Abstract
The newly generated single-positive (SP) thymocytes undergo further maturation in the thymic medulla before their emigration to the periphery. The present study was undertaken to validate a developmental program we proposed for CD4SP medullary thymocytes and to explore the mechanisms regulating this process. During mouse ontogeny, the emergence of different subsets of CD4SP thymocytes followed a strict temporal order from SP1 to SP4. Parallel to the transition in surface phenotype, a steady increase in function was observed. As further evidence, purified SP1 cells were able to sequentially give rise to SP2, SP3, and SP4 cells in intrathymic adoptive transfer and in culture. Notably, the development of CD4SP cells in the medulla seemed to be critically dependent on a functionally intact medullary epithelial cell compartment because Relb and Aire deficiency were found to cause severe blockage at the transition from SP3 to SP4. Taken together, this work establishes an ontogenetically and functionally relevant maturation program for CD4SP thymocytes. Precise dissection of this program should facilitate further inquiry into the molecular mechanisms governing normal thymocyte development and its disturbance in pathological conditions.
Keywords: Relb, T cell differentiation, thymic medulla
Immunocompetent T cells are generated in the thymus through a process called T lymphopoiesis. On the basis of CD4 and CD8 expression, this process can be roughly divided into three major stages. The most immature precursors reside within the double-negative (DN) population. Cells at this stage undergo rearrangement of the T cell receptor (TCR) β-chain locus, and those cells with a productively rearranged β-chain are selected to proceed to the double-positive (DP) stage (β-selection). After the further rearrangement of the TCR α-chain locus, DP thymocytes assemble the TCR complex on the cell surface and are tested for the capacity to bind to self-MHC molecules (positive selection). The positively selected DP cells then down-regulate either CD4 or CD8 expression to become single-positive (SP) thymocytes. The lineage-committed SP cells migrate into the thymic medulla, where they stay for as long as 10–14 days before being exported to the periphery (1–4).
The differentiation of DN and DP cells has been studied extensively, and the overall cellular process and underlying molecular mechanisms are reasonably well defined. In contrast, we remain surprisingly ignorant of the maturation process of SP thymocytes, although it has been repeatedly suggested that a number of important events actually take place during this extended period in the medulla. Compared with the terminally differentiated thymocytes, the newly generated SP cells are poor responders to a variety of stimuli, suggesting a functional maturation process within the medulla (5, 6). In addition, the medulla is proposed to be the major site for the negative selection against self-reactive thymocytes (7, 8). In support of this hypothesis, impaired negative selection and autoimmunity are often observed in mice with defects in the thymic medulla, such as in those mice deficient in Relb (9–11), NF-κB2 (12), NIK (13, 14), lymphotoxin β-receptor (LTβR) (13, 15), TRAF 6 (16), or autoimmune regulator (Aire) (17, 18). Still another important medullary event is the generation of regulatory T (Treg) cells. Several studies suggest that the vast majority of Treg cells are branched from early CD4SP cells and acquire the suppressive function during the development in the medulla (19–21).
Both CD4SP and CD8SP populations exhibit significant heterogeneity in phenotype and function (6, 22–25), suggesting that each of them could contain multiple subsets of different maturity. Through the application of several cell-surface markers, two major developmental stages have been revealed. Cells at the early stage express CD69, CD24 [a heat-stable antigen (HSA)], and MTS32 (22, 25), whereas the late-stage SP thymocytes acquire the expression of Qa-2, but lose CD69 and HSA expression (6, 25, 26). Such a classification provided much help for the initial characterization of the developmental program of SP medullary thymocytes. This two-stage scheme, however, looks oversimplified because accumulating evidence points out the existence of intermediate stages (27, 28). For instance, a subset of TCR+CD69−Qa-2− SP cells has been documented, which display a function lower than Qa-2+ cells but higher than CD69+ cells (29). Because of the poor resolution of the developmental pathway, many aspects remain elusive of this last step of T lymphopoiesis from newly generated SP thymocytes to functionally mature emigrants. To achieve better definition of the pathway, we previously performed a careful analysis of the expression pattern of a panel of cell-surface markers in conjunction with the two hallmarks, HSA and Qa-2. On the basis of this analysis, a developmental program has been proposed, which consists of multiple consecutive intermediate stages (27, 28).
In the present study, several approaches were adopted to validate the predicted developmental program for CD4SP thymocytes. First, the chronological appearance, together with their functional properties, was determined during mouse ontogeny of the four subsets (SP1–SP4) representing distinct developmental stages. Second, the SP1 cells were isolated and analyzed for their differentiation potential, in both intrathymic adoptive transfer and culture. Third, several mouse models with a defective thymic medulla were examined for potential alterations in CD4SP thymocyte development. As a result, our studies have defined an ontogenetically and functionally relevant maturation pathway for CD4SP thymocytes and identified a critical checkpoint for this process.
Results
Phenotypic Maturation of CD4SP Thymocytes During Mouse Ontogeny.
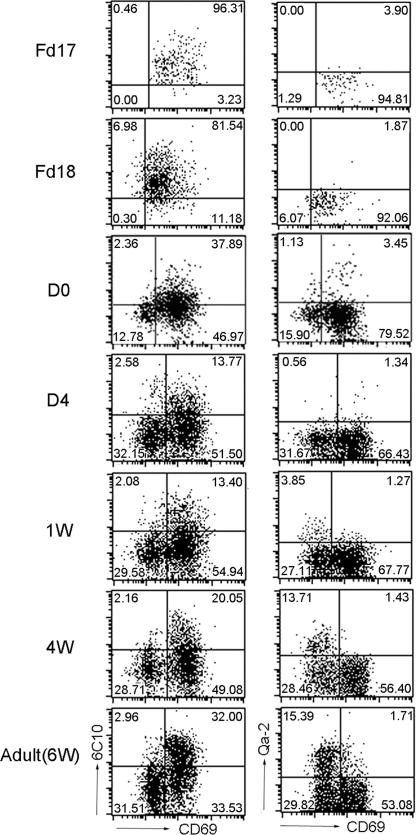
On the basis of differential expression of 6C10, CD69, HSA, and Qa-2, TCRαβ+CD4SP medullary thymocytes could be divided into four major subsets, some of which could be further resolved with the introduction of another marker, 3G11 (27, 29). To simplify the experimental design, we adopted the four-stage scheme in current studies. According to this model, the differentiation of CD4SP thymocytes would proceed from SP1 to SP4. In an attempt to validate the prediction, we determined the sequence of emergence of the different subsets and their dynamic changes during mouse ontogeny (Fig. 1). The TCRαβ+CD4SP thymocytes were first detected in the fetus of day 17 (Fd17) in BALB/cJ mice. At this point, they were almost uniformly SP1 cells (6C10+CD69+Qa-2−). Although a fraction of cells started to down-regulate 6C10 expression from Fd18, a discrete population of SP2 cells (6C10−CD69+Qa-2−) was not obvious until day 0 after birth (D0). Between D0 and D4, SP3 cells (6C10−CD69−Qa-2−) were generated and accumulated after the further down-regulation of CD69. In contrast, the transition from SP3 to SP4 (6C10−CD69−Qa-2+) proceeded rather slowly. A significant number of Qa-2+ thymocytes were only observed on D7. Initially, they were ≈3–5% of the total thymocytes, but their percentage steadily increased afterward, reaching the adult level of 10–15% at the age of 4–6 weeks. Therefore, we observed a sequential progression from SP1 to SP4 during mouse ontogeny.
Fig. 1.
Phenotypic development of TCR+CD4SP medullary thymocytes during mouse ontogeny. CD8-depleted thymocytes from various time points during mouse ontogeny were stained for CD4, TCRβ, CD69, and 6C10 or Qa-2. The expression of 6C10 versus CD69 and CD69 versus Qa-2 was analyzed in TCRβ+CD4SP medullary thymocytes. The age of the fetus or infant is indicated to the left of the plots. The quadrant markers are placed according to the staining pattern of appropriate isotype controls. Results are representative of three separate experiments.
Functional Maturation of CD4SP Thymocytes During Mouse Ontogeny.
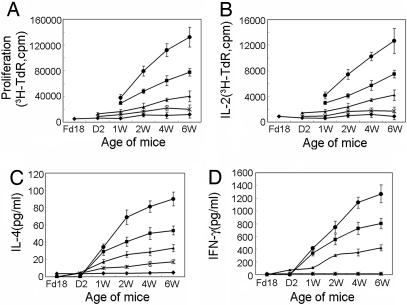
To correlate the phenotypic differentiation with functional maturation, we next analyzed the functional properties of each subset of CD4SP thymocytes isolated at different time points during ontogeny. First assessed is their proliferative response to Con A stimulation (Fig. 2A). SP1 cells demonstrated almost no response. SP2 cells were general poor responders as well, but a slightly improved response was observed with cells isolated from mice at a later stage of ontogeny. SP3 cells displayed a further elevated level of proliferation. Moreover, they underwent a progressive increase in their proliferation capacity from the time of their first appearance to 6 weeks of age. SP4 cells showed the most potent proliferative response to Con A stimulation. Still they were considerably less responsive than CD4+ T cells from the spleen at each time point tested. Interestingly, the proliferation of splenic CD4+ T cells also was found to increase steadily during ontogeny from 2 to 6 weeks of age.
Fig. 2.
Proliferation and cytokine production by distinct subsets of CD4SP thymocytes at discrete time points during ontogeny. Different subsets of CD4SP thymocytes (SP1–SP4) and splenic CD4+ T cells were obtained at each time point as indicated and stimulated with Con A. (A) Cell proliferation was measured by [3H]TdR incorporation assay at day 3. For cytokine production assays, culture supernatant was harvested at day 2 of culture. (B) IL-2 was measured by [3H]TdR incorporation assay by using IL-2-dependent cell line HT-2. (C and D) IL-4 (C) and IFN-γ (D) were measured by ELISA. Data shown are the mean ± SD of two to three repeated experiments. Filled diamond, SP1; ×, SP2; filled triangle, SP3; filled square, SP4; filled circle, splenic CD4 T cell.
Next, we examined the secretion of cytokines, such as IL-2 (Fig. 2B), IL-4 (Fig. 2C), and IFN-γ (Fig. 2D), by various subsets in response to Con A stimulation. All four subsets were competent for IL-2 production. IL-4 and IFN-γ production, however, started late at the SP2 and SP3 stages, respectively. Moreover, the cytokine secretion was found to be progressively enhanced from SP1 to SP4. Nevertheless, even the most mature CD4SP thymocytes (SP4) demonstrated substantially lower responses than splenic CD4+ T cells. Similar to the proliferative response, increasing levels of cytokine secretion were observed, with cells representing the same developmental stage, but obtained at a later time point during ontogeny. Taken together, these results indicate that the function maturation of CD4SP thymocytes is largely coupled with, but may go beyond, their phenotypic differentiation during ontogeny.
Differentiation of Adoptively Transferred SP1 Cells in Vivo.
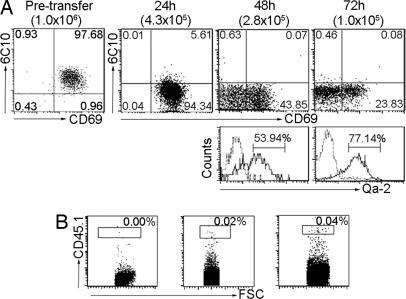
Although the sequential appearance and steady increase in function strongly suggest an ordered maturation program from SP1 to SP4, direct evidence remains to be shown for the precursor–progeny relationship among the various subsets. To this end, an intrathymic cell-adoptive transfer assay was performed. Thymocytes from CD45.1+ donors were first treated with anti-CD8 antibodies and complement to remove DP and CD8SP thymocytes. TCRβ+CD4+6C10+CD69+ SP1 cells were then isolated by cell sorting and intrathymically injected into nonirradiated CD45.2+ recipients. After an interval of 24, 48, or 72 h, the number and surface phenotype of the donor-derived cells were determined. The total number of donor cells harvested at 24 h after injection was about half of what was injected. Given that no donor cell was identified in the periphery at this point, the reduction was most likely due to the procedure-related stress. Of the viable cells, <10% retained the SP1 phenotype (6C10+CD69+), whereas the majority were already converted to SP2-like cells (6C10−CD69+). At 48 h after injection, the original SP1 cells virtually disappeared. Meanwhile, a new population of 6C10−CD69− cells emerged, which constituted ≈55% of the donor cells (2.8 × 105 in total) and were composed of two equally divided Qa-2− (SP3) and Qa-2+ (SP4) subsets (Fig. 3A). At this time point, the donor-derived cells were first detected in the periphery (Fig. 3B). At 72 h, the donor cells in the thymus further dropped to ≈1.0 × 105. Among them, 57% was of the SP4 phenotype, whereas SP2 and SP3 cells decreased to 24% and 19%, respectively (Fig. 3A). In contrast, the number of donor-derived cells increased by 2-fold in the spleen (Fig. 3B).
Fig. 3.
Differentiation of SP1 CD4SP thymocytes in intrathymic cell-adoptive transfer assay. SP1 cells were isolated from CD45.1+ donor mice by cell sorting with a purity of ≥97% (pretransfer). The 1 × 106 cells were then injected into the thymus of CD45.2+ recipient mice. At 24, 48, and 72 h after the adoptive transfer, donor cells in the recipient thymus were analyzed by flow cytometry. (A) Dot plots show 6C10 versus CD69 expression in the whole donor population, and histograms show Qa-2 expression in the 6C10−CD69− fraction. The number of cells recovered at different time points is shown in brackets. (B) Also analyzed is the presence of donor-derived CD45.1+CD4+ T cells in the spleen. Experiments were repeated two to three times with similar results.
The sequential generation of SP2, SP3, and SP4 from SP1 cells further confirms the developmental program we proposed. In addition, the adoptive transfer assay demonstrated that the differentiation from SP1 to SP4 could be achieved in 2–3 days. Once they reached the SP4 stage, the donor cells were ready to be detected in the periphery. This result, although echoing reports by others (25, 30), seems to be at odds with the estimation of 10- to 14-day residence in the medulla for the SP thymocytes (4). However, we observed the retention of a substantial number of SP4 cells in the thymus even 7 days after transfer (data not shown). Therefore, cell maturity is not the only factor that determines thymocyte egress to the periphery.
Stromal Cell-Supported Differentiation of CD4SP Thymocytes in Vitro.
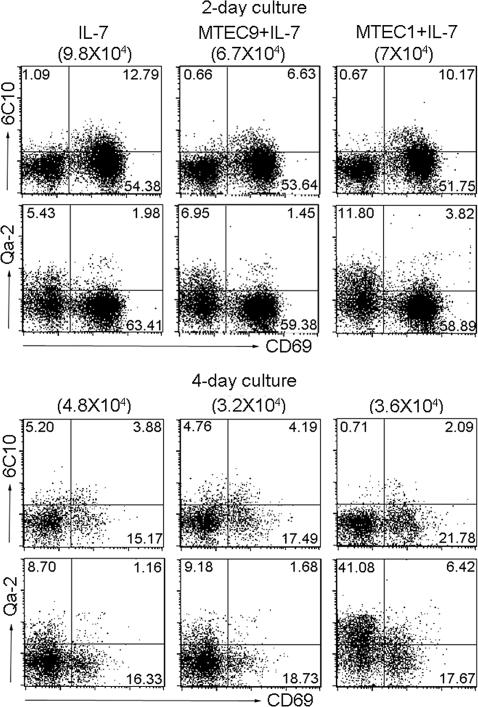
To facilitate further inquiries into the intrinsic program and the external requirements for the differentiation of CD4SP thymocytes, we attempted to establish a culture system that is capable of supporting the differentiation in vitro. Thus, SP1 cells were isolated from the thymi of 6-week-old adult mice and put into culture under various conditions. Among the many cytokines tested, IL-7 was found to be most critical for cell survival, without which virtually no viable cell could be recovered from the culture (data not shown). In the presence of an optimal concentration of IL-7 (30 ng/ml), cells not only survived, but also differentiated (Fig. 4). After 2 days of culture, the majority of SP1 cells were already converted into SP2 and SP3 cells, each representing ≈50% and 30% of the culture, respectively. At day 4, most cells proceeded to the SP3 stage with almost no SP1 cells left. Although such a system was permissive for the generation of SP4 cells, the percentage of this subset remained low over the course of culture, suggesting that the transition from SP3 to SP4 was relatively inefficient in IL-7 alone.
Fig. 4.
In vitro differentiation of SP1 CD4SP thymocytes. Purified 1.5 × 105 SP1 cells were cultured in medium supplemented with 30 ng/ml IL-7 in the presence or absence of the thymic epithelial cell line mTEC1 or mTEC9. Cells were harvested at days 2 and 4 and analyzed for surface expression of 6C10, CD69, and Qa-2. The number of cells recovered from different cultures is shown in brackets, and the number in each quadrant indicates the percentage of the corresponding subsets. Results are representative of four to five separate experiments.
The restricted localization and extended residence of SP thymocytes in the medulla suggest that the medullary microenvironment is a critical factor in the maturation of SP cells. Therefore, we subsequently investigated the potential influence of thymic epithelial cells (TECs) on the in vitro differentiation of SP1 cells from the same genetic background. Coculture with established TEC lines failed to cause further improvement of cell survival. However, cell differentiation could be dramatically altered depending on the specific cell lines to be used. In comparison to 9% of SP4 cells obtained with IL-7 alone, this subset accounted for 41% of the cultures at 96 h in the presence of the medulla TEC1 (mTEC1) cell line. No significant effect was detected with another cell line, mTEC9 (Fig. 4). Therefore, the efficient generation of SP4 cells requires additional signals generated through interaction with a special type of TEC. The reason behind the functional distinction between mTEC1 and mTEC9 remains elusive. Given that freshly prepared thymic stromal cells from C57BL/6 mice could induce SP4 differentiation as efficiently as those from BALB/c mice (data not shown), the different genetic background of the two lines (mTEC1 from BALB/c, mTEC9 from C57BL/6) does not seem to be a critical factor. Another known difference lies in the presence of Ulex europaeus agglutinin-1 (UEA-1) binding sites on mTEC1, but in the absence from mTEC9 cells. It is noteworthy that the UEA-1+ cells are the most severely affected subset of mTECs in several mouse strains with a malfunctioning medulla because of mutations in such genes as Relb (10), Nik (13, 15), Traf6 (16), Ltbr (13), or Nfkb2 (12).
To test the functionality of SP4 cells generated in vitro, we examined IL-2 production by these cells after stimulation with PMA and ionomycin. CytoSpot assay revealed that ≈5% of them were competent for IL-2 production. Although this number is much lower than that of splenic CD4 T cells (15%), it was comparable to that of freshly isolated SP4 cells generated in vivo (data not shown).
Developmental Blockage of CD4SP Thymocytes in Relb- and Aire-Deficient Mice.
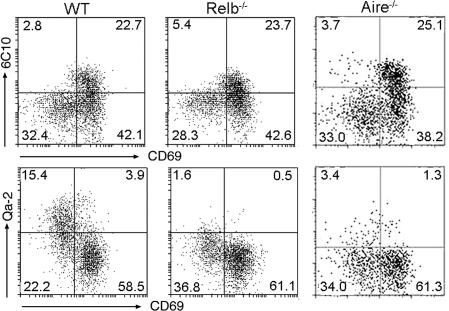
The potent effect of TECs on cell differentiation in vitro prompted us to explore the potential abnormalities of CD4SP cell development in mice with a defective TEC compartment. In Relb-deficient mice, the medullary structure is virtually absent because of a drastic reduction of dendritic and epithelial cells (10, 31). In addition to an impaired negative selection, Relb-deficient mice showed a significant decrease in the number of HSA− SP thymocytes (32). With the developmental scheme herein introduced, we pursued a more accurate definition of the defect. As shown in Fig. 5, Relb deficiency resulted in a 10-fold decrease of SP4 cells, with a relative accumulation of SP3 cells, but normal SP1 and SP2 populations. Such a profile indicates that Relb mutation specifically targets the transition from SP3 to SP4.
Fig. 5.
Developmental defects of CD4SP thymocytes in Relb−/− and Aire−/− mice. Thymocytes were harvested from wild-type (WT), Relb−/−, and Aire−/− mice and analyzed by flow cytometry. Total numbers of CD4SP and CD8SP medullary thymocytes were comparable in each thymus. The dot plots show 6C10 versus CD69 and CD69 versus Qa-2 expression in CD4SP thymocytes. The percentage of cells in each quadrant is indicated. Results are representative of two separate experiments.
Aire was recently identified as an important mediator of central tolerance. Presumably, it exerts this function by driving ectopic expression of a battery of organ-specific genes in mTECs. Thus, Aire-deficient mice develop autoimmunity because of the failure to delete developing thymocytes specific for self-antigens (17, 18). In addition to the induction of peripheral tissue antigens, Aire was implicated in antigen processing/presentation by mTEC and in the coordination of intrathymic cell migration (33). Given the profound influence of Aire on the function of medullary epithelial cells, we wondered how the developmental program of CD4SP thymocytes would be affected in the absence of Aire. Examination of the distribution of different subsets of CD4SP cells in Aire−/− mice revealed a developmental defect similar to that observed in Relb−/− mice. In this case, SP4 cells were reduced by 80%, whereas SP3 cells increased by 70% (Fig. 5), again indicating a developmental blockage at the transition from SP3 to SP4.
Discussion
Evidence is accumulating that the differentiation of SP thymocytes in the medulla is a crucial step in T lymphopoiesis. Some of the important events occurring over this period include the acquisition of functional competence, negative selection against self-reactive cells, and commitment to lineages of specific functions. Our understanding of these events, however, is severely hampered because of the inadequate resolution of the differentiation program. In view of this problem, we proposed a scheme for more precise dissection of the developmental pathway of CD4SP thymocytes (27, 29). Several lines of evidence generated from the current study support the validity of this scheme. First, we showed that the arising of the four subsets follows a temporal order during mouse ontogeny, SP1 at Fd17, SP2 at Fd18, SP3 at D0, and SP4 at D7. Second, our intrathymic adaptive transfer assay demonstrated that SP1 cells can sequentially give rise to SP2, SP3, and SP4 cells. Similar results also were obtained with SP1 cells cultured in vitro. In both assays, cells representing later stages accumulated over time. Meanwhile, cells of earlier stages diminished or disappeared. Thus, the progression appears to be unidirectional. Finally, when each of the four subsets was assessed for their functional capacities, we saw a hierarchy of SP1 < SP2 < SP3 < SP4, indicating increasing maturity from SP1 to SP4.
The current scheme has some obvious advantages over the two-stage model defined by the reciprocal expression of HSA and Qa-2 (6, 25). In general, the precise definition of the developmental pathway makes it possible to isolate and manipulate cells at discrete stages, thereby facilitating the dissection of the cellular and molecular mechanisms underlying the differentiation of CD4SP thymocytes. More specifically, the identification of the intermediate stages helps to reveal some critical checkpoints and allows easier and clearer definition of potential defects in the differentiation process, which could be otherwise ignored. For example, when Relb and Aire mutant mice were examined, a common defect was immediately observed, with a marked reduction of SP4 cells and the concomitant accumulation of SP3 cells, suggesting that the SP3/SP4 transition could be an important regulatory target.
The functional maturation is a prominent feature of the medullary thymocyte development (5, 6). Concordantly, we found that the progression from SP1 to SP4 is coupled with a steady increase in proliferation and cytokine secretion. Intriguingly, a similar elevation in function also was observed with cells with the same surface phenotype, but obtained from later time points of the ontogeny. The reason is unclear for the continued functional maturation after phenotypic differentiation. Potentially, this finding could be a reflection of the distinct maturing status of the medullary stromal cells. As circumstantial evidence, we found that SP1 cells isolated from D0 and D7 neonatal mice differentiated with kinetics similar to SP1 cells from adult mice when cocultured with mTEC1 cells. Moreover, the SP4 cells generated in these cultures gave rise to an equal percentage of IL-2-producing cells as measured by CytoSpot assay (data not shown). Therefore, the functional difference of fetal/neonatal versus adult CD4SP thymocytes is at least partly determined by the maturity of the thymic stroma.
The supporting role of stromal cells is well recognized in T lymphopoiesis. Interestingly, developing thymocytes also impose a profound influence on the development of the thymic stroma. As evidence, SCID or Rag-deficient mice have a markedly underdeveloped medulla, which is restored to wild-type configuration after transfer of wild-type bone marrow or cross-breeding with TCRαβ transgenic mice (34, 35). As such, it is reasonable to speculate that the newly emerged SP thymocytes may promote the development of medullary stromal cells, which in turn support the further maturation of SP thymocytes.
Recent studies have suggested that the development of the thymic medulla is critically dependent on the signal mediated by LTβR (13, 15). This finding, together with the fact that SP thymocytes are the main source of LTβR ligand in the thymus (13), argues that the interaction between LTβR and its ligand may represent a major means of communication between developing thymocytes and epithelial cells. In addition to LTβR, several other molecules contribute to the formation of the appropriate medullary architecture. In the absence of Relb, NIK, or TRAF6, the drastic reduction of mTEC, especially the UEA-1+ cells, leads to severe medullary thymic atrophy (9, 10, 13, 14, 16). A similar, but milder, phenotype also is observed in mice deficient in NF-κB2 (12). Further analyses have indicated that the malformed medullary structure is often associated with the markedly reduced expression of Aire and a variety of peripherally restricted antigens. Moreover, the mutant mice demonstrate various signs of autoimmunity (9, 10, 12–16).
Although these results establish a key role of the thymic medulla in the induction of central tolerance, its impact on the developmental program of SP thymocytes remains obscure. Given the normal distribution of CD4 and CD8 populations and the presence of fully functional T cells in the periphery in Relb−/− mice, it was postulated that the thymic medullary epithelium is not required for full maturation and exportation of thymocytes (31). This postulation is supported by the seemingly undisturbed developmental kinetics and thymic export of mature thymocytes in Ccr7−/− mice in which SP thymocytes fail to enter the medullary region (36). However, one should bear in mind that these analyses were carried out without the appreciation of the complicated developmental program undertaken by SP thymocytes. In fact, more careful analysis has revealed that the Relb deficiency is associated with a reduction in the number of HSA− SP cells (32). We went further to show that the defect lies in the transition from SP3 to SP4. As direct evidence for a critical role of TECs in SP thymocyte differentiation, our in vitro studies showed that, although the progression from SP1 to SP3 may be intrinsically dictated, the efficient generation of SP4 cells requires the presence of a special type of TEC. On the basis of these findings, we speculate that, in the absence of a functional medullary structure, CD4SP thymocyte differentiation in vivo proceeds to the SP3 stage. Although not fully mature, some of these cells are nevertheless exported to the periphery. The limited number of peripheral T cells may lead to extensive homeostatic proliferation, which could constitute an additional mechanism for the autoimmune response.
A developmental blockage similar to that observed in Relb−/− mice also is present in Aire−/− mice. Such a similarity is intriguing considering the striking difference in the architecture of the thymic medulla between these two strains. Although the medullary structure and epithelial cells are virtually absent in Relb−/− mice (9, 10, 32), Aire−/− mice maintain a morphologically normal thymic medulla (17, 18). Aire expression is significantly reduced in Relb-deficient mice, yet it is difficult to conclude whether Relb is required for Aire expression or whether the reduced Aire expression is because of the absence of mTECs. It is equally difficult to distinguish whether the absence of mTECs or the reduced Aire expression causes the arrest of CD4SP cells at the SP3 stage in Relb−/− mice.
Our finding of a developmental defect of CD4SP cells in Aire−/− mice unveils a previously undescribed aspect of Aire function. But the underlying mechanism remains to be determined. Recent observations indicate that, in addition to the induction of peripherally restricted antigens, Aire is implicated in the transcriptional regulation of a number of molecules involved in antigen processing and presentation by mTECs (33). Therefore, it is not surprising that Aire deficiency also may affect other functions of mTECs, such as the capacity to induce final maturation of SP thymocytes. Such a possibility should be testable by using thymic stromal cell lines prepared from Aire−/− mice and reintroducing a functional Aire gene into these lines.
In conclusion, our studies have clearly defined a developmental program for CD4SP medullary thymocytes and established the critical role of the transcriptional regulator Aire and medullary epithelial cells in this differentiation process. Such knowledge should facilitate inquiries into the negative selection and other important medullary events in T lymphopoiesis.
Materials and Methods
Mice.
BALB/cJ and C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and bred in the Animal Breeding Facilities of the Peking University Health Science Center. Aire−/− mice (37) were generously provided by Leena Peltonen (University of California, Los Angeles, CA) and back-crossed at least six times to C57BL/6 mice before use in the animal colony at University of Chicago. Relb−/− mice were bred in the Animal Breeding Center at the Walter and Eliza Hall Institute of Medical Research. All animal studies were approved by the local ethnic committees.
Reagents.
Anti-CD8 (3.155) was prepared from a hybridoma obtained from American Type Culture Collection (Manassas, VA). 6C10 (SM6C10), which recognizes a glycosylated epitope on Thy-1, was a kind gift of Linna Ding (National Institutes of Health, Bethesda, MD). All other antibodies used in the study were from BD PharMingen (San Diego, CA). Con A was purchased from Amersham Pharmacia (Piscataway, NJ); PMA, ionomycin, and brefeldin A were purchased from Sigma–Aldrich (St. Louis, MO); [3H]thymidine (specific activity 22 mCi·mmol−1·liter−1) was from the Beijing Atomic Energy Research Institute (Beijing, China); and mitomycin C was from Kyowa (Tokyo, Japan).
Cell Lines.
mTEC1 (derived from BALB/c mice) (38) and mTEC9 (derived from C57BL/6 mice) were established and maintained in this laboratory. The cells were pretreated with 50 μg/ml mitomycin C for 1 h at 37°C before being used in coculture with CD4SP thymocytes of the same genetic background.
Flow Cytometry and Cell Sorting.
Freshly isolated thymocytes were stained with fluorochrome-labeled mAbs and analyzed on FACS Calibur or FACS Aria by using either Cellquest (BD Biosciences) or Flow Jo (Tree Star, CA) software. For the isolation of different subsets of CD4SP medullary thymocytes, single-thymocyte suspension was treated with anti-CD8 (3.155) mAb and complement (guinea pig sera), and the removal of CD8+ cells was confirmed by staining with another anti-CD8 mAb (53–6.7). The resulting viable cells were then stained for CD4, TCRβ, CD69, and 6C10 or Qa-2 and sorted into various subsets with FACS Aria (BD Biosciences). The purity of cells harvested was >97% when reanalyzed by flow cytometry.
Intrathymic Cell-Adoptive Transfer Assay.
SP1 (TCR+6C10+CD69+) CD4SP thymocytes were isolated from CD45.1+ C57BL/6 donors by sorting. The purified cells were then injected into the thymus of CD45.2+ C57BL/6 recipients as described (39). At 24, 48, or 72 h after injection, thymocytes were harvested, and the phenotypic maturation of donor cells was analyzed by gating on CD45.1+ cells.
In Vitro SP Thymocyte Culture.
SP1 CD4SP thymocytes were isolated from 6-week-old mice and cultured in 30 ng/ml IL-7-containing medium in the presence or absence of mTEC1 or mTEC9. The culture was harvested at various time points, and the surface phenotype of the recovered cells was determined by using flow cytometry.
Proliferation and Cytokine Secretion Assay.
The four major subsets of CD4SP thymocytes or splenic CD4+ T cells were isolated from mice of different ages, seeded at 1 × 105 per well into 96-well tissue plates, and cultured in the presence of 2.5 μg/ml Con A for 72 h. Then 0.5 μCi per well [3H]thymidine was included in the culture in the last 12 h. Cells were harvested and measured for [3H]thymidine incorporation. For the analysis of cytokine secretion, the cells were seeded at 1 × 106 per well, and the culture supernatant was collected after 48 h of stimulation with Con A. IL-2 was determined by bioassays using the IL-2-dependent cell line, HT-2, whereas IL-4 and IFN-γ were assayed with ELISA kits (BD PharMingen).
Acknowledgments
We thank Dr. H Zhou for critical reading of the manuscript. This work was supported by National Basic Research Program of China Grants 2006CB504300 and 2006CB910101 and Natural Science Foundation of China Grants 30330520 and 30525044.
Abbreviations
- Dn
day n after birth
- DN
double-negative
- DP
double-positive
- Fdn
fetus of day n
- HSA
heat-stable antigen
- LTβR
lymphotoxin β-receptor
- mTEC
medullary thymic epithelial cell
- SP
single-positive
- TCR
T cell receptor
- TEC
thymic epithelial cell
- Treg
regulatory T
- UEA-1
Ulex europaeus agglutinin-1.
Footnotes
The authors declare no conflict of interest.
References
- 1.Ciofani M, Zuniga-Pflucker JC. Immuol Res. 2006;34:117–132. doi: 10.1385/IR:34:2:117. [DOI] [PubMed] [Google Scholar]
- 2.Takahama Y. Nat Rev Immunol. 2006;6:127–135. doi: 10.1038/nri1781. [DOI] [PubMed] [Google Scholar]
- 3.Rothenberg EV, Taghon T. Annu Rev Immunol. 2005;23:601–649. doi: 10.1146/annurev.immunol.23.021704.115737. [DOI] [PubMed] [Google Scholar]
- 4.Scollay R, Godfrey DI. Immunol Today. 1995;16:268–273. doi: 10.1016/0167-5699(95)80179-0. [DOI] [PubMed] [Google Scholar]
- 5.Bendelac A, Schwartz RH. Nature. 1991;353:68–71. doi: 10.1038/353068a0. [DOI] [PubMed] [Google Scholar]
- 6.Ramsdell F, Jenkins M, Dinh Q, Fowlkes BJ. J Immunol. 1991;147:1779–1785. [PubMed] [Google Scholar]
- 7.Surh CD, Sprent J. Nature. 1994;372:100–103. [PubMed] [Google Scholar]
- 8.Sprent J, Kishimoto H. Immunol Rev. 2002;185:126–135. doi: 10.1034/j.1600-065x.2002.18512.x. [DOI] [PubMed] [Google Scholar]
- 9.Weih F, Carrasco D, Durham SK, Barton DS, Rizzo CA, Ryseck RP, Lira SA, Bravo R. Cell. 1995;80:331–340. doi: 10.1016/0092-8674(95)90416-6. [DOI] [PubMed] [Google Scholar]
- 10.Burkly L, Hession C, Ogata L, Reilly C, Marconi LA, Olson D, Tizard R, Cate R, Lo D. Nature. 1995;373:531–536. doi: 10.1038/373531a0. [DOI] [PubMed] [Google Scholar]
- 11.Heino M, Peterson P, Sillanpaa N, Guerin S, Wu L, Anderson G, Scott HS, Antonarakis SE, Kudoh J, Shimizu N, et al. Eur J Immunol. 2000;30:1884–1893. doi: 10.1002/1521-4141(200007)30:7<1884::AID-IMMU1884>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 12.Zhu M, Chin RK, Christiansen PA, Lo JC, Ware C, Siebenlist U, Fu YX. J Clin Invest. 2006;116:2964–2971. doi: 10.1172/JCI28326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boehm T, Scheu S, Pfeffer K, Bleul CC. J Exp Med. 2003;198:757–769. doi: 10.1084/jem.20030794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kajiura F, Sun S, Nomura T, Izumi K, Ueno T, Bando Y, Kuroda N, Han H, Li Y, Matsushima A, et al. J Immunol. 2004;172:2067–2075. doi: 10.4049/jimmunol.172.4.2067. [DOI] [PubMed] [Google Scholar]
- 15.Chin RK, Lo JC, Kim O, Blink SE, Christiansen PA, Peterson P, Wang Y, Ware C, Fu YX. Nat Immunol. 2003;4:1121–1127. doi: 10.1038/ni982. [DOI] [PubMed] [Google Scholar]
- 16.Akiyama T, Maeda S, Yamane S, Ogino K, Kasai M, Kajiura F, Matsumoto M, Inoue J. Science. 2005;38:248–251. doi: 10.1126/science.1105677. [DOI] [PubMed] [Google Scholar]
- 17.Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, et al. Science. 2002;298:1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 18.Liston A, Lesage S, Wilson J, Peltonen L, Goodnow CC. Nat Immunol. 2003;4:350–354. doi: 10.1038/ni906. [DOI] [PubMed] [Google Scholar]
- 19.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Caton AJ. Nat Immunol. 2001;2:283–284. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 20.Tai X, Cowan M, Feigenbaum L, Singer A. Nat Immunol. 2005;6:152–162. doi: 10.1038/ni1160. [DOI] [PubMed] [Google Scholar]
- 21.Fontenot JD, Dooley JL, Farr AG, Rudensky AY. J Exp Med. 2005;302:901–906. doi: 10.1084/jem.20050784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vicari A, Abehsira-Amar O, Papiernik M, Boyd RL, Tucek CL. J Immunol. 1994;152:2207–2213. [PubMed] [Google Scholar]
- 23.Dyall R, Nikolic-Zugic J. J Exp Med. 1995;181:235–245. doi: 10.1084/jem.181.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vanhecke D, Verhasselt B, Debacker V, Leclercq G, Plum J, Vandekerckhove B. J Immunol. 1995;155:4711–4718. [PubMed] [Google Scholar]
- 25.Lucas B, Vasseur F, Penit C. J Immunol. 1994;153:53–62. [PubMed] [Google Scholar]
- 26.Gabor MJ, Godfrey DI, Scollay R. Eur J Immunol. 1997;27:2010–2015. doi: 10.1002/eji.1830270827. [DOI] [PubMed] [Google Scholar]
- 27.Ge Q, Chen WF. Immunology. 1999;97:665–671. doi: 10.1046/j.1365-2567.1999.00816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tian T, Zhang J, Gao L, Qian XP, Chen WF. Int Immunol. 2001;13:313–320. doi: 10.1093/intimm/13.3.313. [DOI] [PubMed] [Google Scholar]
- 29.Ge Q, Chen WF. Int Immunol. 2000;12:1127–1133. doi: 10.1093/intimm/12.8.1127. [DOI] [PubMed] [Google Scholar]
- 30.Rooke R, Waltzinger G, Benoist C, Mathis D. Immunity. 1997;7:123–134. doi: 10.1016/s1074-7613(00)80515-4. [DOI] [PubMed] [Google Scholar]
- 31.DeKoning J, DiMolfetto L, Reilly C, Wei Q, Havran WL, Lo D. J Immunol. 1997;158:2558–2566. [PubMed] [Google Scholar]
- 32.Guerin S, Baron ML, Valero R, Herrant M, Auberger P, Naquet P. Eur J Immunol. 2002;32:1–9. doi: 10.1002/1521-4141(200201)32:1<1::AID-IMMU1>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 33.Anderson MS, Venanzi ES, Chen Z, Berzins SP, Benoist C, Mathis D. Immunity. 2005;23:227–239. doi: 10.1016/j.immuni.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 34.Shores EW, Van Ewijk W, Singer A. Eur J Immunol. 1991;21:1657–1661. doi: 10.1002/eji.1830210711. [DOI] [PubMed] [Google Scholar]
- 35.Shores EW, Van Ewijk W, Singer A. Int Immunol. 1994;6:1393–1402. doi: 10.1093/intimm/6.9.1393. [DOI] [PubMed] [Google Scholar]
- 36.Kurobe H, Liu C, Ueno T, Saito F, Ohigashi I, Seach N, Arakaki R, Hayashi Y, Kitagawa T, Lipp M, et al. Immunity. 2006;24:165–177. doi: 10.1016/j.immuni.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 37.Ramsey C, Bukrinsky A, Peltonen L. Hum Mol Genet. 2002;11:3299–3308. doi: 10.1093/hmg/11.26.3299. [DOI] [PubMed] [Google Scholar]
- 38.Chen WF, Fan W, Cao LX, Zhang PX. Eur Cytokine Netw. 1992;3:43–52. [PubMed] [Google Scholar]
- 39.Goldschneider I, Komschlies KL, Greiner DL. J Exp Med. 1986;163:1–17. doi: 10.1084/jem.163.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]