Abstract
The danger theory of immune tolerance asserts that environmental factors hold primacy over lymphocyte autoreactivity in initiating autoimmune disease. We sought to test this contention using the Aire-deficient mouse model of the human disease, autoimmune polyendocrinopathy–candidiasis–ectodermal dystrophy, a multiorgan autoimmune disorder rooted in a lesion in thymic tolerance. Compound screens stimulating a broad range of innate immune system pathways failed to show any modulation of disease characteristics in Aire−/− mice on either the C57BL/6 or NOD genetic backgrounds. Furthermore, deficiency in the Toll-like receptor adaptor Myd88 increased the lifespan of NOD.aire−/− mice but did not prevent the initiation of autoimmunity. Finally, germ-free NOD.aire−/− mice exhibited autoimmunity in all organs normally targeted in this model, indicating that microbial conditioning is not required for activation of autoreactive T cells relevant to this disease. Together, these data suggest that the stochastic genesis of dangerous T cell clones can initiate autoimmune disease without the need for environmental stimulation, underlining the importance of Aire-dependent thymic deletion.
Keywords: autoimmunity, environment, immunological tolerance
The induction of central tolerance is an important means of preventing autoimmunity. Thymocytes expressing randomly generated α:β T cell receptors that recognize MHC:self-peptide molecules with high avidity are deleted by apoptosis or somehow functionally altered to remove autoreactive clones (1). The diversity of MHC:self-peptide molecules presented to thymocytes is very broad owing to ectopic expression of a vast array of peripheral-tissue antigens by medullary epithelial cells (2). The importance of this thymic representation of peripheral self is exemplified in humans and mice deficient in the autoimmune regulator (AIRE in humans, Aire in mice). Aire regulates the transcription of thousands of peripheral-tissue antigens by medullary epithelial cells (E. Venanzi, C.B., and D.M., unpublished data); in its absence, impaired expression and presentation of these self-antigens cause a spontaneous, multiorgan autoimmune disease (3).
Genetic background strongly influences the manifestation of autoimmunity in Aire-deficient mice, with varying severity and organ targeting observed in different inbred strains (4, 5). Genetic polymorphism seems to dictate the range and severity of organ-specific autoimmunity, but variability in the onset and targeting of disease among Aire-deficient mice on the same genetic background indicates that environmental and/or stochastic factors are also at play. In light of the discrete breakdown of thymic self/non-self discrimination upon Aire deficiency, the extent to which environmental influences elicit autoimmunity is an important question in the context of current models of immune activation, especially the danger theory (6), which predicts that a “primary hit” involving exogenous (environmental) stimulation of innate immunity would be required for activation of autoreactive T cells emerging from the Aire-deficient thymus.
We set out to test this idea by analyzing whether (i) stimulation of various innate immune system pathways, (ii) a deficiency in Toll-like receptor (TLR) signaling, and (iii) removal of microorganisms would influence the development of autoimmune disease in Aire-deficient mice.
Results
Innate Immune System Stimuli and Autoimmunity in Aire−/− Mice.
To identify pathways that influence the initiation of autoimmunity in aire−/− mice, we administered, before any clinical signs of disease, a variety of compounds that stimulate various arms of innate immunity. C57BL/6 (B6).aire−/− mice were used in screens of factors that have the potential to worsen autoimmune disease because this strain exhibits a mild autoimmunity in only a few organs, thereby providing a low baseline. The compounds, doses, and modes (i.e., single or repetitive administration) that were tested targeted a range of TLR and non-TLR innate immune system pathways previously reported to modulate disease in other autoimmunity models (Table 1) (e.g., ref. 7). Effects on the treated mice were monitored until 20 weeks of age, whereupon the animals were killed and necropsied and sections from all major organs were scored for autoimmune infiltrates.
Table 1.
Autoimmunity in B6.aire−/− mice treated with innate immune stimuli
| Treatment | Pathway | Clinical scores |
|---|---|---|
| Untreated | — | 3, 4, 4, 5, 6 |
| LPS | TLR-4 | 0, 3, 3, 5 |
| poly(I:C) | TLR-3 | 0, 4, 4, 4 |
| poly(I:C), rep | TLR-3 | 3, 5, 5 |
| CpG | TLR-9 | 2, 3, 4, 5, 6 |
| CpG, rep | TLR-9 | 5, 7, 8, 8 |
| poly(I:C) and CpG, rep | TLR-3 and TLR-9 | 4, 6 |
| Proteoglycan | TLR-2 | 5, 5 |
| PAM3CSK4 | TLR-2 | 1, 3, 5, 7 |
| Zymosan | TLR-2/dectin-1 | 6, 7 |
| β-1,3-glucan | Dectin-1 | 0, 2, 3, 4, 4 |
| Muramyl dipeptide | NOD-2 | 4, 5, 5, 7 |
| Anti-CD40 | CD40 | 2, 5, 6, 10 |
rep, repeated administrations. Infiltration was scored as outlined in Materials and Methods.
Consistent with previous reports, untreated B6.aire−/− mice exhibited retinopathy, sialitis, pneumonitis, prostatitis, and dacryoadenitis (4). In all mice treated, autoimmune infiltrates remained constrained to these organs, with localization and composition similar to those of controls, indicating that no new targets were revealed by any of the treatments (data not shown). The overall severity of autoimmunity was quantified by using a histological score that summed mild, moderate, and severe infiltrates (scores 1, 2, and 3, respectively) in each organ. Histological scores were not substantially different in the treatment and control groups (Table 1), suggesting that stimulation of innate immune system pathways does not increase the aggression of the response.
To assess whether stimulation of different pathways of the innate immune system could inhibit autoimmunity, we performed a similar screen using NOD.aire−/− mice. Aire deficiency on the NOD background results in spontaneous and reproducible autoimmune attack of multiple organs and eventual wasting and death of animals between 5 and 15 weeks of age (4, 5). Notably, the pancreatic islets, which are normally the target of autoimmunity in NOD mice, were spared in animals lacking Aire; instead, the exocrine pancreas was progressively destroyed. These features distinguished NOD.aire−/− mice from other background strains as a robust model for the assessment of factors that may dampen autoimmunity. After treatment with the various compounds, NOD.aire−/− mice were monitored for signs of wasting and were killed if they lost 20% of their peak body weight or were killed at 15 weeks of age. Nearly all mice succumb to wasting by this end point, although there have been occasional survivors (4, 5). Histological assessments of autoimmune infiltrates were quantified by using a score tallied from the typical NOD.aire−/− target organs, namely the eyes, lungs, pancreas, stomach, ovaries, liver, prostate, and the lacrimal, thyroid, and salivary glands.
With few exceptions, wasting occurred within the normal timeframe in all treatment groups. Some mice in the groups receiving the TLR-2 agonist PAM3CSK4 or repeated doses of the TLR-3 ligand poly(I:C) survived to 15 weeks of age (Table 2). However, these animals exhibited high clinical scores, arguing against a substantial effect of the treatments on autoimmunity. One mouse treated with TLR-9-stimulatory CpG oligonucleotides did survive the 15 weeks free of infiltration of any target organs, but this was not a consistent observation with this treatment; indeed, another mouse treated with CpG exhibited the highest clinical score observed in the screen (Table 2).
Table 2.
Autoimmunity in NOD.aire−/− mice treated with innate immune stimuli
| Treatment | Pathway | Incidence | Wasting, day | Clinical scores |
|---|---|---|---|---|
| Untreated | — | 8/8 | 44, 44, 46, 50, 52, 58, 64, 72 | 4, 7, 7, 8, 9, 9, 10, 11 |
| LPS | TLR-4 | 3/3 | 60, 64, 79 | 8, 9, 16 |
| poly(I:C) | TLR-3 | 4/4 | 42, 54, 62, 62 | 8, 9, 10, 12 |
| poly(I:C), rep | TLR-3 | 4/6 | 47, 49, 57, 57, 102,* 105* | 2, 3, 8, 10, 10, 12 |
| CpG | TLR-9 | 4/5 | 25, 44, 57, 67, 105* | 0, 7, 7, 10, 21 |
| poly(I:C) and CpG, rep | TLR-3 and TLR-9 | 3/3 | 38, 50, 55 | 7, 12, 15 |
| Proteoglycan, rep | TLR-2 | 3/3 | 55, 64, 72 | 11, 11, 16 |
| PAM3CSK4 | TLR-2 | 2/4 | 57, 59, 104,* 104* | 8, 10, 11, 17 |
| PAM3CSK4, rep | TLR-2 | 3/3 | 48, 57, 67 | 12, 15, 16 |
| Zymosan | TLR-2/dectin-1 | 3/3 | 55, 64, 77 | 9, 15, 17 |
| β-1,3-glucan | Dectin-1 | 3/3 | 60, 66, 66 | 7, 8, 9 |
| Muramyl dipeptide | NOD-2 | 3/3 | 44, 51, 51 | 5, 5, 11 |
| Anti-CD40 | CD40 | 6/6 | 41, 45, 47, 48, 49, 51 | 7, 8, 9, 9, 10, 11 |
| Cyclophosphamide | Lymphopenia | 4/4 | 50, 70, 61, 82 | 8, 9, 10, 11 |
| CFA | ? | 3/3 | 43, 43, 67 | 8, 11, 13 |
Mice did not waste before termination of experiment. rep, repeated administrations. CFA, complete Freund's adjuvant.
*Survival to end point of study.
The combination of repetitive poly(I:C) and CpG administration, two treatments that separately enabled survival of some mice to the end of the study, did not affect wasting or autoimmunity in the small group tested (Table 2). Likewise, compounds with broad immunomodulatory effects, such as anti-CD40, cyclophosphamide, and complete Freund's adjuvant, showed no effect on the NOD.aire−/− disease. The previously reported low tolerance for cyclophosphamide by NOD.aire−/− mice (≈80% dying within 22 days of treatment) (5) was not recapitulated here. It should be noted, however, that the age of the mice upon treatment (and, presumably, the status of autoimmunity) differed in the two studies: Niki et al. (5) treated mice at 6–9 weeks of age, whereas the current study used 3- to 4-week-old NOD.aire−/− animals.
There were no obvious differences in the pattern of serum autoantibody reactivity on immunoblots of pancreatic extract among the various NOD.aire−/− treatment groups (data not shown), suggesting no strong effect of innate immune stimuli on the range of pancreatic targets.
In neither the B6 nor the NOD screens did treatment of aire+/− mice provoke autoimmunity.
Myd88 Deficiency Does Not Prevent Autoimmune Disease in Aire−/− Mice.
Although the experiments described above indicated that administration of known TLR ligands did not influence autoimmunity in Aire-deficient mice, it remained possible that TLR ligation by other microbial and/or “danger” signals was required for the disease observed. To further address this point, we crossed NOD.aire−/− mice with NOD animals deficient in the Myd88 cytoplasmic adaptor required for transduction of TLR (except TLR-3), IL-1R, and IL-18R signals. Autoimmunity and wasting disease in NOD.Myd88−/−.aire−/− mice were analyzed and compared with those of NOD.Myd88+/−.aire−/− controls (Fig. 1).
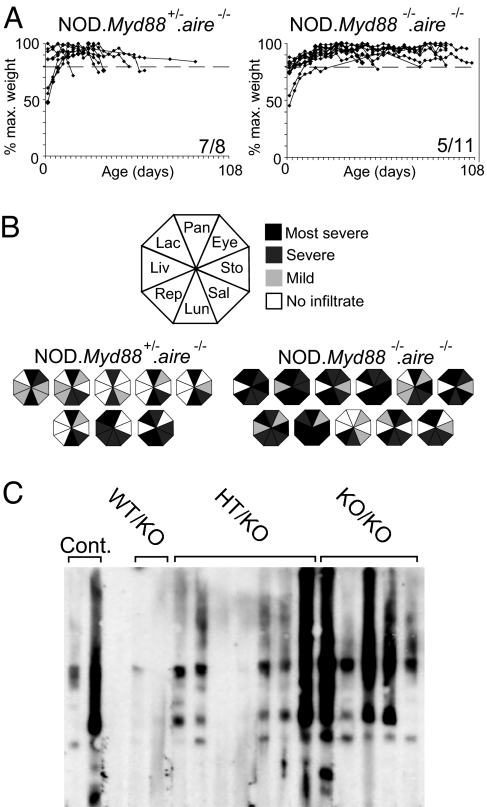
Fig. 1.
Myd88 is not necessary for development of autoimmune disease in NOD.aire−/− mice. (A) Weight curves for NOD.Myd88+/−.aire−/− and NOD.Myd88−/−.aire−/− mice. Animals were killed upon loss of 20% of peak body weight (shown by a dashed line) or at 15 weeks of age. Numbers at the bottom right of graphs denote the number of mice succumbing to wasting over the total number analyzed. (B) Summary of autoimmune infiltration in NOD.Myd88+/−.aire−/− and NOD.Myd88−/−.aire−/− mice at time of killing. Each octagon represents infiltration detected in individual mice, with segments corresponding to the organs indicated in the key filled according to severity. (C) Immunoblotting of NOD pancreatic extracts with serum from NOD.aire−/− controls and NOD.Myd88+/+.aire−/− (WT/KO), NOD.Myd88+/−.aire−/− (HT/KO), and NOD.Myd88−/−.aire−/− (KO/KO) mice. Each lane was incubated with serum from a single mouse.
NOD.
Myd88+/−.aire−/− mice exhibited the same incidence and timing of wasting as those observed for NOD.aire−/− animals (data not shown). By contrast, NOD.Myd88−/−.aire−/− mice showed a lower incidence of wasting by 15 weeks and delayed onset in those that succumbed (Fig. 1A). This delay is likely to reflect the well established role for the inflammatory cytokine IL-1 in models of wasting disease (8); however, the fact that several mice did succumb points to other, Myd88-independent mechanisms.
Interestingly, the double-knockout mice had more severe infiltration than that of the NOD.Myd88+/−.aire−/− controls (Fig. 1B) (mean score = 16 ± 4.0 versus 10.5 ± 2.9; P < 0.005). At 7 weeks, age-matched NOD.Myd88−/−.aire−/− and NOD.Myd88+/−.aire−/− mice had similar disease (mean clinical score = 7.5 ± 0.7 versus 7.8 ± 1.7), suggesting that the more extensive infiltration observed in the double-knockout animals at the time of killing reflected their longer survival time.
To determine whether autoantibody targeting was influenced by Myd88 deficiency, we probed immunoblots of pancreatic extracts with sera from individual NOD.Myd88−/−.aire−/− mice and controls (Fig. 1C). The distinctive pattern of reactivity elicited in NOD.aire−/− mice was also seen with NOD.Myd88−/−.aire−/− animals (Fig. 1C). The restriction of autoantibody targeting to just a few antigen species, highlighting the oligoclonal nature of the pancreatic autoimmunity, remained unchanged in the absence of Myd88.
Autoimmunity in Germ-Free (GF) Aire−/− Mice.
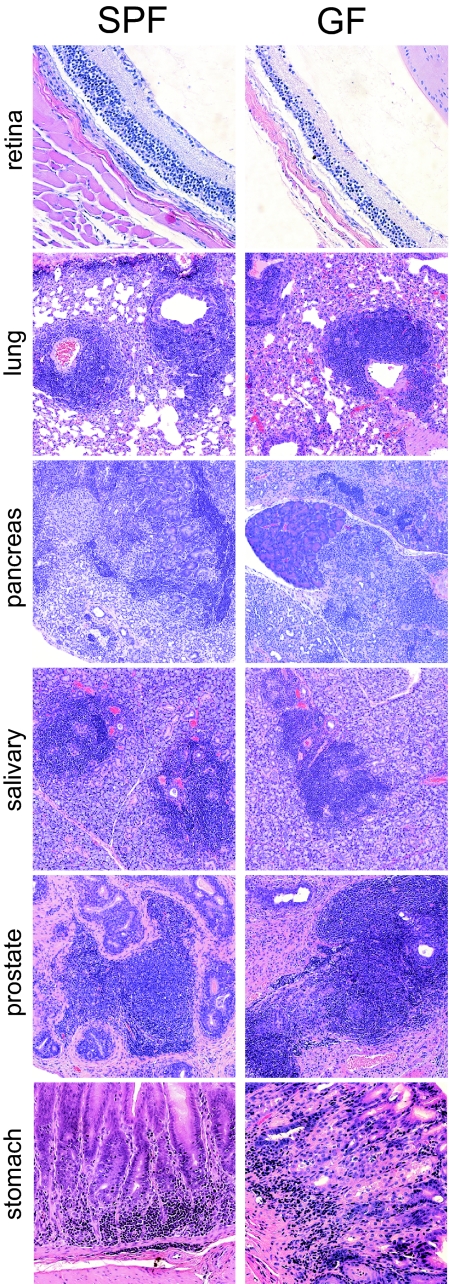
Although Myd88 deficiency cripples most TLR signaling pathways, a growing number of “non-TLR” pattern recognition receptors can also provoke innate immune system activation and/or inflammation (e.g., Nod1/2, Dectin-1) (9, 10). To determine whether stimulation of these pathways, or any microbial conditioning of the host, is required for initiation of the spontaneous autoimmunity caused by Aire deficiency, we derived NOD.aire−/− mice into GF conditions. Weight measurements revealed that, similar to their specific pathogen-free (SPF) counterparts, GF NOD.aire−/− mice began to waste from 5 weeks of age, with variable times of onset (data not shown). Mice that lost 20% or more of their body weight were killed, and their organs were analyzed for signs of infiltration or destruction. GF NOD.aire−/− mice exhibited the full range of autoimmune targets observed in SPF conditions, including retinal and ovarian degradation; infiltration of the liver, lacrimal, and thyroid glands; and exocrine pancreatitis, sialitis, pneumonitis, gastritis, and prostatitis (Fig. 2 and data not shown). The composition and localization of infiltrates were also very similar under the two husbandry conditions, consisting predominantly of lymphocytes and macrophages (Fig. 2). GF NOD.aire+/− and NOD.aire+/+ mice did not present this spectrum of infiltration, instead developing the insulitis, sialitis, and dacryoadenitis normally associated with this genetic background. These data indicate that microbial colonization of NOD.aire−/− mice is not required for autoimmunity.
Fig. 2.
Autoimmune infiltration in SPF and GF NOD.aire−/− mice. Shown are hematoxylin and eosin-stained retina, lung, pancreas, salivary gland (all ×10 objective), and stomach (×20 objective) sections from 7-week-old NOD.aire−/− mice housed in either SPF or GF conditions.
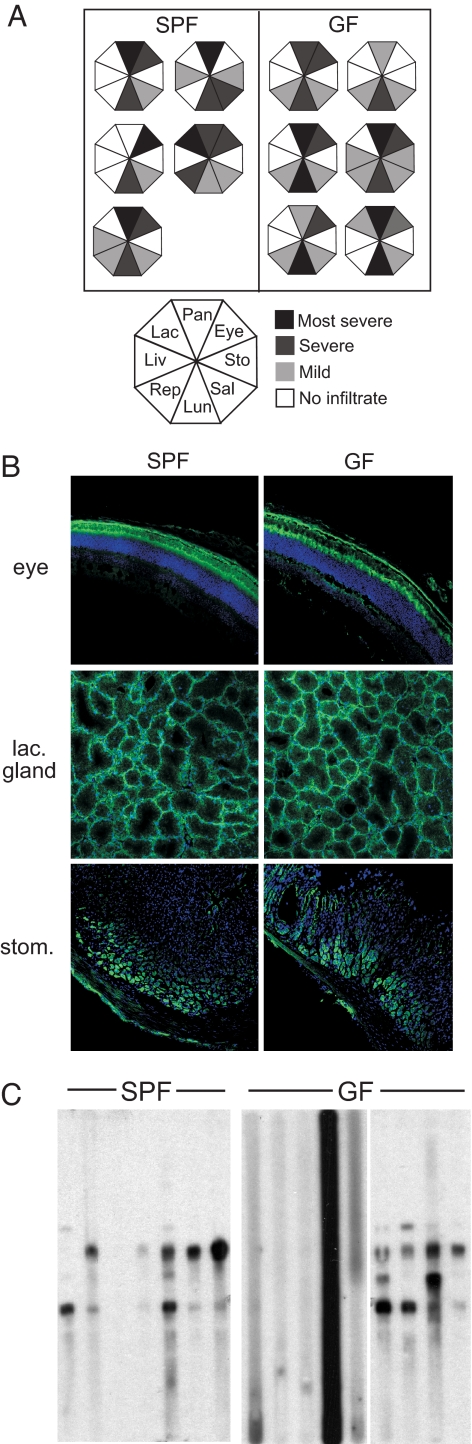
This finding prompted us to ask whether the severity of autoimmunity was any different under GF conditions. Infiltration was analyzed in a cohort of age-matched SPF and GF NOD.aire−/− mice before the onset of wasting. The organs targeted by autoimmunity in both groups were similar; infiltration was seen in almost all eye, pancreas, salivary gland, lung, and prostate/ovary sections (Fig. 3A). The average score for 7-week-old SPF NOD.aire−/− mice (8.2 ± 2.2) was not significantly different from that of age-matched GF NOD.aire−/− mice (8 ± 1.6).
Fig. 3.
Similar severity of autoimmunity in GF and SPF NOD.aire−/− mice. (A) Summary of autoimmune infiltrates from 7-week-old NOD.aire−/− mice housed in SPF or GF conditions. Each octagon represents infiltration detected in individual mice, with segments corresponding to the organs indicated in the key filled according to severity. (B) Representative staining of cryosections of eye, lacrimal gland, and stomach of Rag1-deficient NOD mice with sera from 7-week-old NOD.aire−/− mice housed in SPF or GF conditions (green) and DAPI (blue). (C) Immunoblots of pancreatic extract were probed with serum from 7-week-old NOD.aire−/− mice housed in SPF or GF conditions, with each lane showing autoantibody reactivity of individual mice. lac., lacrimal; stom., stomach.
Furthermore, total serum IgG levels were not significantly different in GF and SPF NOD.aire−/− mice (6.2 ± 3.2 mg/ml and 6.8 ± 2.8 mg/ml, respectively), indicating no gross deficiency in antibody production under GF conditions. Autoantibodies reactive to the photoreceptor layer of the retina, lacrimal gland epithelium, stomach parietal cells, and exocrine pancreas were present in GF serum, as they were in SPF Aire−/− mice (Fig. 3B and data not shown). These observations suggest that GF NOD.aire−/− mice are subject to the same course of autoimmunity against various tissues as their counterparts housed in SPF conditions. To investigate whether the apparent similarity of tissue targeting extends to the actual molecular species targeted, we probed pancreatic extracts with GF NOD.aire−/− mouse sera. Fig. 3C shows a pattern of reactivity with SPF NOD.aire−/− sera similar to that observed previously (4), with as many as four major targets. Sera from GF NOD.aire−/− mice appeared to target the same species, indicating that the course of autoimmunity was not skewed in the absence of microbes. Interestingly, more than half of the GF NOD.aire−/− sera showed no reactivity to pancreatic extracts at all (Fig. 3C). Given that these animals had no global defect in IgG production (8.7 ± 2.4 mg/ml total serum IgG in mice without pancreatic reactivity versus 4.2 ± 2.1 mg/ml in mice with reactivity; P = 0.064), this finding suggests that environmental conditioning may be required for some autoantibody targeting of the pancreas.
Discussion
It is generally held that the interaction of environmental factors with genetic traits plays a critical role in the initiation of autoimmune disease. The most convincing type of evidence for an environmental influence on human autoimmunity is the epidemiology of different diseases [e.g., multiple sclerosis in the Faroe Islands (11)]. The various concordance rates for different autoimmune diseases in monozygotic twins is also often cited in this regard, but this contention disregards stochastic events such as the generation of the T and B cell repertoire. With few exceptions (e.g., gliadin-induced celiac disease, reactive arthritis, and colitis), however, there is little direct evidence that exogenous factors trigger autoimmune responses. Nevertheless, the concept that an environmental stimulus is required for autoimmunity has been adopted and, indeed, is a lynchpin for the danger theory of immune tolerance. This model asserts that alarm signals produced or induced by microbes initiate autoimmune responses, whereas simple failure of self-discrimination by lymphocytes does not (6). Contrary to this notion, the data presented here indicate that differentiation of autoreactive T cells in the Aire-deficient thymus is sufficient to cause autoimmunity in an otherwise normal host, without evident environmental stimulation.
The absence of major differences in the patterns of autoantibodies produced after strong TLR stimulation or with Myd88 deficiency is pertinent to the ongoing debate regarding the dependency of antibody production upon TLR engagement (12, 13). Although we found no such relationship in this setting, it should be noted that these were only qualitative assessments, and measurement of specific antibody titers and isotypes may reveal more subtle differences.
GF animals are devoid of viable microorganisms. This dearth of immunostimulation has a quantitative effect on the immune system, with reductions in lymphoid tissue size and intraepithelial lymphocytes, loss of germinal centers, and decreased serum Ig and IgA production (14, 15). GF mice do, however, have normal proportions, numbers, and repertoires of circulating T and B cells and mount immune responses to antigenic stimulation comparable with those of conventionally housed controls (16, 17). It should be noted that, although the GF animals used in this study bore no viable microbes, it might be that they were exposed to low levels of microbial products, primarily in feed. Although it remains possible that this exposure might provide conditioning necessary for the initiation of autoimmunity in GF NOD.aire−/− mice, the data from NOD.Myd88−/−.aire−/− mice rule out a requirement for all TLR ligands, except those activating the TLR-3 pathways, in disease initiation. In addition, administration of TLR ligands (including TLR-3) did not exacerbate disease. The absence of modulation of autoimmunity by any of the non-TLR ligands examined (e.g., β-1,3-glucans, muramyl dipeptide) suggests that these exogenous signals are also not critical to disease in Aire-deficient mice.
Using GF mice, a requirement for microbial stimulation has been demonstrated for several very different models of autoimmune/inflammatory diseases (7, 18,19–21). By contrast, other autoimmune diseases do not appear to depend on microbes. The seemingly microbe-independent models have features in common with each other and with the Aire-knockout disease: NOD, MRL/lpr, and NZB mice are also spontaneous autoimmune models, each with a defined defect (or defects) in tolerance mechanisms (22–24). The finding that Aire-deficient mice fall into this latter category adds that lesions in central tolerance alone can give rise to autoreactive T cells that override peripheral tolerance mechanisms without exogenous stimulation. This example of a “pure” autoimmune disease highlights the possibility that microbial dependency among the various models of autoimmunity is dictated by whether they are primarily inflammatory or autoimmune in nature.
This finding also implies that thymocytes subject to Aire-dependent deletion are qualitatively different from autoreactive (but tolerized) T cells in normal hosts. This difference is likely to reflect the avidity of the T cell receptors for self-MHC:peptide molecules. Indeed, previous studies have shown that Aire-dependent deletion selectively removes clones with presumably high T cell receptor avidity (25, 26) and that avidity is a critical factor in determining the peripheral activation requirements that lead to autoimmunity (27, 28). In a nontransgenic setting, this concept may be typified by the obligatory role of adjuvant-induced inflammation for experimental autoimmune uveitis directed against interphotoreceptor retinoid-binding protein (29), the same autoantigen driving the retinopathy observed in aire-deficient mice (30). These findings are compatible with an “autoimmune threshold” model (e.g., ref. 31), and the current data extend this concept by showing that, in this instance, a danger signal is not necessary to elicit autoimmune disease. This leaves the Aire-deficient thymus as the driver of autoimmunity, representing a “time bomb” that will produce high-avidity, autoreactive T cells that can be activated by endogenous levels of self-peptide and costimulation.
Materials and Methods
Mice.
Aire-deficient mice were derived and genotyped as previously described (3) and were backcrossed to the B6 and NOD backgrounds for at least eight generations (4). B6.Myd88+/− mice (32) were backcrossed to the NOD background for 12 generations, then intercrossed with NOD.aire+/− mice. SPF mice were housed at the Center for Animal Resources and Comparative Medicine at Harvard Medical School. Pups from NOD.aire+/− matings were cesarean-rederived into GF conditions by Taconic Farms (Hudson, NY) and were maintained via intercrosses. GF mice were given sterilized food (NIH 31M) and water ad libitum and were tested weekly and determined to be free of aerobic and anaerobic bacteria, parasites, and fungal contamination. Sentinel mice were also tested routinely and found to be negative for viral serologies. A complete list of excluded organisms is available upon request.
Treatments.
SPF mice between 3 and 4 weeks of age were treated as follows: i.p. injections of LPS (50 μg; Sigma–Aldrich, St. Louis, MO), poly(I:C) (150-μg single dose or on alternate days for 2 weeks; InvivoGen, San Diego, CA), Pam3CSK4 (50 μg; InvivoGen), CpG-ODN1668 (10-nmol single dose or weekly for 4 weeks; MWG Biotech, High Point, NC), peptidoglycan from Staphylococcus aureus (4 weekly doses of 50 μg; Sigma–Aldrich), zymosan (2 mg; Sigma–Aldrich), β-1,3-glucan (curdlan, 3 mg; Wako, Osaka, Japan), muramyl dipeptide (250 μg; InvivoGen), cyclophosphamide (200 mg/kg split over 2 days; Sigma–Aldrich), anti-CD40 (FGK-45, four weekly doses of 100 μg), and complete Freund's adjuvant (200 μl; Difco, Detroit, MI).
Histology.
Tissues were fixed in buffered 10% formalin and embedded with paraffin. Sections were stained with hematoxylin and eosin, and lymphocytic infiltrates were scored as 0, 1, 2, or 3, indicating none/trace, mild, moderate, or severe infiltration. Retinal degradation was scored according to ref. 4. For immunohistology, 7-μm cryosections of eye, lacrimal gland, or stomach were prepared from 6- to 7-week-old NOD.Rag1−/− mice and were stained with 1:50 dilutions of sera followed by FITC-conjugated goat anti-mouse IgG (Jackson ImmunoResearch, West Grove, PA) and DAPI. Images were acquired with an Axiovert 200M confocal microscope (Zeiss, Oberkochen, Germany) using a xenon-arc lamp in a Lambda DG-4 wavelength switcher (Sutter Instrument, Novato, CA) and were processed with Slidebook imaging software (Intelligent Imaging, Dayton, OH).
Western Blotting.
Protein was extracted from NOD mouse pancreata in a Dounce homogenizer, with 20 μl of sample buffer (62.5 mM Tris·HCl, pH 6.8/25% glycerol/2% SDS/1% bromophenol blue/5% 2-mercaptoethanol) per milligram of tissue. The protein was briefly heated at 96°C, resolved on a 10% curtain gel by SDS/PAGE, and transferred onto polyvinylidene fluoride membrane (Bio-Rad, Hercules, CA). The membrane was blocked for 1 h in a 5% milk solution in TBS (25 mM Tris, pH 7.6/150 mM NaCl) and probed in a Protean II Multiscreen apparatus (Bio-Rad) with mouse sera diluted 1:500 for 2 h at 4°C. The membrane was washed with TBST (TBS/0.1% Tween 20), incubated with HRP-conjugated donkey anti-mouse IgG (1:3,000; Jackson ImmunoResearch), and revealed with SuperSignal chemiluminescent substrate reagents (Pierce, Rockford, IL) by autoradiography.
Serum IgG Measurement.
ELISA plates were coated with 5 μg/ml goat anti-mouse IgG (heavy and light chains; Jackson ImmunoResearch) at 4°C overnight. Unbound Ig was removed by washing the plates three times briefly with PBS containing 1% BSA and once with PBS alone. Test sera were diluted at 1:1,000 and added to the ELISA plates, in duplicates, for 1 h at room temperature. Uncaptured serum Ig was washed as above. Alkaline phosphatase-conjugated goat anti-mouse IgG (Fc-specific; Jackson ImmunoResearch) was added at a dilution of 1:2,000 at room temperature for 1 h; the antibody incubation was followed by another set of four washes. Bound Ig was detected by measuring the absorption at 405 nm of an alkaline phosphatase substrate buffered with 9.6% diethanolamine (vol/vol H2O) and 2 M MgCl2, as recommended by the manufacturer (Sigma–Aldrich). IgG concentrations were calculated by relating absorptions of test samples to those of a standard curve.
Statistical Tests.
A two-tailed Mann–Whitney rank sum U test was applied to data to assess statistical significance (P < 0.05 was considered significant).
Acknowledgments
We thank Dr. Roderick Bronson for expert assistance with histological scoring and Kimie Hattori, Vanessa Tran, and Stephanie Slingerland for animal husbandry. This work was supported by National Institutes of Health Grants T32 CA09382-22 and F32 AI62010-01 (to I.G.), P30 DK36836 (to the Joslin Diabetes and Endocrinology Research Center Core Facilities), and RO1 DK60027 and Young Chair funds (to D.M. and C.B.). D.H.D.G. received support from an Australian National Health and Medical Research C. J. Martin Overseas Biomedical Fellowship.
Abbreviations
- B6
C57BL/6
- GF
germ-free
- SPF
specific pathogen-free
- TLR
Toll-like receptor.
Footnotes
The authors declare no conflict of interest.
References
- 1.Palmer E. Nat Rev Immunol. 2003;3:383–391. doi: 10.1038/nri1085. [DOI] [PubMed] [Google Scholar]
- 2.Kyewski B, Klein L. Annu Rev Immunol. 2006;24:571–606. doi: 10.1146/annurev.immunol.23.021704.115601. [DOI] [PubMed] [Google Scholar]
- 3.Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, et al. Science. 2002;298:1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 4.Jiang W, Anderson MS, Bronson R, Mathis D, Benoist C. J Exp Med. 2005;202:805–815. doi: 10.1084/jem.20050693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niki S, Oshikawa K, Mouri Y, Hirota F, Matsushima A, Yano M, Han H, Bando Y, Izumi K, Matsumoto M, et al. J Clin Invest. 2006;116:1292–1301. doi: 10.1172/JCI26971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matzinger P. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 7.Yoshitomi H, Sakaguchi N, Kobayashi K, Brown GD, Tagami T, Sakihama T, Hirota K, Tanaka S, Nomura T, Miki I, et al. J Exp Med. 2005;201:949–960. doi: 10.1084/jem.20041758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooney RN, Maish GO, III, Gilpin T, Shumate ML, Lang CH, Vary TC. Shock. 1999;11:235–241. doi: 10.1097/00024382-199904000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Inohara N, Chamaillard M, McDonald C, Nunez G. Annu Rev Biochem. 2005;74:355–383. doi: 10.1146/annurev.biochem.74.082803.133347. [DOI] [PubMed] [Google Scholar]
- 10.Brown GD. Nat Rev Immunol. 2006;6:33–43. doi: 10.1038/nri1745. [DOI] [PubMed] [Google Scholar]
- 11.Kurtzke JF, Heltberg A. J Clin Epidemiol. 2001;54:1–22. doi: 10.1016/s0895-4356(00)00268-7. [DOI] [PubMed] [Google Scholar]
- 12.Pasare C, Medzhitov R. Nature. 2005;438:364–368. doi: 10.1038/nature04267. [DOI] [PubMed] [Google Scholar]
- 13.Gavin AL, Hoebe K, Duong B, Ota T, Martin C, Beutler B, Nemazee D. Science. 2006;314:1936–1938. doi: 10.1126/science.1135299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stepankova R, Sinkora J, Hudcovic T, Kozakova H, Tlaskalova-Hogenova H. Folia Microbiol (Praha) 1998;43:531–534. doi: 10.1007/BF02820814. [DOI] [PubMed] [Google Scholar]
- 15.Olson GB, Wostmann BS. J Immunol. 1966;97:275–286. [PubMed] [Google Scholar]
- 16.Bos NA, Ploplis VA. Eur J Immunol. 1994;24:59–65. doi: 10.1002/eji.1830240110. [DOI] [PubMed] [Google Scholar]
- 17.Vos Q, Jones LA, Kruisbeek AM. J Immunol. 1992;149:1204–1210. [PubMed] [Google Scholar]
- 18.Murakami M, Nakajima K, Yamazaki K, Muraguchi T, Serikawa T, Honjo T. J Exp Med. 1997;185:791–794. doi: 10.1084/jem.185.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goverman J, Woods A, Larson L, Weiner LP, Hood L, Zaller DM. Cell. 1993;72:551–560. doi: 10.1016/0092-8674(93)90074-z. [DOI] [PubMed] [Google Scholar]
- 20.Sadlack B, Merz H, Schorle H, Schimpl A, Feller AC, Horak I. Cell. 1993;75:253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- 21.Taurog JD, Richardson JA, Croft JT, Simmons WA, Zhou M, Fernandez-Sueiro JL, Balish E, Hammer RE. J Exp Med. 1994;180:2359–2364. doi: 10.1084/jem.180.6.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzuki T, Yamada T, Fujimura T, Kawamura E, Shimizu M, Yamashita R, Nomoto K. In: Immune-Deficient Animals in Biomedical Research. Rygaard J, Brunner N, Graem N, Spang-Thomsen M, editors. Switzerland: Karger, Basel; 1985. pp. 112–116. [Google Scholar]
- 23.Maldonado MA, Kakkanaiah V, MacDonald GC, Chen F, Reap EA, Balish E, Farkas WR, Jennette JC, Madaio MP, Kotzin BL, et al. J Immunol. 1999;162:6322–6330. [PubMed] [Google Scholar]
- 24.Unni KK, Holley KE, McDuffie FC, Titus JL. J Rheumatol. 1975;2:36–44. [PubMed] [Google Scholar]
- 25.Liston A, Lesage S, Wilson J, Peltonen L, Goodnow CC. Nat Immunol. 2003;4:350–354. doi: 10.1038/ni906. [DOI] [PubMed] [Google Scholar]
- 26.Anderson MS, Venanzi ES, Chen Z, Berzins SP, Benoist C, Mathis D. Immunity. 2005;23:227–239. doi: 10.1016/j.immuni.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Zehn D, Bevan MJ. Immunity. 2006;25:261–270. doi: 10.1016/j.immuni.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gronski MA, Boulter JM, Moskophidis D, Nguyen LT, Holmberg K, Elford AR, Deenick EK, Kim HO, Penninger JM, Odermatt B, et al. Nat Med. 2004;10:1234–1239. doi: 10.1038/nm1114. [DOI] [PubMed] [Google Scholar]
- 29.Su SB, Silver PB, Grajewski RS, Agarwal RK, Tang J, Chan CC, Caspi RR. J Immunol. 2005;175:6303–6310. doi: 10.4049/jimmunol.175.10.6303. [DOI] [PubMed] [Google Scholar]
- 30.Devoss J, Hou Y, Johannes K, Lu W, Liou GI, Rinn J, Chang H, Caspi R, Fong L, Anderson MS. J Exp Med. 2006;203:2727–2735. doi: 10.1084/jem.20061864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lehmann PV, Targoni OS, Forsthuber TG. Immunol Rev. 1998;164:53–61. doi: 10.1111/j.1600-065x.1998.tb01207.x. [DOI] [PubMed] [Google Scholar]
- 32.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]