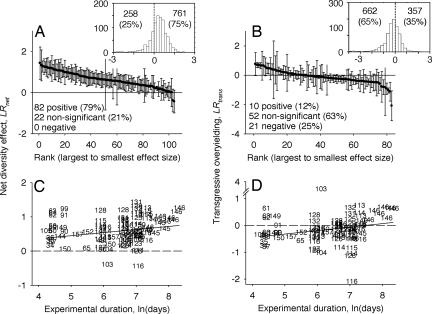
The net effect of plant richness on plant biomass was significantly positive for 82 of 104 estimates of LRnet (Fig. 1A), and averaged 0.54 across all dates and experiments [95% confidence interval (C.I.) = 0.45 to 0.64]. This indicates that the most diverse polycultures used in experiments have achieved 1.7 times the biomass of the average species monoculture. In contrast, the grand mean for all 96 estimates of LRtrans was −0.13 (95% C.I. = −0.25 to −0.002), which indicates that the most diverse polycultures have achieved an average 0.88 times the biomass of the most productive monoculture. Of the 83 estimates of LRtrans where experiments had sufficient replication of monocultures (mean n = 3) to estimate confidence intervals, 10 were significantly more than zero, 52 were not different from zero, and 21 were significantly less than zero (Fig. 1B).
Fig. 1.
Effects of plant species richness on the production of plant biomass. (A) Net effects of diversity on biomass, LRnet, expressed as the log ratio of biomass in the most diverse polyculture to the average of all species grown in monoculture. (B) A test for transgressive overyielding, LRtrans, which is the log ratio comparing the mean biomass of polycultures with the mean biomass of the single most productive species. Main plots give the mean ± 95% confidence interval for individual estimates of LRnet (n = 104) and LRtrans (n = 83) ranked from largest to smallest effect size for all dates in all experiments. (A and B Insets) Frequency distributions for LRnet and LRtrans (x axis) for 1,019 individual polyculture plots (counts on y axis) where biomass could be matched to the same species in monoculture. (C and D) Shown are how LRnet and LRtrans change through time. Each data point is a single estimate from one experiment, but note that some experiments contribute multiple data points [numbers correspond to experiments listed in supporting information (SI) Datasets 1 and 2].
Results in Fig. 1 A and B indicate that polycultures have generally achieved more biomass than the average species, but not more than the most productive species. However, it is important to note that tests described above focus on single points-in-time, ignoring that the most productive monoculture can change through time. Of the 17 experiments with time-series data, the probability that the same species was the most productive monoculture on two consecutive dates was just 0.25 (14 of 58 intervals). This raises the possibility that no single species can produce more biomass than a diverse polyculture when a longer time span is considered. To examine this possibility, we averaged the biomass of each species monoculture across all dates on which data were collected in an experiment and compared the species with the highest average to the mean biomass of the most diverse polyculture for the same interval. For this analysis, LRtrans was not different from zero (t = 0.25, P = 0.81). Five experiments had values that were significantly positive, seven were not different from zero, and five were significantly negative.
When LRnet and LRtrans are calculated on an experiment-wide basis, they are not guaranteed to compare the biomass of a polyculture to the biomass of those same species in monoculture. This is because in ≈25% of experiments, the most diverse polyculture is composed of fewer species than the total species pool used to randomly select taxa. For these experiments, a bias can result when polycultures are compared with species that are not part of that community. Unfortunately, the data published in most papers are summarized in a form that does not allow us to distinguish which species were seeded in which polycultures. However, we were able to obtain the original datasets from 26 of 44 experiments, and we used estimates of biomass from the final dates of each study to calculate diversity effects for 1,019 individual experimental units (field plots, greenhouse pots, etc.) that contained ≥2 species. When each polyculture plot was matched to the same species in monoculture, LRnet remained significantly positive with a mean of 0.26 (95% C.I. = 0.21 to 0.31, t = 10.07, df = 1,018, P < 0.01). LRtrans remained significantly negative with a mean of −0.30 (95% C.I. = −0.25 to −0.35, t = −11.52, df = 1,018, P < 0.01). Nearly 75% of polycultures achieved greater biomass than the mean of those same species in monoculture, whereas just 35% of polyculture plots yielded more biomass than their most productive species (Fig. 1 A and B Insets). Thus, despite an improved matching of species mixtures with their respective monocultures, results indicate that polycultures achieve more biomass than their average species, but less than their most productive species.
Diversity Effects Through Time.
Analyses of time trends revealed that both the net effect of plant richness on plant biomass and the probability of transgressive overyielding tended to increase with the duration of an experiment (Fig. 1 C and D and Table 1). When we used each of 17 individual experiments that have measured biomass on two or more sampling dates as the subjects in a repeated-measures ANOVA, we found that both LRnet and LRtrans increased with time (Table 1, “within experiments”). Because these 17 experiments represent a limited subset of studies, we performed a second analysis that takes advantage of the broader array of data to determine whether trends are more general across different study systems. For this analysis, we used a mixed model ANOVA with experiment included as a random factor to analyze each diversity effect as a function of the number of days the experiment had run. Consistent with the first analysis, LRnet and LRtrans increased as the duration of experiments increased (Table 1, “across experiments”). Although LRnet was positive across the entire temporal range of experiments performed to date, LRtrans was negative across most of the range (Fig. 1D). This is not to say that transgressive overyielding has not occurred in some experiments (note studies with LRtrans > 0 in Fig. 1D, and see Fig. 1B). Rather, the trend in Fig. 1D simply suggests that it takes ≈5 years before the most diverse polyculture exhibits transgressive overyielding in the average experiment. Of the studies that met the criteria for inclusion in our analyses, only Tilman's (22) Biodiversity II experiment performed at Cedar Creek has >5 years of data (labeled “146” in Fig. 1). To assess whether conclusions were overly influenced by this study, we examined the residuals and leverage of each experiment on model fit and found no evidence of statistical outliers. We also ran models with and without this experiment and found that exclusion did not alter model fit (based on AIC and a log-likelihood test). This finding and the “within” and “across” experiment analyses giving consistent results indicate that conclusions about time trends are robust.
Table 1.
Results of statistical analyses showing how the effects of plant species richness on plant biomass change through time
| Dependent variable | n | F | Pr | Intercept |
Duration ln, days |
||
|---|---|---|---|---|---|---|---|
| Est ± SE | 95% C.I. | Est ± SE | 95% C.I. | ||||
| LRnet | |||||||
| Within experiments | 81 | 36.50 | <0.01 | −1.25 ± 0.30 | −1.86 to −0.64 | 0.27 ± 0.04 | 0.18 to 0.36 |
| Across experiments | 104 | 7.44 | <0.01 | 0.00 ± 0.20 | −0.41 to 0.41 | 0.09 ± 0.03 | 0.02 to 0.16 |
| LRtrans | |||||||
| Within experiments | 75 | 22.10 | <0.01 | −1.88 ± 0.38 | −2.65 to −1.11 | 0.27 ± 0.06 | 0.15 to 0.38 |
| Across experiments | 96 | 8.64 | <0.01 | −0.97 ± 0.29 | −1.56 to −0.39 | 0.13 ± 0.05 | 0.04 to 0.23 |
| CE | |||||||
| Within experiments | 32 | 6.97 | 0.01 | −332 ± 178 | −696 to 31 | 73 ± 28 | 17 to 130 |
| Across experiments | 47 | 13.28 | <0.01 | −177 ± 87 | −352 to −2 | 56 ± 15 | 25 to 86 |
| SE | |||||||
| Within experiments | 32 | 1.62 | 0.21 | 208 ± 135 | −68 to 484 | −27 ± 21 | −70 to 16 |
| Across experiments | 47 | 0.61 | 0.44 | 131 ± 82 | −34 to 297 | −11 ± 14 | −40 to 18 |
Each of four dependent variables on the left side was modeled in two ways. For “within experiment” analyses, each experiment that measured the dependent variable through time was used as the subject in a repeated measures mixed model ANOVA. For the “across experiment” analyses, each experiment was accounted for as a random effect in a mixed model ANOVA. The independent variable in both was days since the start of the experiment. Parameter estimates (Est) and confidence intervals (C.I.) for the intercept and effect of experimental duration are given in the right side of the table. n, number of experiments; F, F value from the mixed model ANOVA; Pr, P value from the mixed model ANOVA.
Factors Contributing to Diversity Effects.
Loreau and Hector (27) devised a statistical method to differentiate two general mechanisms that contribute to the net diversity effect—selection effects (SE) and complementarity effects (CE) (see Methods). SE represent changes in polyculture biomass that can be attributed to the productivity of individual species, such as those that can occur when the most productive species come to dominate the biomass of diverse polycultures. In contrast, CE represent that portion of a diversity effect that cannot be attributed to any single species. Although positive values of CE are often taken as evidence for “niche complementarity” (e.g., resource partitioning or positive species interactions), CE actually represent the balance of all forms of niche partitioning that might influence biomass and all forms of indirect and nonadditive species interactions (28). Although this makes it impossible to equate CE to any single biological mechanism, CE and SE do quantify ecologically distinct factors that contribute to diversity effects (single vs. multispecies processes).
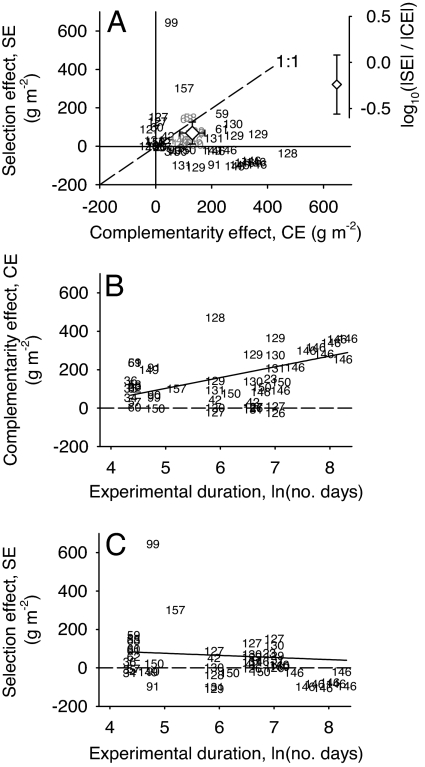
We obtained 47 estimates of SE and CE, either from published tables or figures (nine experiments) or from our own analyses of original datasets (15 experiments). The net effect of diversity on biomass in nearly every experiment was jointly explained by some combination of SE and CE rather than being driven by any one mechanism (Fig. 2A). When averaged across all experiments, CE were significantly positive, increasing polyculture biomass by a mean 131 g·m−2 above the component species (95% C.I. = 85 to 176 g·m−2, t = 5.87, df = 46, P < 0.01). SE were also significantly positive with a mean 69 g·m−2 of the net diversity effect attributable to the impacts of individual species (95% C.I. = 12 to 126 g·m−2, t = 2.51, df = 46, P = 0.02). For 25 experiments with sufficient data, we used Fox's (29) modification of the additive partitioning method to further divide SE into species-specific impacts on biomass that stem from competitive dominance versus those impacts not associated with dominance (e.g., when the presence of legumes increases biomass of species other than itself). For this subset of experiments, competitive dominance by highly productive species explained a mean 93% of SE (see SI Text).
Fig. 2.
Mechanisms underlying the effects of plant species richness on the production of biomass. (A) Magnitude of complementarity effects (CE) and selection effects (SE) for each of 47 estimates available from 24 experiments. The grand mean ± 95% C.I. values for CE and SE across all experiments is displayed as an open diamond, estimated from mixed model ANOVAs with experiment included as a random effect. (B and C) Shown are how complementarity and selection effects vary with the duration of an experiment. Each data point is a single estimate from one experiment, but note that some experiments contribute multiple data points (numbers correspond to experiments listed in SI Datasets 1 and 2) (see SI Text).
Although analyses above suggest that CE contributed 1.9 times more to biomass than SE, it is worth noting that 43% of SE estimates were negative, which can occur when species with lower than average productivity come to dominate polycultures (24). Given this, it is worthwhile to compare the total contributions of single verses multiple species to the net diversity effect based on the proportional difference in their absolute magnitudes, log10 (|SE|/|CE|). This ratio averaged −0.24 (indicating that |SE|<|CE|, Fig. 2A Inset), but was not significantly different from zero (95% C.I. = −0.57 to 0.08, t = −1.58, df = 46, P = 0.13). To a first approximation, these results suggest that CE have equaled or exceeded the contributions of SE to net diversity effects, but with CE having a predominantly positive influence, whereas SE were sometimes negative.
Analyses of time series indicate that CE tended to increase with the duration of an experiment (Fig. 2B and Table 1). This was true both for individual experiments that have measured biomass on two or more sampling dates (CE = −322 + 73 g·m−2 × ln[days]; see Table 1), and when data for all experiments were combined and analyzed collectively (CE = −177 + 56 g·m−2 × ln[days]).
When we consider that the mean biomass of monocultures averaged 226 g·m−2 for the initial sampling date of all 44 experiments, results of these time series suggest that CE increased by a minimum factor of 1.25× for each log increase in time ([226 g·m−2 + 56 g·m−2]/226 g·m−2). Therefore, increases in CE are sufficient to explain changes in the net effect of diversity through time, which increased by a factor of e0.09 to e0.27 = 1.09–1.31× (Table 1). In contrast to CE, we failed to find evidence that SE changes significantly through time. Although the coefficient relating SE to time was negative for models run “within” and “across” experiments (Table 1), the coefficient was not distinguishable from zero in either case (P > 0.20 for both).
Discussion
We have summarized the results of nearly two decades of experiments that have manipulated the richness of plant communities to assess how this aspect of biodiversity impacts the production of biomass. Our metaanalyses show that the net effects of plant species richness on plant biomass are generally positive, but that diverse plant polycultures usually achieve less biomass than their most productive species. However, both the net effect of diversity and the probability that species mixtures will yield more than their most productive species increase with the duration of experiments. These results corroborate the findings of a number of individual studies (e.g., refs. 22, 25, 30, and 31), and suggest there is considerable generality in the way that plant diversity impacts the productivity of ecosystems.
Although our analyses confirm a number of previously reported patterns, they also refute certain prior interpretations about the mechanisms that generate these patterns. Experiments performed with prokaryotes and eukaryotes inhabiting a wide variety of freshwater, marine, and terrestrial ecosystems have found that the net effects of species richness on biomass are positive, but that diverse mixtures seldom achieve more biomass than their most productive species (14). This pattern has often been interpreted as evidence that selection effects (the increased probability that a highly productive species will be included in and come to dominate a diverse mixture) are the primary mechanism responsible for the higher biomass of polycultures (14, 16–18). Analyses presented here are, to our knowledge, the first to directly assess mechanisms underlying diversity effects across a wide variety of studies, and they do not support this interpretation—at least for this subset of studies performed by using plants. Consistent with the conclusions of several individual experiments (e.g., refs. 23, 27, 30, 32, and 33), we found that the net effect of diversity on biomass in nearly every study resulted from some combination of species-specific selection effects and multispecies complementarity. Selection effects have most often resulted from highly productive species coming to competitive dominance in polyculture (see SI Text). But an equal if not larger fraction of net diversity effects are attributable to multiple species having complementary effects on biomass. These findings contribute valuable information to one of the most contentious debates in ecology. For many years, researchers have argued over whether net diversity effects stem from species richness per se, or are simply a function of the most productive species in a community (3, 16, 34). The outcome of this debate is nontrivial as it has major implications for whether conservation and management objectives are best achieved through the preservation of functionally important species or by maximizing biodiversity (18, 35). Our analyses suggest that this debate has been framed around a false dichotomy. The balance of evidence shows that both the number of species and the types of species in an ecosystem have significant impacts on the production of biomass. However, although highly productive species do indeed make consistent and sizeable contributions to plant biodiversity effects, these are equaled or exceeded in frequency and magnitude by multispecies contributions to biomass.
Our finding that the net effects of plant diversity on biomass are generally positive corroborates what appears to hold true for many types of organisms in ecosystems around the globe (10, 11, 13–15, 36). However, our finding that diverse polycultures tend to achieve less biomass than their most productive species contrasts with a common prediction in diversity-function research. It has been proposed that diverse communities will capture a greater fraction of available resources and produce more biomass than even their most productive species (called “transgressive overyielding”; see refs. 37 and 38). This prediction was originally based on niche theory, which argues that species must use resources in ways that are complementary in space or time to stably coexist with one another (2). Thus, one question that follows from our analyses is: Why do we generally fail to find transgressive overyielding in experiments despite the evidence for complementarity among species?
First, it is important to realize that a lack of transgressive overyielding is not necessarily in conflict with positive species complementarity. Complementarity can arise from a variety of biological mechanisms that include various forms of niche partitioning and/or interspecific interactions, such as facilitation and indirect effects that enhance resource capture (27, 28). Although positive values of complementarity indicate that two or more species are contributing to biomass in ways that are unique or that enhance the productivity by other species, these effects can be driven by a subset of the full community. Furthermore, Loreau has shown mathematically that transgressive overyielding requires a greater degree of complementarity than is needed for competing species to coexist (39). Thus, even when species exhibit a degree of niche complementarity that is sufficient to stabilize their interactions, this does not ensure that a diverse polyculture will outperform the highest yielding species. Therefore, one possible explanation for the results of experiments to date is that, although complementarity has been sufficiently strong to generate positive net effects of diversity, it has not been strong enough to generate transgressive overyielding. To the extent that this explanation is correct, it suggests there may be a “cost” to maintaining higher diversity in ecosystems, at least in the short-term. For example, a farmer or resource manager might be able to identify a species or subset of species that can achieve greater yield than a more diverse system. However, this higher yield may or may not be sustainable. Many studies have shown that systems with fewer species tend to exhibit greater fluctuations in aggregate biomass through time (9, 40, 41). Thus, much like the tradeoff between yield and stability in an investment portfolio, a select few species may be able to produce higher yield than a diverse community, but this might come at the expense of the stability of yield through time (9).
A second possible explanation for the lack of transgressive overyielding in experiments is that there may be statistical limitations in our ability to detect it. Several authors have noted that tests for transgressive overyielding are inherently asymmetrical such that LRtrans > 0 is sufficient to demonstrate overyielding, but LRtrans ≤ 0 is not necessarily sufficient to demonstrate its absence (24, 31, 42). In part, this is because the validity of conclusions from tests that compare the extreme value of one set of distributions (the highest mean value from all possible monocultures) to the mean of another (the average polyculture) are limited by how accurately these distributions have been characterized. Uncertainty in biomass distributions can be especially problematic when multiple species vie for the highest monoculture value because stochastic sampling probabilities can cause the selection of the highest monoculture values to overestimate the true mean. These caveats are important when we consider two limitations of existing data. First, experiments to date have had rather low replication of monocultures (median = 2, mean = 3), which means that uncertainty in biomass is high. Second, for those experiments that have taken repeated measurements, the species with the highest biomass in monoculture has tended to change through time. In our analyses, we found no evidence that the magnitude of LRtrans depended on the number of monoculture replicates run in experiments (t = −0.34, P = 0.74 for a general linear model). We also showed that, when analyses were based on the time-averaged monoculture values, conclusions did not change. Even so, it is important to keep in mind that studies to date have not been designed to obtain reliable estimates of biomass for species monocultures and that LRtrans and other related metrics are probably conservative tests for transgressive overyielding (42).
Another possible explanation for the lack of transgressive overyielding is that, although the phenomenon may be common, experiments have been performed for too short a time to detect it. This is a distinct possibility given that the mechanisms that produce diversity effects are temporally dynamic. Several individual experiments have reported that complementarity effects grow stronger through time (e.g., refs. 22, 23, 25, and 30). This might be the case if, for example, plant assemblages take some time to develop the different rooting depths that lead to differential use of soil resources. Originally, it was also presumed that selection effects would grow stronger through time as the most productive species came to dominance in polyculture (16). However, the fact that selection effects are often negative led some to argue that, as the least productive species come to dominate a community through time, complementarity must grow disproportionately strong to generate transgressive overyielding (22, 24, 30, 31, 43, 44). Our analyses confirm that complementarity tends to increase through time, but they do not support the idea that selection effects change through time.
Indeed, one of the surprising results of our analyses was the lack of any clear change in the strength of selection effects with the duration of experiments. This may be because biodiversity experiments performed with plants are typically established by using high-density seed mixtures. High seeding densities may encourage rapid selection for dominant species within the first year of establishment and thus a rank–dominance structure that exhibits little change through time. Rapid establishment of a rank–dominance structure may be enhanced by many experiments omitting early successional plants from their species pool to encourage fast development of a “mature” community that is more typical of the system of interest. Of those studies that have included early successional species, species-specific impacts on biomass can change over the course of an experiment as fast-growing taxa are replaced by late-successional dominants (see ref. 21 for an example). Because the majority of experiments have yet to incorporate the nonequilibrium conditions and successional dynamics that characterize most natural communities, our conclusion about the constancy of selection effects should be considered tentative.
Although selection effects did not change through time, our analyses clearly showed that complementarity tends to grow stronger as experiments age. One consequence is that both the net effect of diversity on biomass and the probability of transgressive overyielding tended to increase as experiments were run longer. Even so, our best statistical estimates suggest that it takes ≈1,750 days before the most diverse polyculture begins to yield more biomass than the highest monoculture. For the annual and perennial species commonly used in these studies, this corresponds to ≈2–5 generations or growing seasons, which is disconcerting given that the median experiment has only run for 730 days. This suggests that it may take several more years before we can reliably say whether diverse plant communities yield more biomass than their most productive species—a result that emphasizes the importance of long-term biodiversity studies. Regardless of what experiments ultimately show, our analyses suggest that experiments to date have, if anything, underestimated the impact of species diversity on the productivity of ecosystems.
Methods
Selection of Studies.
Studies analyzed here are a subset of those reviewed by Cardinale et al. (14) but for which extensive amounts of new data were collected. Studies were identified from a literature search in which authors collated reference lists from recent surveys of biodiversity-ecosystem functioning research (9, 10, 18, 35, 36), supplemented with a search of the Institute for Scientific Information Web of Knowledge database, using the keyword sequence species AND (diversity OR richness) AND (community OR ecosystem) AND (function OR functioning OR production OR productivity OR biomass). More than 200 articles published through 2005 were reviewed. To be included here, the article had to report the results of an experiment in which the richness of three or more plant species was manipulated as an independent variable to examine how richness impacts the aboveground biomass of plants per square meter. We found 44 independent experiments reported in 22 articles that were performed with species from temperate grasslands, tundra, estuaries, or temperate bryophyte assemblages. All data used in our analyses are provided in SI Datasets 1 and 2.
Analysis of Diversity Effects.
For each of the 44 experiments, we used digitized figures from the original publications, original datasets published in online repositories or original datasets provided by the authors to determine plant biomass produced per sqaure meter in the most diverse polyculture, and the biomass achieved by each species grown in monoculture. For each time t of experiment i, we used two log ratios to characterize the effect of richness on biomass. The first, LRnet = 1n(Bp̄i/Bm̄i gives the proportional difference in biomass between the mean value of all replicates of the most species rich polyculture, Bp̄i, and the mean value of all replicates for all species grown in monoculture, Bm̄i. The second, LRtrans = 1n(Bp̄i/Bmax i, tests for transgressive overyielding by estimating the proportional difference between Bp̄i and the mean value of all replicates of the species that achieves the highest biomass in monoculture, Bmax i.
In total, we obtained 104 estimates of LRnet and 96 estimates of LRtrans, which were analyzed in three ways. First, we calculated confidence intervals for all 104 estimates of LRnet and 83 estimates of LRtrans where studies had replicated monocultures of the most productive species. Second, we used a mixed model ANOVA to examine how LRnet and LRtrans change through time across studies, using the model yi = μ+ ti + bi + εi, where ti is the number of days since the start of an experiment to the date on which biomass was measured, bi is the random effect of experiment i (iid N[0, σb2]), and εi is the residual error. Third, we examined how LRnet and LRtrans change through time within individual experiments, using a mixed model like that above, but where each of the 17 experiments with measurements on multiple dates were treated as subjects in a repeated-measures ANOVA. Log ratios were weighted by 1/σ2 to account for heterogeneity among experiments (although weighted analyses explained more variation, analyses without weighting led to the same conclusions).
Loreau and Hector (27) developed a technique to statistically partition the net effect of diversity into two components: species-specific selection effects (SE) and multispecies complementarity effects (CE). We obtained 47 estimates of SE and CE either from published tables or figures (9 experiments) or from our own analyses of original datasets (15 experiments). To assess how SE and CE changed through time within individual experiments and across experiments, we used the same mixed model ANOVA's described above for LRnet and LRtrans. SE and CE were weighted by the sample sizes to account for heterogeneity among experiments.
Supplementary Material
Acknowledgments
We thank numerous researchers who graciously provided us with their original datasets and then reviewed this article for accuracy. D. Tilman, J. Levine, B. Schmid, and three anonymous reviewers provided comments that improved this manuscript. This work was supported by United States National Science Foundation Grants DEB 0614428 (to B.J.C.) and DEB 0435178 (to S. Naeem's BioMERGE network), and a grant from Canada's Natural Sciences and Engineering Research Council (to M.L.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0709069104/DC1.
References
- 1.Loreau M, Naeem S, Inchausti P, Bengtsson J, Grime JP, Hector A, Hooper DU, Huston MA, Raffaelli D, Schmid B, et al. Science. 2001;294:804–808. doi: 10.1126/science.1064088. [DOI] [PubMed] [Google Scholar]
- 2.Tilman D. Ecology. 1999;80:1455–1474. [Google Scholar]
- 3.Naeem S, Chapin FS, III, Costanza R, Ehrlich PR, Golley FB, Hooper DU, Lawton JH, O'Neill RV, Mooney HA, Sala OE, et al. Issues in Ecology. 1999;4:1–11. [Google Scholar]
- 4.Sala OE, Chapin FS, Armesto JJ, Berlow E, Bloomfield J, Dirzo R, Huber-Sanwald E, Huenneke LF, Jackson RB, Kinzig A, et al. Science. 2000;287:1770–1774. doi: 10.1126/science.287.5459.1770. [DOI] [PubMed] [Google Scholar]
- 5.Daily GC. Nature's Services: Societal Dependence on Natural Ecosystems. Washington, DC: Island; 1997. [Google Scholar]
- 6.Chapin SI, Sala OE, Burke I, Grime J, Hooper D, Lauenroth W, Lombard A, Mooney H, Mosier A, Naeem S, et al. Bioscience. 1998;48:45–52. [Google Scholar]
- 7.Hector A, Schmid B, Beierkuhnlein C, Caldeira MC, Diemer M, Dimitrakopoulos PG, Finn JA, Freitas H, Giller PS, Good J, et al. Science. 1999;286:1123–1127. doi: 10.1126/science.286.5442.1123. [DOI] [PubMed] [Google Scholar]
- 8.Tilman D, Wedin D, Knops J. Nature. 1996;379:718–720. [Google Scholar]
- 9.Hooper DU, Chapin FS, Ewel JJ, Hector A, Inchausti P, Lavorel S, Lawton JH, Lodge DM, Loreau M, Naeem S, et al. Ecol Monogr. 2005;75:3–35. [Google Scholar]
- 10.Schmid B, Joshi J, Schlapfer F. In: The Functional Consequences of Biodiversity: Empirical Progress and Theoretical Extensions. Monographs in Population Biology 33. Kinzig AP, Pacala SW, Tilman D, editors. Princeton: Princeton Univ Press; 2001. pp. 120–150. [Google Scholar]
- 11.Schlapfer F, Schmid B. Ecol Appl. 1999;9:893–912. [Google Scholar]
- 12.Naeem S. Ecology. 2002;83:1537–1552. [Google Scholar]
- 13.Balvanera P, Pfisterer AB, Buchmann N, He JS, Nakashizuka T, Raffaelli D, Schmid B. Ecol Lett. 2006;9:1146–1156. doi: 10.1111/j.1461-0248.2006.00963.x. [DOI] [PubMed] [Google Scholar]
- 14.Cardinale BJ, Srivastava DS, Duffy JE, Wright JP, Downing AL, Sankaran M, Jouseau C. Nature. 2006;443:989–992. doi: 10.1038/nature05202. [DOI] [PubMed] [Google Scholar]
- 15.Stachowicz J, Bruno JF, Duffy JE. Annu Rev Ecol Evol Syst. 2007 in press. [Google Scholar]
- 16.Huston MA. Oecologia. 1997;110:449–460. doi: 10.1007/s004420050180. [DOI] [PubMed] [Google Scholar]
- 17.Wardle DA. Oikos. 1999;87:403–410. [Google Scholar]
- 18.Schwartz MW, Brigham CA, Hoeksema JD, Lyons KG, Mills MH, van Mantgem PJ. Oecologia. 2000;122:297–305. doi: 10.1007/s004420050035. [DOI] [PubMed] [Google Scholar]
- 19.Cardinale BJ, Palmer MA, Collins SL. Nature. 2002;415:426–429. doi: 10.1038/415426a. [DOI] [PubMed] [Google Scholar]
- 20.Mulder CPH, Uliassi DD, Doak DF. Proc Natl Acad Sci USA. 2001;98:6704–6708. doi: 10.1073/pnas.111055298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weis JJ, Cardinale BJ, Forshay KJ, Ives AR. Ecology. 2007;88:929–939. doi: 10.1890/06-0943. [DOI] [PubMed] [Google Scholar]
- 22.Tilman D, Reich PB, Knops J, Wedin D, Mielke T, Lehman C. Science. 2001;294:843–845. doi: 10.1126/science.1060391. [DOI] [PubMed] [Google Scholar]
- 23.Spehn EM, Hector A, Joshi J, Scherer-Lorenzen M, Schmid B, Bazeley-White E, Beierkuhnlein C, Caldeira MC, Diemer M, Dimitrakopoulos PG, et al. Ecol Monogr. 2005;75:37–63. [Google Scholar]
- 24.Hooper DU, Dukes JS. Ecol Lett. 2004;7:95–105. [Google Scholar]
- 25.van Ruijven J, Berendse F. Proc Natl Acad Sci USA. 2005;102:695–700. doi: 10.1073/pnas.0407524102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fox JW. Ecology. 2004;85:549–559. [Google Scholar]
- 27.Loreau M, Hector A. Nature. 2001;412:72–76. doi: 10.1038/35083573. [DOI] [PubMed] [Google Scholar]
- 28.Petchey OL. Oikos. 2003;101:323–330. [Google Scholar]
- 29.Fox JW. Ecol Lett. 2005;8:846–856. [Google Scholar]
- 30.Fargione J, Tilman D, Dybzinski R, Lambers JHR, Clark C, Harpole WS, Knops JMH, Reich PB, Loreau M. Proc R Soc London B. 2007;274:871–876. doi: 10.1098/rspb.2006.0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hector A, Bazeley-White E, Loreau M, Otway S, Schmid B. Ecol Lett. 2002;5:502–511. [Google Scholar]
- 32.Lanta V, Leps J. Acta Oecol. 2006;29:85–96. [Google Scholar]
- 33.Dimitrakopoulos PG, Schmid B. Ecol Lett. 2004;7:574–583. [Google Scholar]
- 34.Wardle DA, Huston MA, Grime JP, Berendse F, Garnier E, Lauenroth WK, Setala H, Wilson SD. Bull Ecol Soc Am. 2000;81:235–239. [Google Scholar]
- 35.Srivastava DS, Vellend M. Annu Rev Ecol Evol Syst. 2006;36:267–294. [Google Scholar]
- 36.Covich AP, Austen MC, Barlocher F, Chauvet E, Cardinale BJ, Biles CL, Inchausti P, Dangles O, Solan M, Gessner MO, et al. Bioscience. 2004;54:767–775. [Google Scholar]
- 37.Tilman D, Lehman D, Thompson K. Proc Natl Acad Sci USA. 1997;94:1857–1861. doi: 10.1073/pnas.94.5.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fridley JD. Oikos. 2001;93:514–526. [Google Scholar]
- 39.Loreau M. Oikos. 2004;104:606–611. [Google Scholar]
- 40.Cottingham KL, Brown BL, Lennon JT. Ecol Lett. 2001;4:72–85. [Google Scholar]
- 41.Tilman D, Reich PB, Knops JMH. Nature. 2006;441:629–632. doi: 10.1038/nature04742. [DOI] [PubMed] [Google Scholar]
- 42.Loreau M. Oikos. 1998;82:600–602. [Google Scholar]
- 43.Troumbis AY, Dimitrakopoulos PG, Siamantziouras ASD, Memtsas D. Oikos. 2000;90:549–559. [Google Scholar]
- 44.Engelhardt KAM, Ritchie ME. Nature. 2001;411:687–689. doi: 10.1038/35079573. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.