Abstract
Hepatitis B virus (HBV) is a hepadnavirus that is a major cause of acute and chronic hepatitis in humans. Hepatitis B viral infection itself is noncytopathic, and it is the immune response to the viral antigens that is thought to be responsible for hepatic pathology. Previously, we developed a transgenic mouse model of primary HBV infection and demonstrated that the acute liver injury is mediated by nonclassical natural killer (NK)T cells, which are CD1d-restricted, but nonreactive to α-GalCer. We now demonstrate a role for NKG2D and its ligands in this nonclassical NKT cell-mediated immune response to hepatitis B virus and in the subsequent acute hepatitis that ensues. Surface expression of NKG2D and one of its ligands (retinoic acid early inducible-1 or RAE-1) are modulated in an HBV-dependent manner. Furthermore, blockade of an NKG2D–ligand interaction completely prevents the HBV- and CD1d-dependent, nonclassical NKT cell-mediated acute hepatitis and liver injury. This study has major implications for understanding activation of NKT cells and identifies a potential therapeutic target in treating hepatitis B viral infection.
Infection with hepatitis B virus (HBV) is a major cause of liver disease worldwide. More than 350 million people are persistently infected with HBV (1, 2). Hepatitis B viral infection itself is noncytopathic, and it is the immune response to the viral antigens that is thought to be responsible for the necroinflammatory process involved in chronic infection, cirrhosis, and hepatocellular carcinoma (3, 4). Thus, understanding the pathogenesis of acute and chronic HBV infection mandates understanding the immune responses underlying these processes. Unfortunately, the study of HBV immunopathogenesis has been problematic because natural hepadnaviral infections occur only in outbred species whose immune systems are difficult to experimentally manipulate.
We have established a transgenic mouse model of primary HBV infection that allows the study of mechanisms underlying the immunopathogenesis of hepatitis B virus-induced disease. To generate this mouse model of primary HBV infection, we used either mice that express the small, middle, and large envelope proteins of HBV as transgenes in the liver by using an albumin promoter (hereafter designated HBV-Env+) (5) or mice that express a terminally redundant HBV DNA construct as a transgene and display intrahepatic HBV replication (hereafter designated HBV-Replication+) (6). We ablated the resident adaptive immune system of these HBV-transgenic mice, in which the B and T cells are tolerant to HBV, by crossing the HBV-transgenic mice to mice with mutations in the recombinase activating genes (RAG-1) (7). We then reconstituted the immune system in these mice by the adoptive transfer of unimmunized splenocytes isolated from syngeneic, wild-type mice. In this way, a liver with HBV-expressing hepatocytes is exposed to a healthy, untolerized, naïve immune system, just as in acute HBV infection of humans. This system results in a biphasic illness, with a rapid acute hepatitis, followed by a smoldering chronic hepatitis (8).
In this model of HBV infection, a spontaneous, natural immune response to hepatitis B virus is generated. Because this experimental system mimics primary HBV infection, we can uniquely study innate and adaptive immune responses to HBV. Our previous results using this model demonstrated that natural killer (NK)T cells (and not NK cells or conventional αβ-TcR T cells) alone are sufficient to induce hepatitis when adoptively transferred into HBV transgenic RAG−/− mice. Furthermore, we demonstrated, using several different experimental approaches, that this population of NKT cells that mediates acute hepatitis is nonclassical in that these cells do not recognize the classic NKT cell ligand, α-galactosyl-ceramide CD1d and do not express the canonical T cell receptor Vα14; but activation of these cells depends on expression of CD1d and HBV.
Innate immune effector cells mediate the acute hepatitis in our model, although the mechanism of activation of these cells in response to the presence of HBV in liver is unclear. Our previous data suggest that the presence of HBV leads to alterations in the class I-like molecule CD1d and, subsequently, to the activation of nonclassical NKT cells and hepatitis (8). NKT and NK cells share many of the same activating and inhibitory receptors. One of the activating receptors is NKG2D, a type II transmembrane-anchored glycoprotein, which has been shown to be an activating or costimulatory receptor expressed on the surface of all NK cells, activated CD8+ T lymphocytes, and most γ/δ T cells, both in mice and humans (9–11). Although NKG2D is known to also be expressed on the surface of NKT cells (12, 13), a role for NKG2D in NKT cell activation has never been demonstrated.
NKG2D binds to a family of ligands with structural homology to MHC class I molecules. In contrast to classical MHC molecules, NKG2D ligands do not require association with β2 microglobulin for expression or function, and do not bind antigenic peptides (14). In mice, NKG2D ligands include the retinoic acid early-inducible 1 family of proteins (RAE-1α, β, γ, δ, ε), H60, and MULT1 (15–17). The ligands of NKG2D are known to be “stress-inducible” molecules, whose expression is triggered by cellular transformation, viral infection (9), and/or DNA damage (18).
In this study, we addressed the question of whether NKG2D and its ligands play a role in the nonclassical NKT cell-mediated immune response to HBV and the subsequent acute hepatitis that ensues. Our results demonstrate that NKG2D is modulated on NK and NKT cells at the time of acute hepatitis; and the presence of HBV in the livers of our transgenic mice can lead to an increase in RAE-1 surface expression on hepatocytes. Furthermore, blockade of an NKG2D–ligand interaction completely prevents the HBV-specific and CD1d-dependent, nonclassical NKT cell-mediated acute hepatitis and liver injury.
Results
NKG2D Expression Is Modulated on Intrahepatic Immune Cells from HBV-Env+ RAG−/− Mice with Acute Hepatitis.
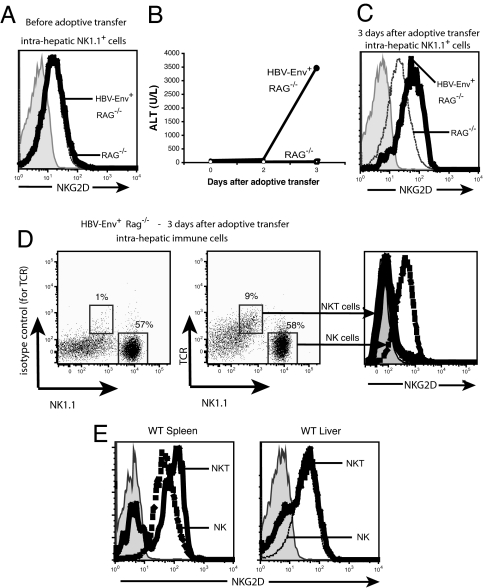
Our previous studies have demonstrated that activation of nonclassical NKT cells is necessary for the acute hepatitis to develop and that NK cells or conventional T cells alone cannot initiate the acute hepatitis (8). NK1.1+ cells from the livers of HBV-Env+ RAG−/− and RAG−/− mice before adoptive transfer of syngeneic naïve splenocytes expressed equivalent amounts of NKG2D on their surface (Fig. 1A). However, when we analyzed the expression of NKG2D on liver lymphoid cells during the acute immune response and hepatitis seen in the livers of HBV-expressing mice with reconstituted immunity (Fig. 1B), we found that NK1.1+ cells from HBV-Env+ RAG−/− mice (which include both resident and donor NK cells and donor NKT cells) expressed higher levels of NKG2D than the same population eluted from RAG−/− mice that also had reconstituted immunity (Fig. 1C).
Fig. 1.
Modulation of the NKG2D receptor during acute hepatitis. (A and C) NKG2D expression on the surface of NK1.1+ cells from HBV-Env+ RAG−/− (heavy black line) and RAG−/− (dotted line) before (A) and 3 days after (C) adoptive transfer of 108 splenocytes. (B) Hepatic necrosis in these animals was assessed by the measurement of ALT in the sera of RAG−/− (○) or HBV-Env+ RAG−/− (●) mice. ALT values are shown as mean ± SEM. (D) Surface expression of NKG2D on intrahepatic NKT (heavy black line) and NK cells (dashed line) from HBV-Env+ RAG−/− mice 3 days after adoptive transfer. The left dot plot depicts the isotype-matched control Ig staining of TCR on NKT cells. (E) Surface expression of NKG2D on NKT cells (heavy black line) and NK cells (dashed line) from the spleen and liver of wild-type mice. The shaded histograms depict staining using an isotype-matched control rat IgG1. All experiments were repeated at least three times, and representative data are shown.
We next analyzed the surface expression of NKG2D on the NKT and NK populations in the liver at the peak of acute hepatitis. We found that NK cells eluted from the livers of HBV-Env+ RAG−/− mice with acute hepatitis expressed high levels of NKG2D (Fig. 1D), but the majority of activated NKT cells expressed very low levels of NKG2D on their cell surface (Fig. 1D). Because the majority of NKT cells in the spleen (the cells adoptively transferred) and liver of wild-type mice expressed high levels of NKG2D (Fig. 1E), this result suggests that NKT cells eluted from the livers of the HBV-Env+ RAG−/− mice have down-regulated the surface expression of NKG2D. This is consistent with the fact that NKG2D is known to be internalized after interaction with its ligands (8, 14, 19). Taken together, these results suggest that NKG2D expression is up-regulated on the NK cells, and down-regulated on the NKT cells, during acute hepatitis.
Constitutive Surface Expression of RAE-1 on Hepatocytes Is Elevated Specifically on HBV-Env+ Hepatocytes.
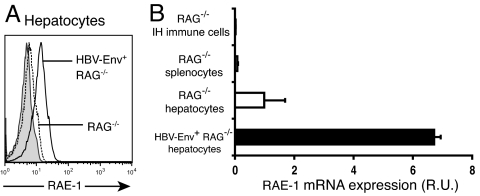
In light of these data, we examined the expression of NKG2D ligands on wild-type nontransgenic hepatocytes and on HBV-Env+ hepatocytes. In the genetic background of the HBV-transgenic mice (B10.D2 and C57BL/6), the NKG2D ligands expressed are RAE-1δ, RAE-1ε, and MULT1 (14). Although RAE-1 is not expressed in most tissues isolated from healthy, adult mice, RAE-1 is transcribed preferentially in liver of healthy, adult mice (http://source.stanford.edu/cgi-bin/source/sourceSearch). We examined the expression of these NKG2D ligand proteins on primary hepatocytes and intrahepatic immune cells of HBV-Env+ RAG−/− and wild-type nontransgenic RAG−/− mice before adoptive transfer. We found constitutive low-level surface expression of RAE-1 on hepatocytes from RAG−/− mice, which was increased specifically on the surface of HBV-Env+ hepatocytes (Fig. 2A). This constitutive expression of RAE-1 on hepatocytes, and increased expression in the HBV-Env+ transgenic mice, was also found in wild-type mice that were not crossed to RAG−/− mice (data not shown). We found no expression of RAE-1 on splenocytes or on intrahepatic immune cells from HBV-Env+ RAG−/− mice, RAG−/− mice, or wild-type mice (Fig. 2B and data not shown). The constitutive surface expression of RAE-1 on hepatocytes is an interesting finding, because the expression of RAE-1 family members is strictly regulated in normal cells, and little expression is found on healthy adult tissue (14). Increased RAE-1 expression on hepatocytes from HBV-Env+ RAG−/− mice demonstrates that RAE-1 can be modulated on hepatocytes in an HBV-specific manner. We did not detect MULT1 expression, or a change in either MHC class I or CD1d expression, on primary hepatocytes derived from either HBV-Env+ RAG−/− or RAG−/− mice or on intrahepatic immune cells (data not shown).
Fig. 2.
Constitutive surface expression of the NKG2D ligand RAE-1 on hepatocytes is up-regulated specifically on HBV-Env-expressing hepatocytes, before adoptive transfer. (A) Surface expression of RAE-1 on hepatocytes from HBV-Env+ RAG−/− (solid line) and RAG−/− (dotted line) mice. Shaded histograms depict staining using the appropriate isotype-matched control Ig. (B) RAE-1 mRNA expression in hepatocytes from HBV-Env+ RAG−/− (black bar) and RAG−/− (white bar) mice and from intrahepatic (IH) immune cells and splenocytes from RAG−/− mice in comparison with HPRT expression. All data are representative of at least three independent experiments.
Constitutive expression of RAE-1 on primary hepatocytes from RAG−/− mice was confirmed by quantitative PCR of reverse-transcribed RAE-1 mRNA normalized to the expression of Hprt transcripts (Fig. 2B). Increased RAE-1 expression on hepatocytes from HBV-Env+ RAG−/− mice, as compared with RAG−/− mice, was also confirmed by quantitative RT-PCR. There was an almost 7-fold increase in RAE-1 mRNA from HBV-Env+ RAG−/− hepatocytes, as compared with RAG−/− hepatocytes (Fig. 2B).
Blocking of an NKG2D–Ligand Interaction in Vivo Prevents the Acute Immune Response to Human HBV.
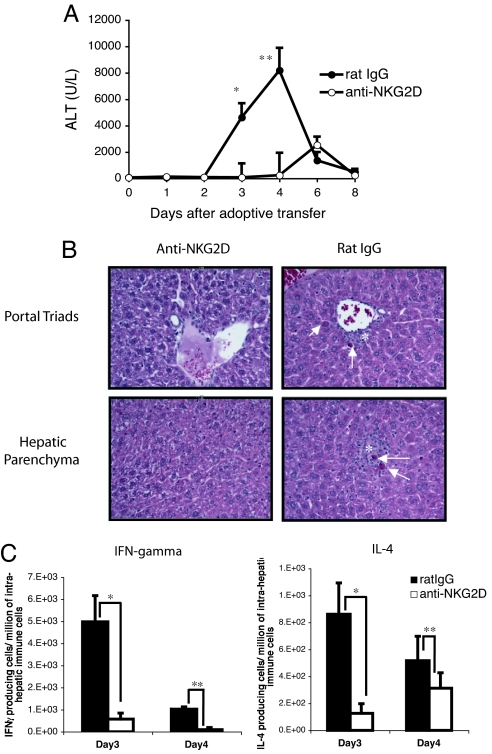
In view of the fact that NKG2D and one of its ligands are modulated during the acute immune response to HBV, we studied the effects of blocking this interaction on the onset of the acute hepatitis by using an anti-mouse NKG2D monoclonal antibody (CX5), which efficiently blocks the binding of NKG2D to its ligands and does not deplete NKG2D-bearing cells in vivo (20). HBV-Env+ RAG−/− recipient mice were treated with 200 μg of anti-NKG2D mAb (CX5) or control rat IgG the day before and 4 days after adoptive transfer of syngeneic naïve splenocytes. Blocking the NKG2D receptor completely prevented acute liver injury in all HBV-Env+ RAG−/− mice, whereas the control antibody had no effect as all mice showed signs of massive acute hepatitis, as revealed by the elevated serum ALT values at days 3 and 4 after adoptive transfer (Fig. 3A). Histological analyses of liver sections also showed that mice treated with control IgG developed a severe hepatitis, pathologically characterized by parenchymal inflammation, hepatocellular damage, and portal inflammation and necrotic hepatocytes at day 4 after adoptive transfer (Fig. 3B). These histological abnormalities were absent at the same time point in all mice treated with anti-NKG2D mAb (Fig. 3B). These results demonstrate a fundamental role played by NKG2D in the acute immune response to HBV-expressing hepatocytes, and the consequent development of hepatitis and hepatic necrosis.
Fig. 3.
Blocking NKG2D in vivo prevents the liver injury caused by the acute immune response to HBV. (A) Serum ALT levels of HBV-Env+ RAG−/− mice treated with anti-NKG2D mAb (○) or rat IgG (●). The ALT values as mean ± SEM are shown. Student's t test analyses: *, P < 0.02; **, P < 0.01. (B) Hematoxylin and eosin-stained section (magnification ×20) of portal triads (Upper) and hepatic lobular parenchyma (Lower) from HBV-Env+ RAG−/− mice treated with anti-NKG2D mAb (Left) or rat IgG (Right), 4 days after the adoptive transfer of 108 splenocytes. Arrows point to necrotic hepatocytes, and asterisks indicate inflammatory infiltrate. (C) Elispot analyses of IFN-γ- and IL4-producing intrahepatic immune cells from HBV-Env+ RAG−/− mice treated with control rat IgG (black bars) or anti-NKG2D mAb (white bars) at days 3 and 4 after adoptive transfer. Representative data are shown as mean ± SD. Student's t test analyses: *, P < 0.005; **, P < 0.02. All data are representative of at least three independent experiments.
HBV-Env+ RAG−/− mice have an HBV-dependent increase in the frequency of IFN-γ- and IL-4-producing cells in their livers 3 days after adoptive transfer (8). Because NKT cells mediate this cytokine burst detected at the time of acute hepatitis, we investigated the cytokine profile of lymphoid cells in anti-NKG2D or control IgG-treated HBV-Env+ RAG−/− mice. We quantified the number of IFN-γ- and IL-4-producing intrahepatic immune cells by Elispot at days 3 and 4 after adoptive transfer of syngeneic wild-type splenocytes. Three days after the adoptive transfer, the number of IFN-γ- and IL-4-producing cells increased by 8- and 7-fold, respectively, in mice that received control IgG (and developed hepatitis) as compared with NKG2D-blocked mice (Fig. 3C). A similar difference was observed on day 4 after adoptive transfer. These data demonstrate that blocking of NKG2D also severely impaired the production of cytokines by intrahepatic immune cells in mice expressing HBV antigens. Flow cytometric analysis of the intrahepatic immune cells derived from the anti-NKG2D or control IgG-treated HBV-Env+ RAG−/− mice revealed that the absolute number of NK cells eluted from both groups of mice was similar, whereas the absolute number of NKT cells was reduced by 2- and 3-fold in the mice that received the anti-NKG2D treatment and did not develop hepatitis [supporting information (SI) Table 1]. This specific reduction in the number of NKT cells, but not NK cells, in the livers of the anti-NKG2D-treated mice suggests that the antibody is specifically affecting the NKT cells.
NKG2D Receptor Expression on NKT Cells Is Required for Efficient Disease Induction.
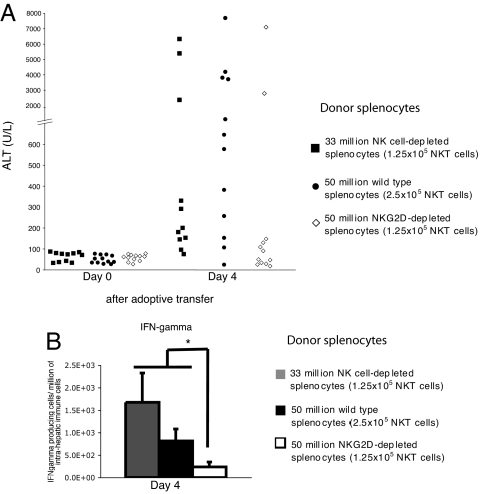
NKG2D is expressed on ≈60% of NKT cells in the spleen (Fig. 1E). To determine whether donor NKT cells expressing NKG2D are responsible for induction of the acute hepatitis after transfer into HBV-Env+ RAG−/− mice, splenocytes from wild-type mice were depleted of NKG2D+ lymphocytes by flow cytometric cell sorting. NKG2D-depleted splenocytes were adoptively transferred into HBV-Env+ RAG−/− recipients. In this way, the transferred donor NKT cells would not express surface NKG2D, but resident NK cells in the recipient mice would still express NKG2D. HBV-Env+ RAG−/− mice received NKG2D-depleted splenocytes or appropriate controls for the total number of splenocytes, or total number of NK and NKT cells transferred. The HBV-Env+ RAG−/− recipient mice received one of three different populations of donor splenocytes: 50 million NKG2D-depleted splenocytes (which included 1.25 × 105 NKG2D− NKT cells, and no NK cells); 50 million unsorted splenocytes (which included 2.5 × 105 unsorted NKT cells and 1.25 × 106 NK cells); or 33 million NK cell-depleted splenocytes (which included 1.25 × 105 unsorted NKT cells, and no NK cells). In this latter group, the NK cells were depleted from the donor mice by injection of anti-asialoGM1 antisera, which is known to deplete NK cells, but not NKT cells (21).
The depletion of NKG2D+ NKT cells, but not the depletion of NKG2D-bearing NK cells, from donor splenocytes significantly diminished the acute liver injury and cytokine burst seen in the HBV-Env+ RAG−/− mice as compared with either control group (Fig. 4 A and B). Thus, NKG2D receptor expression on NKT cells and not on NK cells is required for efficient disease induction. The finding that depletion of NKG2D-bearing cells did not completely eliminate all disease in all recipient mice could be accounted for the fact that the NKG2D− NKT cells to begin to express surface NKG2D after adoptive transfer (data not shown). Further evidence that NKG2D expression only on NKT cells is necessary for disease induction comes from the finding that depletion of NKG2D+ NKT cells from donor splenocytes completely prevented induction of acute hepatitis in the HBV-Env+ RAG−/− mice (SI Fig. 6). Thus, NKG2D expression on NKT cells, and not on any other cell types, is necessary for induction of hepatitis.
Fig. 4.
NKG2D+ NKT cells are required for efficient disease induction and cytokine production during the acute immune response to HBV. (A) Hepatic injury of HBV-Env+ RAG−/− mice at day 4 after adoptive transfer of 50 × 106 NKG2D-depleted splenocytes (which included 1.25 × 105 NKG2D− NKT cells, and no NK cells) (open diamonds) was compared with hepatic injury in HBV-Env+ RAG−/− mice that received the same total number of unsorted wild-type splenocytes (50 × 106, which included 2.5 × 105 unsorted NKT cells, and 1.25 × 106 NK) (filled circles) (Mann–Whitney test analyses: P < 0.02) or that received the same total number of unsorted NKT cells and NK cells (33 × 106, which included 1.25 × 105 unsorted NKT cells, and no NK cells) (filled squares). (Mann–Whitney test analyses: P < 0.03). (B) Elispot analyses of IFN-γ producing intrahepatic immune cells from HBV-Env+ RAG−/− mice depicted in A. Representative data are shown as mean ± SD. Student's t test analyses: *, P < 0.001.
NKG2D Blockade Prevents Acute Hepatitis and the Cytokine Burst Seen in HBV-Replication+ RAG−/− Transgenic Mice.
To assess the relevance of our observations to responses to authentic human HBV infection, we characterized the role of NKG2D in the acute immune response developed in HBV-Replication+ RAG−/− mice, which display intrahepatic HBV replication and produce infectious virions (6). Reminiscent of the usual initial presentation of human HBV infection, these mice develop a mild, subclinical hepatitis after adoptive transfer of naïve splenocytes. Analogous to our observations in the HBV-Env+ RAG−/− mice, this hepatitis is mediated by nonclassical NKT cells in an HBV-specific and CD1d-dependent manner, leading to cytokine production (ref. 8 and unpublished data). Although the severity of hepatitis seen in the two lines of HBV-transgenic mice is different, owing to an increase in hepatocyte sensitivity to cytotoxic effects of IFN-γ in the HBV-Env+ mice (22), a similar disease pattern is seen in both lines of HBV-transgenic mice. Specifically, a biphasic ALT rise that is seen in the HBV-Env+ RAG−/− mice is also observed in the HBV-Replication+ RAG−/− mice, but, as expected, the ALT rise is much more modest than that seen in the HBV-Env+ mice, typically, serum transaminases were elevated no more than 2-fold above background, which is a clinically significant finding in human HBV disease.
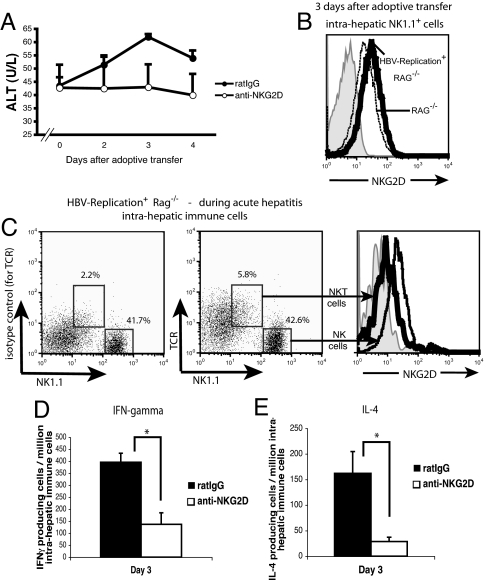
HBV-Replication+ RAG−/− mice were treated the day before the adoptive transfer of syngeneic naïve splenocytes with 200 μg of anti-NKG2D or control IgG, and we monitored the serum ALT levels. We found that the modest rise in the serum ALT in the HBV-Replication+ RAG−/− mice treated with control IgG was not evident in mice treated with anti-NKG2D (Fig. 5A). Together with this modest rise of ALTs, we observed that 3 days after the adoptive transfer, the number of IFN-γ- and IL-4-producing cells increased by 3- and 6-fold, respectively, in mice that received control IgG as compared with NKG2D-blocked mice (Fig. 5 D and E). To evaluate the role of NKG2D in acute hepatitis developed in HBV-Replication+ mice, we examined the expression of NKG2D on NK1.1+ cells from HBV-Replication+ RAG−/− mice 3 days after adoptive transfer. Just as we observed in the HBV-Env+ RAG−/− mice, NK cells from the livers of HBV-Replication+ RAG−/− mice had increased expression of NKG2D during acute hepatitis, as compared with RAG−/− mice; and the NKT cells had down-regulated NKG2D (Fig. 5 B and C).
Fig. 5.
Blocking an NKG2D–ligand interaction in HBV-Replication+ RAG−/− mice prevents liver injury and cytokine production mediated by the acute immune response to HBV. (A) Serum ALT levels of HBV-Replication+ RAG−/− mice treated with anti-NKG2D mAb (○) or rat IgG (●) at 2, 3, and 4 days after adoptive transfer of 1 × 108 syngeneic splenocytes are shown as mean ± SEM. (B) NKG2D surface expression on intrahepatic NK1.1+ cells from HBV-Replication+ RAG−/− mice (heavy black line) as compared with RAG−/− mice (dotted line) at day 3 after the adoptive transfer of syngeneic naïve splenocytes. Shaded histogram depicts staining using the appropriate isotype-matched control Ig (rat IgG1). (C) Surface expression of NKG2D on intrahepatic NKT (heavy black line) and NK cells (lighter black line) from HBV-Replication+ RAG−/− mice 3 days after adoptive transfer. The left dot plot depicts the isotype-matched control Ig staining of TCR on NKT cells. (D and E) Elispot analyses of IFN-γ (D) and IL-4-producing (E) intrahepatic immune cells from HBV-Replication+ RAG−/− mice treated with rat IgG (black bars) or anti-NKG2D mAb (white bars) at day 3 after adoptive transfer. Representative data are shown as mean ± SD. Student's t test analyses: *, P < 0.001. All data are representative of at least two independent experiments.
Discussion
Collectively, our findings clearly establish a role for NKG2D in the HBV-specific, CD1d-restricted nonclassical NKT cell-mediated acute hepatitis and cytokine production seen in both the HBV-Env+ RAG−/− and HBV-Replication+ RAG−/− mice. These results demonstrate a role for an NKG2D–ligand interaction in NKT cell activation. NKG2D is expressed on several cell types in the liver. However, NKG2D-bearing NK cells alone, or splenocytes depleted only of NKG2D+ NKT cells do not induce acute hepatitis in the HBV-Env+ RAG−/− and HBV-Replication+ RAG−/− mice (ref. 8 and SI Fig. 6).
A direct effect of NKG2D blockade on NKT cell activation in our studies is suggested by several lines of evidence. First, anti-NKG2D mAb treatment efficiently prevented production of IL-4, which is expressed by the HBV-activated NKT cells but not NK cells. Second, anti-NKG2D mAb treatment decreased the number of NKT cells, but not the number of NK cells, in the livers of mice with hepatitis. Finally, NKG2D receptor expression on NKT cells, and not on other cells types, is required for efficient disease induction in our transgenic model of primary HBV infection.
For these reasons, we propose a model in which nonclassical NKT cells are first activated in a HBV-specific, CD1d-restricted and NKG2D-dependent manner, leading to production of cytokines, which in turn activate NK cells. NKG2D ligand interaction can function to directly activate cells or function as a costimulatory molecule (9–11). That the activation of nonclassical NKT cells in our mouse model of HBV infection requires expression of HBV and CD1d, in addition to an NKG2D-ligand interaction, suggests that nonclassical NKT cell activation requires a CD1d-dependent signal through its T cell receptor and that NKG2D may function as a costimulatory molecule. Further studies will be required to address whether NKG2D is also important in the subsequent NK cell activation. While our studies were under review, Chen et al. (23) reported the ability of NKG2D blockade to diminish hepatitis in HBV-transgenic mice; however, in these experiments, induction of the disease required the injection of the mitogen Con A, which polyclonally activates all T cells and possibly other cell types such as NK cells in the host.
We detected the NKG2D ligand, RAE-1, on the surface of all hepatocytes in normal, wild-type as well as HBV transgenic mice. This constitutive expression of RAE-1 was increased on hepatocytes from the HBV-Env+ RAG−/− mice. Unlike the HBV-Env+ RAG−/− mice, the amount of RAE-1 on hepatocytes from the HBV-Replication+ RAG−/− mice was not elevated compared with nontransgenic hepatocytes (data not shown). NKG2D is nonetheless necessary for the nonclassical NKT cell activation and onset of hepatitis in both the HBV-Env+ RAG−/− mice and HBV-Replication+ RAG−/− mice because disease was completely prevented and cytokine production was greatly diminished by anti-NKG2D blockade. Therefore, we hypothesize that the constitutive, basal levels of RAE-1 on the hepatocytes are sufficient to trigger the HBV-specific, CD1d-restricted, NKG2D-dependent, nonclassical NKT cell-mediated hepatitis. This constitutive surface expression of RAE-1 on hepatocytes is also an interesting finding, because the expression of RAE-1 family members is strictly regulated in normal cells, and little expression is found on healthy adult tissue.
Because the HBV-Env+ RAG−/− mice have increased expression of one of the three isoforms of HBV envelope protein (large or L protein) that is retained in the endoplasmic reticulum, these mice display increased sensitivity to the cytotoxic effects of IFN-γ (22). The up-regulation of RAE-1 in the liver of these mice may be a direct or indirect consequence of increased large envelope expression. Increased expression and accumulation of envelope proteins is also one of the pathophysiologic consequences of HBV infection in humans (24). Because expression of HBV large envelope genes is zonal (6, 25), it is possible that RAE-1 is increased on some hepatocytes that have higher expression of envelope protein, but we cannot detect the increased expression of RAE-1 because these cells are a minority of the total hepatocyte population.
Our present findings reveal a mechanism by which human HBV activates the innate immune system and sets up the cytokine milieu in which the subsequent adaptive immune response develops. The question of whether HBV alerts the innate immune system and what role the innate immune system plays in HBV pathogenesis is controversial. Studies of acute HBV infection in primates and humans reveal an initial quiescent phase of ≈4–7 weeks before HBV starts to replicate vigorously, reaching levels of 109 to 1010 copies per milliliter (26–28). Activation of components of the innate immune system are likely to play a central role in control of this initial HBV burst because HBV-DNA quantity decreases by almost 90% well in advance of the appearance of an antigen-specific CD8+ T cell response and hepatopathology (27–31). However, identification of the individual components of the innate immune system responsible for this rapid down-regulation of viral replication, and the mechanism of activation, has been elusive. NK cells have been implicated in this process, because there is an increase in the number of peripheral NK cells before the peak of viral replication (31). However, Northern blot and gene expression analysis of total liver RNA derived from core liver biopsies during this period have failed to reveal evidence of activation of innate immune effector pathways, leading to the hypothesis that HBV does not alert the innate immune system (32, 33).
Our current data demonstrating that nonclassical NKT cells are activated to produce cytokines in an HBV-specific, CD1d-restricted, and NKG2D-dependent manner is consistent with a role for these cells in the initial response to HBV. The finding that activation of these nonclassical NKT cells leads to a cytokine burst in the absence of overt hepatocellular injury in the HBV-Replication+ mice is consistent with the usual initial subclinical presentation of HBV infection. Using real-time PCR analysis on whole liver biopsies, we, like others, cannot detect an innate immune response (the presence of T cell receptor, IL-4, or IFN-γ transcripts) in the HBV-transgenic RAG−/− mice 3 days after adoptive transfer of syngeneic splenocytes (SI Fig. 7). In contrast, we clearly demonstrate the presence of NKT cells (using flow cytometry) and the production of IL-4 and IFN-γ (using ELispot assays) in the eluted lymphocytes from the same livers used in the real-time PCR experiments, as depicted in Figs. 1, 3, and 5 (data not shown). Thus, our data suggest that innate immune responses to HBV infection exist, and likely have been previously unappreciated because NKT cells represent only a small fraction of the total cell mass of the liver; thus, any NKT cell transcripts are diluted by the overwhelming abundance of hepatocyte RNA.
These mouse models of HBV infection lay the foundation for directed studies analyzing the role of NKT cells, NK cells, NKG2D, and its ligands in human HBV infection. In addition, because the activation of innate effector cells has also been implicated in hepatic flares in chronic HBV infection (34), our models offer the opportunity to examine the role of NKG2D and its ligands in chronic HBV infection, and suggest possible new strategies for therapeutic intervention in this disease.
Methods
Mice and Disease Model.
HBV-Env+ transgenic mice: mouse lineage 107-5D [official designation Tg (Alb-1.HBV) Bri66; inbred B10.D2, H-2d) (5) and HBV-Replication+ mice: lineage 1.3.46 [official designation, Tg (HBV 1.3 genome) chi46; inbred C57BL/6H-2b] (6) crossed to RAG-1 −/− mice. HBV-Tg × Rag-1−/− mice (8-to 10-week-old) were intravenously injected with donor splenocytes from 6- to 10-week-old wild-type B10.D2 or C57BL/6 male mice (The Jackson Laboratory, Bar Harbor, ME), respectively. Mice were bled by tail vein at the described intervals, and sera were collected. Other mice were killed at the indicated time points, and livers were perfused or collected for histology. All mice were kept in a pathogen-free facility at University of California (San Francisco, CA).
Alanine Aminotransferase (ALT).
Serum ALT was measured by the standard photometric method by using a COBAS MIRA plus autoanalyzer.
Isolation of Hepatocytes and Intrahepatic Immune Cells.
To obtain hepatocytes, livers were perfused via the thoracic portion of the inferior vein cava with a commercial liver perfusion medium (GIBCO, Carlsbad, CA) for 5 min, followed by incubation in a digestion media (DMEM Low Glucose 50%/F-12 50% mixture and 0.12–0.2 mg/ml collagenase) for 8 min. Livers were cut into small pieces and filtered through a 70-μm nylon cell strainer and centrifuged at 30 × g for 3 min. Immune cells were obtained as described (8).
Flow Cytometry.
Fc-block (2.4G2 anti-CD16/32 mAb) and fluorochrome-conjugated antibodies against TCRβ (H57), NK1.1 (PK136), CD1d (1B1), H-2Kd (SF1–1.1), or the appropriate isotype-matched control Ig were purchased from BD (Franklin Lakes, NJ). PE-labeled anti-NKG2D (CX5) antibody (rat IgG1 isotype) was purchased from eBioscience (San Diego, CA). Purified antibodies against RAE-1, which recognizes all known RAE-1 proteins (rat IgG2a isotype), and MULT1 were purchased from R & D Systems (Minneapolis, MN) (35). Cells were analyzed on a LSR II (BD) by using FlowJo software.
Cell Sorting.
Splenocytes were stained with anti-NKG2D mAb CX5 and the negative lymphocytes isolated. In some experiments, splenocytes were costained with anti-NKG2D (CX5), anti-TCRβ (H57), and anti-NK1.1 (PK136). Cells expressing all three cell surface markers (NKG2D+ NKT cells) were depleted. All flow cytometry sorting experiments showed >98% purity by using a FACS Aria cell sorter (BD).
TaqMan Quantitative RT-PCR.
Quantitative (real-time) RT-PCR was carried out by using an ABI 7300 according to the manufacture's instructions. Specific primers and probes were used for HPRT (19), pan-RAE-1 (20), IFNγ, TCRβ, and IL-4.
In Vivo Antibodies.
A neutralizing, nondepleting rat anti-mouse NKG2D mAb, clone CX5 (rat IgG1), generated as described (19), recognizes the NKG2D extracellular domain and blocks the binding of NKG2D to its ligands. We injected i.p. 200 μg of CX5 or control rat IgG (Sigma, St. Louis, MO) per recipient mouse the day before and 4 days after the adoptive transfer of syngeneic naïve splenocytes. To deplete NK cells, but not NKT cells, from donor splenocytes, we used a depleting rabbit anti-mouse/rat asialo GM1 polyclonal antibody purchased from Cedarlane Laboratories (21). Depletion of NK cells (<0.1%) was verified by flow cytometry before the adoptive transfer was performed.
ELISpot Assay.
Intrahepatic immune cells were eluted from mice at day 3 and/or day 4 after adoptive transfer. Cells were counted and immediately plated in an anti-cytokine mAb-coated 96-well microplate (ELISpot mouse IFN-γ and IL-4 kits; BD). Eight serial 2- or 3-fold dilutions were done in duplicate, per condition. Spots were counted automatically by using an AID ELISpot Reader.
Histology.
Liver was fixed, embedded in paraffin, and stained with hematoxylin and eosin. Liver sections were scored by an unbiased pathologist, according to the histopathologic standard scale for assessing viral hepatitis (36).
Supplementary Material
Acknowledgments
We thank Laura Vilarinho for technical advice; Gerald Willkom and Anna Bogdanova for technical support; João P. Pereira for technical advice and manuscript comments; and William Seaman, Samuel Baron, T. S. Benedict Yen, Stewart Cooper, and Don Ganem for critically reading the manuscript. This work was supported in part by the Burroughs Wellcome Fund, the American Liver Foundation, the Cancer Research Institute, and the University of California Liver Center (San Francisco, CA) (P30-DK26743). S.V. is supported by Portuguese Foundation for Science and Technology (POCI) 2010 Grant SFRH/BD/21982/2005. L.L.L. is an American Cancer Society Research Professor, and work was supported by National Institutes of Health Grant R37 AI066897.
Abbreviations
- ALT
alanine aminotransferase
- HBV
hepatitis B virus
- NK
natural killer
- RAE-1
retinoic acid early-inducible-1
- MULT1
murine ULBP-like transcript 1
- RAG−/−
recombinase activating gene-1.
Footnotes
Conflict of interest statement: The authors declare a conflict of interest (such as defined by PNAS policy). University of California (San Francisco, CA) has licensed intellectual property rights relating to this research for potential therapeutic development.
This article contains supporting information online at www.pnas.org/cgi/content/full/0708968104/DC1.
References
- 1.Chang TT, Gish RG, de Man R, Gadano A, Sollano J, Chao YC, Lok AS, Han KH, Goodman Z, Zhu J, et al. N Engl J Med. 2006;354:1001–1010. doi: 10.1056/NEJMoa051285. [DOI] [PubMed] [Google Scholar]
- 2.Lai CL, Shouval D, Lok AS, Chang TT, Cheinquer H, Goodman Z, DeHertogh D, Wilber R, Zink RC, Cross A, et al. N Engl J Med. 2006;354:1011–1020. doi: 10.1056/NEJMoa051287. [DOI] [PubMed] [Google Scholar]
- 3.Chisari FV, Ferrari C. Annu Rev Immunol. 1995;13:29–60. doi: 10.1146/annurev.iy.13.040195.000333. [DOI] [PubMed] [Google Scholar]
- 4.Ganem D, Schneider RJ. Fields Virology. Philadelphia: Lippincott, Williams & Wilkins; 2001. [Google Scholar]
- 5.Chisari FV, Filippi P, McLachlan A, Milich DR, Riggs M, Lee S, Palmiter RD, Pinkert CA, Brinster RL. J Virol. 1986;60:880–887. doi: 10.1128/jvi.60.3.880-887.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guidotti LG, Matzke B, Schaller H, Chisari FV. J Virol. 1995;69:6158–6169. doi: 10.1128/jvi.69.10.6158-6169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 8.Baron JL, Gardiner L, Nishimura S, Shinkai K, Locksley R, Ganem D. Immunity. 2002;16:583–594. doi: 10.1016/s1074-7613(02)00305-9. [DOI] [PubMed] [Google Scholar]
- 9.Cerwenka A, Lanier LL. Immunol Rev. 2001;181:158–169. doi: 10.1034/j.1600-065x.2001.1810113.x. [DOI] [PubMed] [Google Scholar]
- 10.Diefenbach A, Tomasello E, Lucas M, Jamieson AM, Hsia JK, Vivier E, Raulet DH. Nat Immunol. 2002;3:1142–1149. doi: 10.1038/ni858. [DOI] [PubMed] [Google Scholar]
- 11.Wu J, Song Y, Bakker AB, Bauer S, Spies T, Lanier LL, Phillips JH. Science. 1999;285:730–732. doi: 10.1126/science.285.5428.730. [DOI] [PubMed] [Google Scholar]
- 12.Jamieson AM, Diefenbach A, McMahon CW, Xiong N, Carlyle JR, Raulet DH. Immunity. 2002;17:19–29. doi: 10.1016/s1074-7613(02)00333-3. [DOI] [PubMed] [Google Scholar]
- 13.Raulet DH. Nat Rev Immunol. 2003;3:781–790. doi: 10.1038/nri1199. [DOI] [PubMed] [Google Scholar]
- 14.Lanier LL. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 15.Carayannopoulos LN, Naidenko OV, Fremont DH, Yokoyama WM. J Immunol. 2002;169:4079–4083. doi: 10.4049/jimmunol.169.8.4079. [DOI] [PubMed] [Google Scholar]
- 16.Cerwenka A, Bakker AB, McClanahan T, Wagner J, Wu J, Phillips JH, Lanier LL. Immunity. 2000;12:721–727. doi: 10.1016/s1074-7613(00)80222-8. [DOI] [PubMed] [Google Scholar]
- 17.Diefenbach A, Jamieson AM, Liu SD, Shastri N, Raulet DH. Nat Immunol. 2000;1:119–126. doi: 10.1038/77793. [DOI] [PubMed] [Google Scholar]
- 18.Gasser S, Orsulic S, Brown EJ, Raulet DH. Nature. 2005;436:1186–1190. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogasawara K, Hamerman JA, Hsin H, Chikuma S, Bour-Jordan H, Chen T, Pertel T, Carnaud C, Bluestone JA, Lanier LL. Immunity. 2003;18:41–51. doi: 10.1016/s1074-7613(02)00505-8. [DOI] [PubMed] [Google Scholar]
- 20.Ogasawara K, Hamerman JA, Ehrlich LR, Bour-Jordan H, Santamaria P, Bluestone JA, Lanier LL. Immunity. 2004;20:757–767. doi: 10.1016/j.immuni.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 21.Seki S, Hashimoto W, Ogasawara K, Satoh M, Watanabe H, Habu Y, Hiraide H, Takeda K. Immunology. 1997;92:561–566. doi: 10.1046/j.1365-2567.1997.00383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilles PN, Guerrette DL, Ulevitch RJ, Schreiber RD, Chisari FV. Hepatology. 1992;16:655–663. doi: 10.1002/hep.1840160308. [DOI] [PubMed] [Google Scholar]
- 23.Chen Y, Wei H, Sun R, Dong Z, Zhang J, Tian Z. Hepatology. 2007;46:706–715. doi: 10.1002/hep.21872. [DOI] [PubMed] [Google Scholar]
- 24.Davies SE, Portmann BC, O'Grady JG, Aldis PM, Chaggar K, Alexander GJ, Williams R. Hepatology. 1991;13:150–157. [PubMed] [Google Scholar]
- 25.Hollinger FB, Liang TJ. Fields Virology. Philadelphia: Lippincott, Williams & Wilkins; 2001. [Google Scholar]
- 26.Whalley SA, Murray JM, Brown D, Webster GJ, Emery VC, Dusheiko GM, Perelson AS. J Exp Med. 2001;193:847–854. doi: 10.1084/jem.193.7.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guidotti LG, Rochford R, Chung J, Shapiro M, Purcell R, Chisari FV. Science. 1999;284:825–829. doi: 10.1126/science.284.5415.825. [DOI] [PubMed] [Google Scholar]
- 28.Bertoletti A, Ferrari C. Hepatology. 2003;38:4–13. doi: 10.1053/jhep.2003.50310. [DOI] [PubMed] [Google Scholar]
- 29.Jilbert AR, Wu TT, England JM, Hall PM, Carp NZ, O'Connell AP, Mason WS. J Virol. 1992;66:1377–1388. doi: 10.1128/jvi.66.3.1377-1388.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kajino K, Jilbert AR, Saputelli J, Aldrich CE, Cullen J, Mason WS. J Virol. 1994;68:5792–5803. doi: 10.1128/jvi.68.9.5792-5803.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Webster GJ, Reignat S, Maini MK, Whalley SA, Ogg GS, King A, Brown D, Amlot PL, Williams R, Vergani D, et al. Hepatology. 2000;32:1117–1124. doi: 10.1053/jhep.2000.19324. [DOI] [PubMed] [Google Scholar]
- 32.Wieland S, Thimme R, Purcell RH, Chisari FV. Proc Natl Acad Sci USA. 2004;101:6669–6674. doi: 10.1073/pnas.0401771101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wieland SF, Chisari FV. J Virol. 2005;79:9369–9380. doi: 10.1128/JVI.79.15.9369-9380.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dunn C, Brunetto M, Reynolds G, Christophides T, Kennedy PT, Lampertico P, Das A, Lopes AR, Borrow P, Williams K, et al. J Exp Med. 2007;204:667–680. doi: 10.1084/jem.20061287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lodoen M, Ogasawara K, Hamerman JA, Arase H, Houchins JP, Mocarski ES, Lanier LL. J Exp Med. 2003;197:1245–1253. doi: 10.1084/jem.20021973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scheuer PJ. J Hepatol. 1991;13:372–374. doi: 10.1016/0168-8278(91)90084-o. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.