Abstract
ACTH (i.e., corticotropin) is the principal regulator of the hypothalamus–pituitary–adrenal axis and stimulates steroidogenesis in the adrenal gland via the specific cell-surface melanocortin 2 receptor (MC2R). Here, we generated mice with an inactivation mutation of the MC2R gene to elucidate the roles of MC2R in adrenal development, steroidogenesis, and carbohydrate metabolism. These mice, the last of the knockout (KO) mice to be generated for melanocortin family receptors, provide the opportunity to compare the phenotype of proopiomelanocortin KO mice with that of MC1R–MC5R KO mice. We found that the MC2R KO mutation led to neonatal lethality in three-quarters of the mice, possibly as a result of hypoglycemia. Those surviving to adulthood exhibited macroscopically detectable adrenal glands with markedly atrophied zona fasciculata, whereas the zona glomerulosa and the medulla remained fairly intact. Mutations of MC2R have been reported to be responsible for 25% of familial glucocorticoid deficiency (FGD) cases. Adult MC2R KO mice resembled FGD patients in several aspects, such as undetectable levels of corticosterone despite high levels of ACTH, unresponsiveness to ACTH, and hypoglycemia after prolonged (36 h) fasting. However, MC2R KO mice differ from patients with MC2R-null mutations in several aspects, such as low aldosterone levels and unaltered body length. These results indicate that MC2R is required for postnatal adrenal development and adrenal steroidogenesis and that MC2R KO mice provide a useful animal model by which to study FGD.
Keywords: adrenocorticotropic hormone (ACTH), familial glucocorticoid deficiency (FGD), hypothalamus–pituitary–adrenal, zona fasciculata
The adrenal gland regulates a number of essential physiological functions in adult organisms through the production of steroids and catecholamines. Maintenance of adrenal structure and function is regulated through the integration of extra- and intracellular signals. The pituitary hormone ACTH (i.e., adrenocorticotropic hormone), which is derived from the proopiomelanocortin (POMC) polypeptide precursor, is the principal regulator that stimulates adrenal glucocorticoid (GC) biosynthesis and secretion via the membrane-bound specific receptor for ACTH, ACTH receptor/melanocortin 2 receptor (MC2R) (1).
It was previously demonstrated that, although POMC knockout (KO) mice are born at the expected Mendelian frequency, three-quarters of POMC KO mice undergo neonatal death. Furthermore, those mice surviving to adulthood exhibit obesity, pigmentation defects, and adrenal insufficiency (2–4). POMC KO mice possess macroscopically detectable adrenal glands that lack normal architecture (2, 4, 5). These results demonstrate the importance of POMC-derived peptides in regulating the hypothalamus–pituitary–adrenal axis and adrenal development.
Familial glucocorticoid deficiency (FGD), or hereditary unresponsiveness to ACTH [Online Mendelian Inheritance in Man (OMIM) no. 202200; www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=202200], is an autosomal recessive disorder resulting from resistance to the action of ACTH on the adrenal cortex. Affected individuals are deficient in cortisol and, if untreated, are likely to die as a result of hypoglycemia or overwhelming infection in infancy or childhood (6). Mutations of MC2R are responsible for 25% of FGD cases. Mutations of the MC2R accessory protein MRAP, which plays a role in the trafficking of MC2R from the endoplasmic reticulum to the cell surface, account for 20% of FGD cases (7), and a third locus responsible for FGD has been suggested (8). There has been no animal model for FGD, and MC2R KO mice are likely to become a valuable tool for the pathophysiological investigation of FGD.
To study specifically the roles of MC2R in adrenal gland development, steroidogenesis, and carbohydrate metabolism, we generated mice with an inactivation mutation of the MC2R gene. We demonstrated that disruption of MC2R leads to neonatal lethality in approximately three-quarters of MC2R KO pups, possibly as a result of hypoglycemia. Those surviving to adulthood exhibited macroscopically detectable adrenal glands with markedly atrophied zona fasciculata (zF) and lack of detectable levels of GC and reduced serum concentrations of aldosterone and epinephrine. Those surviving to adulthood exhibited hypoglycemia after prolonged (36 h) fasting as a result of the reduced expression of the genes involved in gluconeogenesis.
Results
Generation of MC2R KO Mice.
To generate MC2R KO mice, a targeting vector was constructed in which the portion of the MC2R gene encoding the entire coding region (9) was replaced with a neomycin-resistance gene cassette [supporting information (SI) Fig. 7A]. One of 545 neomycin-resistant colonies screened was positive as assessed by Southern analysis with an external probe (Fig. 7B). Chimeric founder mice were produced from the targeted ES cell clones, and germ-line transmission of the disrupted allele was obtained. MC2R KO mice were backcrossed to C57BL/6J mice for five generations before use in this study. To confirm the deficiency of MC2R, expression of the MC2R gene in the adrenal gland was examined by quantitative real-time PCR (qRT-PCR). No mRNA was detected in MC2R−/− mice, and expression was decreased by approximately half in MC2R+/− mice (Fig. 7C).
Most of the MC2R KO Pups Died Shortly After Birth.
Mice that were homozygous null for MC2R were obtained by interbreeding heterozygous mice. Pups lacking MC2R were born at the expected Mendelian ratio, suggesting that MC2R is not essential for embryonic development. Of 190 mice born from heterozygous MC2R KO parents, 61 pups were dead before weaning at 4 wk of age. Genotype analysis revealed that most of the 61 dead pups were homozygous for the MC2R allele. Genotype analysis of 129 mice at 4 wk revealed 9 homozygote, 74 heterozygote, and 46 WT mice. Approximately three-quarters of MC2R KO pups died before weaning, mostly within 48 h after birth. Most of the mutant newborn mice were indistinguishable from their WT littermates; some homozygous pups were pink and had milk in their stomachs, whereas some homozygous pups were lethargic and pale.
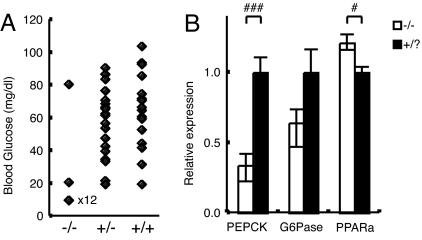
We analyzed blood glucose levels on postnatal day 0.5 at 1200 hours. Three of 57 mice had already died at the time of analysis (two were MC2R−/− and one was MC2R+/−). MC2R−/− pups were significantly hypoglycemic compared with MC2R+/? pups (Fig. 1A). We found only one of 14 homozygous pups that maintained normal blood glucose levels, comparable with those in WT mice. It is possible that this pup could survive neonatal death and grow to adulthood. Blood glucose levels for 12 of 14 homozygous pups were below detection level (<20 mg/dl). Analysis of blood glucose levels on postnatal day 7 revealed that MC2R−/− pups maintained glucose levels comparable with those of WT mice (data not shown). Expression of phosphoenolpyruvate carboxykinase (PEPCK), a rate-limiting enzyme for gluconeogenesis in liver, was significantly decreased, and expression of glucose-6-phosphatase (G6Pase) was relatively decreased in MC2R KO pups compared with MC2R+/? pups (Fig. 1B). The expression of peroxisome proliferator-activated receptor α (PPARα) responsible for β-oxidation of free fatty acids was significantly increased in MC2R KO pups (Fig. 1B). These results suggest that MC2R KO mice die as a result of hypoglycemia with decreased gluconeogenesis in the liver and defective neonatal nutritional adaptation. A slight increase in mortality was observed at 3–4 wk of age, due to undetermined cause(s), but no increase in mortality was observed after that period.
Fig. 1.
Neonatal hypoglycemia in MC2R KO mice. (A) Blood glucose levels on postnatal day 0.5 at 1200 hours. Detection limit was 20 mg/dl. Each point indicates the glucose level of a single pup. The blood glucose level of 12 of 14 homozygous pups was under detection level (<20 mg/dl). The values below detection level were plotted at 10 mg/dl. (B) MC2R pups were killed at 1200 hours, and liver RNAs were prepared. The experiments were performed with postnatal day 0.5 MC2R−/− (n = 6) and MC2R+/? (n = 9) mice. The expression of phosphoenolpyruvate carboxykinase (PEPCK), glucose-6-phosphatase (G6Pase), and peroxisome proliferator-activated receptor α (PPARa) in the liver was determined by qRT-PCR. Data are expressed as means ± SEM. Statistical significance was determined by t test. #, P < 0.05.
The body weights of 12-wk-old MC2R KO mice were indistinguishable from those of their littermates: MC2R−/−, 28.7 ± 0.8 g (n = 4); and MC2R+/+, 28.8 ± 0.6 g (n = 5). Whereas FGD patients with MC2R mutations exhibited increased height and POMC KO mice exhibited increased body length (10, 11), MC2R KO mice did not exhibit any significant difference in body length compared with that of their WT siblings: MC2R−/−, 9.58 ± 0.17 cm (n = 5); and MC2R+/?, 9.74 ± 0.07 cm (n = 10).
Adrenal Hypoplasia in MC2R KO Mice That Survived to Adulthood.
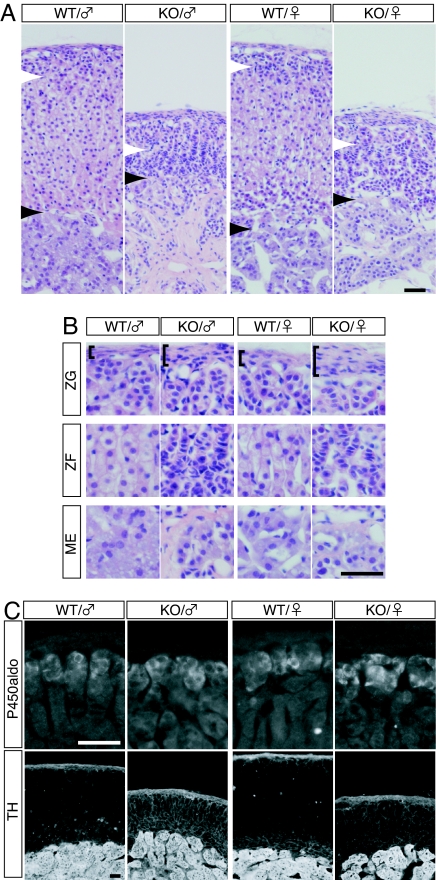
In MC2R KO mice, adrenal glands were considerably reduced in size compared with those of their WT siblings: male MC2R−/−, 0.58 ± 0.02 mg per pair of glands (n = 4); and MC2R+/+, 2.24 ± 0.18 mg per pair of glands (n = 5). The histological analysis revealed marked hypoplasia of zF in the mutant adrenal gland (Fig. 2A). The number of nuclei per 50-μm-wide column in the cortical area, however, was not significantly changed: male MC2R+/−, 138.2 ± 13.3; and MC2R−/−, 147.7 ± 19.8, P > 0.35; and female MC2R+/−, 149.7 ± 18; and MC2R−/−, 143.8 ± 20.7, P > 0.61. These results indicate that the total number of nuclei in the zF was similar in MC2R KO and WT mice. Higher-magnification images revealed that, in the MC2R KO mice, the nuclei in zF were more densely packed with reduced cytoplasmic volume (Fig. 2B), suggesting that a decrease in cell size, but not cell number, accounted for the hypoplasia of zF. On the other hand, the zona glomerulosa (zG) and the adrenal medulla (ME) remained fairly intact as shown in the histological sections (Fig. 2B). To confirm this idea, we examined the expression patterns of aldosterone synthase cytochrome P450 (P450aldo) and tyrosine hydroxylase (TH), markers for zG and ME, respectively. Both of these markers were similarly expressed in WT and MC2R KO mice (Fig. 2C), suggesting that the cells in zG and ME had differentiated into the appropriate cell types. We also noticed that the thickness of capsule was increased in MC2R KO mice (Fig. 2B, brackets).
Fig. 2.
Histological analysis of the adrenal gland of MC2R KO mice. (A and B) H&E staining of sections from the adrenal gland of WT or MC2R KO mice. Ten-week-old male or 13-wk-old female WT and MC2R KO mice were analyzed. (B) Higher-magnification images of zG, zF, and ME shown in A. The white and black arrowheads in A indicate the border between zG/zF and cortical zone/ME, respectively. The brackets in B indicate thickness of the capsule, which was remarkably thicker in the mutant mice. (C) Immunofluorescent detection of aldosterone synthase cytochrome P450 (P450aldo) and TH in the adrenal gland of WT and MC2R KO mice. Both enzymes were normally expressed in the mutant mice. (Scale bars, 50 μm.)
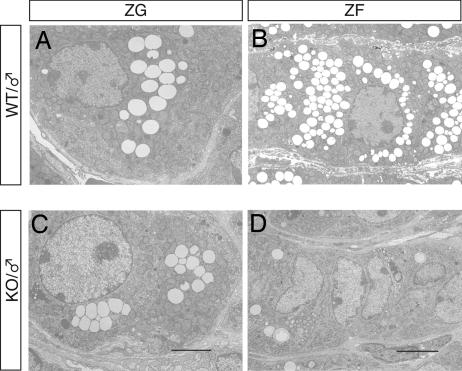
Ultrastructural examination of zF cells in MC2R KO mice revealed that the number of lipid droplets was significantly decreased and mitochondrial appearance was inactive compared with that of WT mice (Fig. 3 B and D). In contrast, zG cells in MC2R KO mice contained lipid droplets comparable with those in WT mice, and zG cells were not significantly different from those of WT mice (Fig. 3 A and C). Chromaffin cells in MC2R KO mice exhibited a marked depletion in epinephrine-storing secretory granules (data not shown), and highly vascularized connective tissue was developed in MC2R KO adrenal ME (data not shown). The H&E staining of the adrenal glands of newborn (postnatal day 0.5) MC2R KO mice was not significantly different from that of WT siblings (data not shown), suggesting that postnatal adrenal development was impaired in MC2R KO mice.
Fig. 3.
Electron micrographs of the adrenal gland from MC2R KO mice. (A and C) Electron micrographs of zG of WT or MC2R KO mice. (Scale bars, 2 μm.) (B and D) Electron micrographs of zF of WT or MC2R KO mice. (Scale bars, 5 μm.) Note the remarkable decrease in lipids in zF in MC2R KO mice.
Adrenal Hormones in MC2R KO Mice.
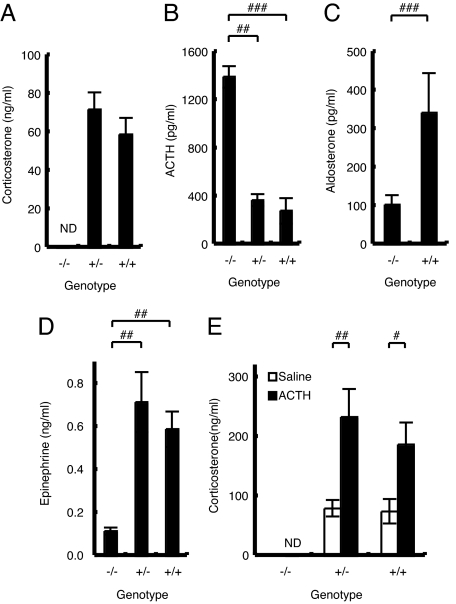
Serum corticosterone levels in MC2R KO mice were undetectable (Fig. 4A): male MC2R−/−, undetectable (n = 4); MC2R+/−, 72.0 ± 8.9 ng/ml (n = 6); and MC2R+/+, 59.0 ± 8.6 ng/ml (n = 5). ACTH levels were significantly increased in MC2R KO mice (Fig. 4B): male MC2R−/−, 1,394 ± 89 pg/ml (n = 4); MC2R+/−, 370 ± 50 pg/ml (n = 6); and MC2R+/+, 281 ± 106 pg/ml (n = 5). Surprisingly, serum aldosterone levels were significantly decreased in MC2R KO mice (Fig. 4C): male MC2R−/−, 104 ± 25 pg/ml (n = 4); and MC2R+/+, 343 ± 103 pg/ml (n = 5). In this regard, the MC2R-deficient mouse model is different from patients with MC2R-null mutations, in whom there is no mineralocorticoid deficiency and the renin–angiotensin system (RAS) is not affected (OMIM no. 202200). Consistent with the reduced serum corticosterone in MC2R KO mice, thymus and spleen weights were significantly increased and adipose weight was significantly decreased compared with those of WT mice (data not shown).
Fig. 4.
Hormone levels in MC2R KO mice. (A–D) Blood was collected at 1600 hours from male mice (male MC2R−/−, n = 4; MC2R+/−, n = 6; and MC2R+/+, n = 5) fasted for 8 h. Serum corticosterone (A), ACTH (B), aldosterone (C), and epinephrine (D) levels were determined. (E) Serum corticosterone response after ACTH (10 μg per kg of body weight) or saline injection in 12-wk-old male MC2R−/− [saline, not determined (ND); ACTH, n = 8], MC2R+/− (saline, n = 11; ACTH, n = 11), or MC2R+/+ (saline, n = 5; ACTH, n = 8) mice. ACTH or saline was injected from 1000 to 1030 hours, and blood was collected after 60 min. Data are expressed as means ± SEM. Statistical significance was determined by one-way ANOVA and Fisher's protected least significant difference (PLSD) test (A, B, D, and E) or t test (C). ###, P < 0.001; ##, P < 0.01; #, P < 0.05.
We analyzed the corticosterone response to exogenously administered ACTH in MC2R KO mice. The responsiveness to ACTH was completely abrogated in MC2R KO mice (Fig. 4E): male MC2R−/−, ACTH, undetectable (n = 8); MC2R+/−, saline, 80.1 ± 13.8 ng/ml (n = 11), and ACTH, 233.9 ± 46.6 ng/ml (n = 11); and MC2R+/+, saline, 75.2 ± 20.9 ng/ml (n = 5), and ACTH, 186.9 ± 37.6 ng/ml (n = 8). These results indicate that MC2R is essential for corticosterone release in response to ACTH.
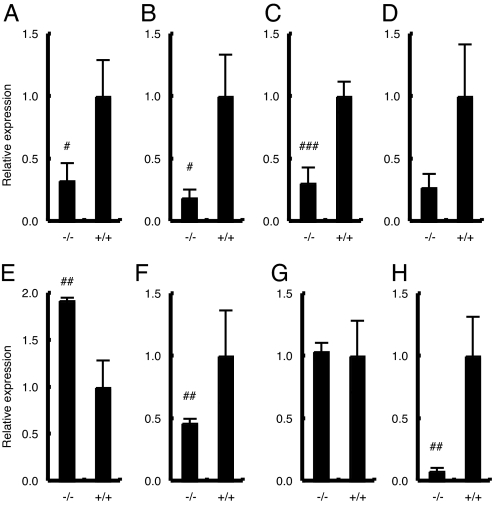
Because ACTH plays an essential role in regulating 11β-hydroxylase (Cyp11b1) expression, as well as other genes encoding enzymes involved in steroidogenesis (12), we analyzed the expression of adrenal steroidogenic enzymes. Expression levels of cholesterol side-chain cleavage enzyme P450scc (Cyp11a1) (Fig. 5A), Cyp21a1 (Fig. 5B), and Cyp11b1 (Fig. 5C) were significantly reduced in MC2R KO mice, reflecting the hypoplasia of zF. The expression of Cyp11b2 [aldosterone synthase (P450aldo)] was relatively reduced in adrenal glands from MC2R KO mice (Fig. 5D). These results collectively indicate that the reduction of corticosterone level (Fig. 4A) is due to hypoplasia of zF with reduced lipid droplets (Figs. 2A and 3C), together with reduced levels of Cyp11b1 and rate-limiting Cyp11a1 (Fig. 5 A and C).
Fig. 5.
Adrenal gene expression in MC2R KO mice. Expression of Cyp11a1 (A), Cyp21a1 (B), Cyp11b1 (C), Cyp11b2 (D), AT1bR (E), TH (F), Phox2a (G), and phenylethanolamine N-methyltransferase (PNMT) (H) in adrenal glands from female 12-wk-old MC2R−/− (n = 4) and MC2R+/+ (n = 3) mice was determined by qRT-PCR. Data are expressed as means ± SEM. Statistical significance was determined by t test. ###, P < 0.001; ##, P < 0.01; #, P < 0.05.
To determine the physiological effect of reduced aldosterone levels in MC2R KO mice, we measured serum electrolytes and blood pressure at 12 wk of age. There were no differences in the sodium concentrations of MC2R KO and WT mice, whereas chloride levels increased significantly in male MC2R KO mice and tended to increase in female MC2R KO mice (data not shown). Female MC2R KO mice exhibited significantly increased potassium levels (data not shown), whereas male MC2R KO mice did not. Although no significant differences in blood pressure were observed, the heart rate was significantly attenuated in MC2R KO mice (data not shown), consistent with reduced epinephrine levels in MC2R KO mice (Fig. 4D). We found that the expression of angiotensin receptor 1b (AT1bR) was significantly increased in MC2R KO mice (Fig. 5E), suggesting that renin–angiotensin system (RAS) signaling was enhanced in MC2R KO glomerulosa cells to compensate for the complete absence of ACTH signaling.
Measurement of catecholamine levels demonstrated that epinephrine levels were significantly reduced (Fig. 4D): male MC2R−/−, 0.12 ± 0.02 ng/ml (n = 4); MC2R+/−, 0.72 ± 0.14 ng/ml (n = 6); and MC2R+/+, 0.59 ± 0.08 ng/ml (n = 5). However, norepinephrine and dopamine levels were not significantly altered in MC2R KO mice (data not shown). The expression of TH was significantly reduced in MC2R KO adrenal glands (Fig. 5F), whereas the expression of Phox2a, a specific marker for chromaffin cells, was not significantly different (Fig. 5G), suggesting that MC2R is not required for chromaffin cell development but is necessary for TH expression. These results are consistent with a previous report that GC is not required for chromaffin cell development (13). We also observed that the expression of phenylethanolamine N-methyltransferase (PNMT), which catalyzes the conversion of norepinephrine to epinephrine and is modulated by GC, was significantly reduced in adrenal glands from MC2R KO mice (Fig. 5H). These results suggest that the reduced epinephrine level in MC2R KO mice is due to the reduced expression levels of PNMT and TH.
MC2R KO Mice Develop Hypoglycemia upon Prolonged Fasting.
We measured blood glucose levels in animals both fed and fasted for 8 h. Interestingly, adult MC2R KO mice exhibited relatively higher glucose levels than those of WT mice under fed and 8-h fasting conditions. However, the difference was not statistically significant (data not shown). MC2R KO mice exhibited relatively reduced serum insulin levels compared with control littermates. However, the difference was not statistically significant: male MC2R−/−, 2,044 ± 190 pg/ml (n = 4); and MC2R+/+, 2,644 ± 265 pg/ml (n = 5).
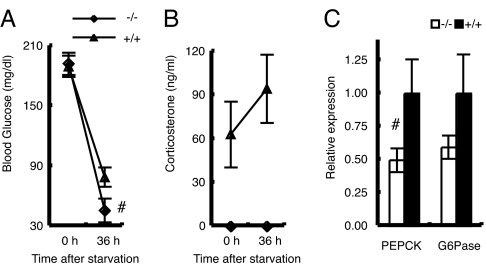
We next evaluated the role of MC2R during prolonged starvation. After 36 h of starvation, liver gluconeogenesis becomes the major source of blood glucose (14). During a 36-h fast, MC2R KO mice exhibited a faster decline in blood glucose levels (Fig. 6A): male MC2R−/−, 45.8 ± 11.7 mg/dl (n = 5); and MC2R+/+, 79.2 ± 9.5 mg/dl (n = 5). As anticipated, corticosterone levels in WT mice were increased in response to fasting, whereas corticosterone levels in MC2R KO mice were not increased in response to a 36-h fast (Fig. 6B): male MC2R−/−, undetectable (n = 5); and MC2R+/+, 94.4 ± 23.4 ng/ml (n = 5). Serum epinephrine was significantly decreased in MC2R KO mice, whereas norepinephrine, dopamine, insulin, and glucagon levels (data not shown) were not significantly different. The expression of PEPCK, which is a rate-limiting enzyme in gluconeogenesis, was significantly decreased, and G6Pase was relatively decreased in MC2R KO mice after a prolonged (36 h) fast (Fig. 6C), indicating that the lower blood glucose levels in MC2R KO mice were due to impaired gluconeogenesis.
Fig. 6.
MC2R KO mice develop hypoglycemia during fasting. The experiment was performed with 12-wk-old male MC2R−/− (n = 5) and MC2R+/+ (n = 5) mice. (A and B) At time 0 (2000 hours), food was withdrawn and blood glucose (A) and serum corticosterone level (B) were measured. After 36 h, mice (at 0800 hours) were killed and serum samples and liver RNA were prepared. Expression of phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase) in the liver was determined by qRT-PCR (C). Data are expressed as means ± SEM. The statistical difference was evaluated by two-way ANOVA (factor 1 was genotype and factor 2 was treatment) followed by t test to compare the significant difference between the glucose value for 36 h of MC2R KO mice and the glucose value for 36 h of WT mice. #, P < 0.05.
Discussion
Immediately after birth, the maternal supply of substrates ceases abruptly, and the newborn mouse has to withstand a brief period of starvation before being fed with milk that is high in fat and low in carbohydrates. The adaptation of neonates to these changes in nutrition and environment requires modification of glucose and fatty acid metabolism, which is controlled by the neonatal increase in glucagon and the fall in insulin (15). Defective gluconeogenesis leads to neonatal death (16). Plasma corticosterone levels are high at delivery and rapidly decline during the first 24 h after birth, and epinephrine and norepinephrine levels are increased severalfold in newborns in response to the stresses of birth, such as transient hypoxia, cold exposure, and cord cutting (15). Because GC plays a critical role in the maintenance of neonatal blood glucose levels through the induction of gluconeogenesis, one-half of hepatocyte-specific GC receptor KO mice die shortly after birth as a result of hypoglycemia (17). Here, we demonstrated that MC2R KO mice are defective in this adaptation, consistent with a previous report that 75% of POMC KO mice die shortly after birth (2). Genetic replacement of pituitary POMC in POMC KO mice (POMC−/−Tg+) rescues neonatal lethality in POMC KO mice, suggesting that peripheral POMC, possibly ACTH, is important for neonatal survival (18). These results collectively suggest that ACTH MC2R signaling plays a critical role in the neonatal adaptation to nutrition supply, consistent with the fact that patients with FGD often suffer from neonatal hypoglycemia (OMIM no. 202200) (19). Neonatal hypoglycemia in MC2R KO mice might be secondary to low levels of both circulating corticosterone and epinephrine. It is also interesting that a slight increase in mortality was also observed at 3–4 wk of age because weaning is a crucial period when mice need to adapt to nutritional modifications. In fact, corticosterone concentration is low during the suckling period, increases after 12 days, and peaks at 24 days (15). Further studies are required to clarify the possible role of ACTH MC2R in suckling/weaning adaptation.
We observed significant adrenocortical hypoplasia in adult MC2R KO mice compared with WT siblings. Although zG cells remained fairly intact, zF cells were severely atrophied (Fig. 2), indicating that MC2R is not required for proper development of zG cells but is required for that of zF cells. Adrenal glands of rodents possess a transient zone between the adrenal cortex and the adrenal ME called the murine X zone. The overall function of the X zone remains unclear (20). Detailed studies are required to clarify the possible effect of ACTH deficiency on X zone regression. Because the adrenal glands from MC2R KO pups were indistinguishable in size and histological appearance from those from WT littermates at birth (data not shown), consistent with POMC KO mice (21), the ACTH MC2R signaling pathway regulates postnatal development of the adrenal gland. It was previously proposed that POMC-derived peptides other than ACTH contribute to adrenal development, function, and maintenance. Specifically, cleavage of the N-terminal POMC (amino acids 1–74) results in the generation of shorter peptides with mitogenic properties (22). If POMC-derived peptides other than ACTH have any role in adrenal development, the adrenal phenotype of MC2R KO mice should be less severe than that of POMC KO mice. Compared with the adrenal structure of POMC KO mice previously reported (2, 4), the adrenal structure/morphology of MC2R KO mice was intact, especially in zG. The total number of nuclei in zF of MC2R KO mice was not significantly changed (Fig. 2A), suggesting that the proliferation of zF in MC2R KO mice was comparable with that in WT mice. In contrast, it was previously demonstrated that adrenal glands of POMC KO mice on postnatal day 14 had reduced proliferating cell nuclear antigen (PCNA)-positive cells (21). These differences could be explained by the possible role of POMC-derived peptides other than ACTH in adrenal development (22), although we could not exclude the possibility of difference due to genetic background or of compensatory function by other MCRs in the absence of MC2R. Simultaneous comparison of POMC KO mice and MC2R KO mice is required to clarify these possibilities.
We analyzed the adrenal gene expression involved in the syntheses of corticosterone, aldosterone, and catecholamines in MC2R KO mice at 12 wk of age. Two previous studies have shown adrenal gene expression profiles in POMC KO mice. Karpac et al. (21) demonstrated that the expression of Cyp11b2, Cyp11b1, and TH in POMC KO mice at 5 wk of age was not significantly different from that in WT mice and suggested that the essential role for POMC peptides is in the maintenance of the adrenal gland and not in differentiation. Coll et al. (23) also analyzed the expression of Cyp11b1 and Cyp11a1 and demonstrated that the expression of both was reduced in POMC KO mice at 8 wk of age. Although the latter results were consistent with ours, the former results were not. We could not fully explain the reason for the difference: however, one possible explanation is that adrenal glands of POMC KO and MC2R KO mice at 5 wk of age are indistinguishable from those of WT mice and that they regress thereafter, as suggested by Karpac et al. (21). Further developmental studies on the adrenal gland in POMC KO and MC2R KO mice are needed to clarify these issues.
We found that the adrenal glands in MC2R KO mice produce aldosterone at reduced levels, as has been observed in POMC KO mice (2, 4). In this regard, the MC2R-deficient mouse model is different from FGD type 1 patients, who have been reported to exhibit normal serum aldosterone levels (24). This disparity could be explained by the fact that the majority of humans have one or two missense alleles, and homozygous nonsense mutations are very rare. It was recently reported that a small number of such patients may provide biochemical evidence of mineralocorticoid deficiency (25). The role of ACTH in aldosterone production is further supported by the fact that glucocorticoid receptor (GR) KO mice had enlarged adrenal glands with greatly increased expression of not only Cyp11b1 but also Cyp11b2 at embryonic day 18.5 (26). GR KO mice had increased ACTH levels as a result of the deficiency of negative feedback by corticosterone (26). It is possible that increased ACTH levels in GR KO mice are directly responsible for the increased expression of Cyp11b2 in zG. These observations collectively indicate that ACTH MC2R signaling is an important regulator of aldosterone production.
Here we described the initial characterization of MC2R KO mice and confirmed and extended the importance of ACTH- MC2R in neonatal adaptation to nutrition supplies, adrenal development, and the production of corticosterone and aldosterone. The possible role of ACTH-MC2R in adipose metabolism (27), β-cell function (28), and skin homeostasis (29) could be clarified by further analysis of MC2R KO mice.
Materials and Methods
Animals.
Generation of MC2R KO mice is described in SI Materials and Methods. For analysis of tissue weight, 12-wk-old mice of each genotype were evaluated. Adrenal glands were dissected, cleaned of fat under a stereoscopic microscope, and weighed. Epididymal white adipose tissue, inguinal white adipose tissue, thymus, and spleen were dissected and weighed. Whole-tissue samples were isolated and placed on saline-saturated filter papers to remain hydrated until weighing. All of the mice were kept under specific pathogen-free conditions in an environmentally controlled clean room in the Laboratory Animal Research Center, Institute of Medical Science, University of Tokyo. The experiments were conducted according to institutional ethical guidelines for animal experiments and safety guidelines for gene manipulation experiments.
Blood Analysis.
For analysis of basal hormone levels, 12-wk-old adult mice of each genotype were evaluated. After 8 h of fasting, at 1600 hours, mice were anesthetized with diethyl ether, and blood samples were collected rapidly from the heart. Serum electrolyte concentrations were measured by the ion-selective electrode method (SRL, Tokyo, Japan). Serum corticosterone, ACTH, and aldosterone levels were determined by RIA with detection limits of 4.8 ng/ml (Amersham, Little Chalfont, United Kingdom), 5 pg/ml (Mitsubishi, Tokyo, Japan), and 0.05 ng/ml (Aldosterone-RIAKIT II; SRL), respectively. Serum catecholamine levels were determined by HPLC (SRL). Male MC2R+/+, MC2R+/−, and MC2R−/− mice of 12 wk of age were injected i.p. at 1000 hours with either ACTH (Peptide Institute, Osaka, Japan) at a dose of 10 μg/kg or saline. Animals were killed by decapitation, and blood was collected after 60 min.
Blood Pressure Measurement.
Blood pressure and heart rate were measured in conscious mice by the indirect tail-cuff method (BP-98A; Softron, Tokyo, Japan) as described in ref. 30.
Histology and qRT-PCR Analysis.
Histochemical procedures are described in SI Materials and Methods. For determination of relative mRNA concentrations, total adrenal gland or liver RNA, isolated by sepazol, was subjected to reverse transcription by using SuperScript III (Invitrogen, Carlsbad, CA). The cDNA was analyzed by automated fluorescent real-time PCR with SYBR Green (Invitrogen) by using an iCycler iQ (Bio-Rad, Hercules, CA). Ribosomal protein S3 (RPS3) was used as a control. Primer sequences were designed by using Universal ProbeLibrary Assay Design Center (www.roche-applied-science.com/sis/rtpcr/upl/adc.jsp) (Roche Applied Science, Indianapolis, IN) or Primer Bank (http://pga.mgh.harvard.edu/primerbank) (31) as described in SI Materials and Methods.
Statistical Analysis.
All values were calculated as means ± SEM. Comparisons of two groups were analyzed by using Student's t test; for comparisons of more than two groups, one- or two-way ANOVA was performed, followed by Fisher's protected least significant difference (PLSD) tests, to analyze statistical differences in each group. In all analyses, a two-tailed probability of <5% (i.e., P < 0.05) was considered statistically significant.
Supplementary Material
Acknowledgments
We thank all of the members of our laboratory for discussion and help with animal care; Dr. Michael S. Patrick for critical reading of this manuscript; and Dr. Atsushi Miyajima (University of Tokyo), Dr. Hiroshi Takemori (National Institute of Biomedical Innovation, Osaka, Japan), and Kuniaki Mukai (Keio University) for reagents. We express our condolences on Dr. Kubo's sudden death during the preparation of this manuscript. This work was supported by grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and the Ministry of Health, Labor, and Welfare of Japan.
Abbreviations
- ACTH
adrenocorticotropic hormone
- POMC
proopiomelanocortin
- GC
glucocorticoid
- KO
knockout
- FGD
familial glucocorticoid deficiency
- zF
zona fasciculata
- zG
zona glomerulosa
- ME
medulla
- TH
tyrosine hydroxylase.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0706953104/DC1.
References
- 1.Dallman MF. Endocr Res. 1984;10:213–242. doi: 10.1080/07435808409036499. [DOI] [PubMed] [Google Scholar]
- 2.Yaswen L, Diehl N, Brennan MB, Hochgeschwender U. Nat Med. 1999;5:1066–1070. doi: 10.1038/12506. [DOI] [PubMed] [Google Scholar]
- 3.Challis BG, Coll AP, Yeo GS, Pinnock SB, Dickson SL, Thresher RR, Dixon J, Zahn D, Rochford JJ, White A, et al. Proc Natl Acad Sci USA. 2004;101:4695–4700. doi: 10.1073/pnas.0306931101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coll AP, Challis BG, Yeo GS, Snell K, Piper SJ, Halsall D, Thresher RR, O'Rahilly S. Endocrinology. 2004;145:4721–4727. doi: 10.1210/en.2004-0491. [DOI] [PubMed] [Google Scholar]
- 5.Smart JL, Tolle V, Otero-Corchon V, Low MJ. Endocrinology. 2007;148:647–659. doi: 10.1210/en.2006-0990. [DOI] [PubMed] [Google Scholar]
- 6.Clark AJL, Weber A. Endocr Rev. 1998;19:828–843. doi: 10.1210/edrv.19.6.0351. [DOI] [PubMed] [Google Scholar]
- 7.Metherell LA, Chapple JP, Cooray S, David A, Becker C, Ruschendorf F, Naville D, Begeot M, Khoo B, Nurnberg P, et al. Nat Genet. 2005;37:166–170. doi: 10.1038/ng1501. [DOI] [PubMed] [Google Scholar]
- 8.Genin E, Huebner A, Jaillard C, Faure A, Halaby G, Saka N, Clark AJ, Durand P, Begeot M, Naville D. Hum Genet. 2002;111:428–434. doi: 10.1007/s00439-002-0806-3. [DOI] [PubMed] [Google Scholar]
- 9.Shimizu C, Kubo M, Saeki T, Matsumura T, Ishizuka T, Kijima H, Kakinuma M, Koike T. Gene. 1997;188:17–21. doi: 10.1016/s0378-1119(96)00769-x. [DOI] [PubMed] [Google Scholar]
- 10.Clark AJ, Cammas FM, Watt A, Kapas S, Weber A. J Mol Med. 1997;75:394–399. doi: 10.1007/s001090050124. [DOI] [PubMed] [Google Scholar]
- 11.Coll AP, Challis BG, Lopez M, Piper S, Yeo GS, O'Rahilly S. Diabetes. 2005;54:2269–2276. doi: 10.2337/diabetes.54.8.2269. [DOI] [PubMed] [Google Scholar]
- 12.Miller WL. Endocr Rev. 1988;9:295–318. doi: 10.1210/edrv-9-3-295. [DOI] [PubMed] [Google Scholar]
- 13.Finotto S, Krieglstein K, Schober A, Deimling F, Lindner K, Bruhl B, Beier K, Metz J, Garcia-Arraras JE, Roig-Lopez JL, et al. Development (Cambridge, UK) 1999;126:2935–2944. doi: 10.1242/dev.126.13.2935. [DOI] [PubMed] [Google Scholar]
- 14.Pilkis SJ, Granner DK. Annu Rev Physiol. 1992;54:885–909. doi: 10.1146/annurev.ph.54.030192.004321. [DOI] [PubMed] [Google Scholar]
- 15.Girard J, Ferre P, Pegorier JP, Duee PH. Physiol Rev. 1992;72:507–562. doi: 10.1152/physrev.1992.72.2.507. [DOI] [PubMed] [Google Scholar]
- 16.Wang ND, Finegold MJ, Bradley A, Ou CN, Abdelsayed SV, Wilde MD, Taylor LR, Wilson DR, Darlington GJ. Science. 1995;269:1108–1112. doi: 10.1126/science.7652557. [DOI] [PubMed] [Google Scholar]
- 17.Tronche F, Opherk C, Moriggl R, Kellendonk C, Reimann A, Schwake L, Reichardt HM, Stangl K, Gau D, Hoeflich A, et al. Genes Dev. 2004;18:492–497. doi: 10.1101/gad.284704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smart JL, Tolle V, Low MJ. J Clin Invest. 2006;116:495–505. doi: 10.1172/JCI25243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clark AJ, Metherell LA, Cheetham ME, Huebner A. Trends Endocrinol Metab. 2005;16:451–457. doi: 10.1016/j.tem.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 20.Hershkovitz L, Beuschlein F, Klammer S, Krup M, Weinstein Y. Endocrinology. 2007;148:976–988. doi: 10.1210/en.2006-1100. [DOI] [PubMed] [Google Scholar]
- 21.Karpac J, Ostwald D, Bui S, Hunnewell P, Shankar M, Hochgeschwender U. Endocrinology. 2005;146:2555–2562. doi: 10.1210/en.2004-1290. [DOI] [PubMed] [Google Scholar]
- 22.Estivariz FE, Iturriza F, McLean C, Hope J, Lowry PJ. Nature. 1982;297:419–422. doi: 10.1038/297419a0. [DOI] [PubMed] [Google Scholar]
- 23.Coll AP, Fassnacht M, Klammer S, Hahner S, Schulte DM, Piper S, Tung YC, Challis BG, Weinstein Y, Allolio B, et al. J Endocrinol. 2006;190:515–525. doi: 10.1677/joe.1.06749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clark AJ, McLoughlin L, Grossman A. Lancet. 1993;341:461–462. doi: 10.1016/0140-6736(93)90208-x. [DOI] [PubMed] [Google Scholar]
- 25.Lin L, Hindmarsh PC, Metherell LA, Alzyoud M, Al-Ali M, Brain CE, Clark AJ, Dattani MT, Achermann JC. Clin Endocrinol (Oxford) 2007;66:205–210. doi: 10.1111/j.1365-2265.2006.02709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cole TJ, Blendy JA, Monaghan AP, Krieglstein K, Schmid W, Aguzzi A, Fantuzzi G, Hummler E, Unsicker K, Schutz G. Genes Dev. 1995;9:1608–1621. doi: 10.1101/gad.9.13.1608. [DOI] [PubMed] [Google Scholar]
- 27.Boston BA, Cone RD. Endocrinology. 1996;137:2043–2050. doi: 10.1210/endo.137.5.8612546. [DOI] [PubMed] [Google Scholar]
- 28.Genuth S, Lebovitz HE. Endocrinology. 1965;76:1093–1099. doi: 10.1210/endo-76-6-1093. [DOI] [PubMed] [Google Scholar]
- 29.Arck PC, Slominski A, Theoharides TC, Peters EM, Paus R. J Invest Dermatol. 2006;126:1697–1704. doi: 10.1038/sj.jid.5700104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsuki T, Isoda K, Horai R, Nakajima A, Aizawa Y, Suzuki K, Ohsuzu F, Iwakura Y. Circulation. 2005;112:1323–1331. doi: 10.1161/CIRCULATIONAHA.105.564658. [DOI] [PubMed] [Google Scholar]
- 31.Wang X, Seed B. Nucleic Acids Res. 2003 Dec 15;31 doi: 10.1093/nar/gng154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.