Abstract
A missense mutation in the ob gene causes leptin deficiency and morbid obesity. Leptin replacement to three adults with this mutation normalized body weight and eating behavior. Because the neural circuits mediating these changes were unknown, we paired functional magnetic resonance imaging (fMRI) with presentation of food cues to these subjects. During viewing of food-related stimuli, leptin replacement reduced brain activation in regions linked to hunger (insula, parietal and temporal cortex) while enhancing activation in regions linked to inhibition and satiety (prefrontal cortex). Leptin appears to modulate feeding behavior through these circuits, suggesting therapeutic targets for human obesity.
Keywords: functional MRI, obesity, hunger, prefrontal cortex, insula
Leptin, the primary signaling hormone from adipocyte energy stores, regulates feeding behavior and energy expenditure (1). A recessive missense mutation (c313C>T Arg105Trp) in the ob gene was identified in three adults (two women and one man) and one child from a family in Turkey (2). The only other known mutation producing congenital leptin deficiency is a frame shift/premature stop (c398delG Δ133G), identified in three families of Pakistani origin (3). Before treatment with leptin supplement was initiated, the adults with the Arg105Trp mutation were morbidly obese and hypogonadal, and one of them had type-2 diabetes mellitus. Concomitant with weight loss, replacement of leptin to the adults with the Arg105Trp mutation normalized endocrine and other health measures (4) and produced a 50% reduction in food intake (during the first 15 weeks) that correlated with ratings of hunger and desire to eat (5). Moreover, supplementation was associated with sustained increases in brain gray matter concentration in the anterior cingulate gyrus, inferior parietal lobule, and cerebellum (6). Leptin treatment had similar effects on body weight and health measures for patients identified with the Δ133G mutation (7) as well as mice that bear a homologous gene mutation (ob/ob mice) (8).
Consistent with a direct action in the brain, leptin receptors are found in the cerebral cortex, hippocampus, basal ganglia, hypothalamus, brainstem, and cerebellum (9). However, the neural circuits through which leptin alters human feeding behavior are not known. We addressed this question by pairing functional magnetic resonance imaging (fMRI) with presentation of food images to the three leptin-deficient adults with the Arg105Trp mutation with and without leptin treatment. In healthy adults, presentation of food increases ratings of hunger and cerebral glucose metabolism, particularly in the orbitofrontal cortex, somatosensory cortex, superior temporal cortex, occipital cortex, insula, basal ganglia, and thalamus (10); images of high-calorie foods increase ratings of hunger and activate a network of brain regions, including the medial and dorsolateral prefrontal cortex (PFC), corpus callosum, amygdala, thalamus, hypothalamus, and cerebellum (11). We hypothesized that leptin replacement would alter ratings of hunger induced by the food-related stimuli and that this would occur along with changes in activation of the previously identified brain regions by the food-related cues in the congenitally leptin-deficient research participants.
Results
Ratings of Hunger and Body Mass Index (BMI).
Self-reports of hunger in response to images of food presented during fMRI scanning were generally higher when subjects were not receiving leptin supplementation than during leptin treatment (Table 1). ANOVAs indicated a significant effect of test session in two participants [F(2, 63) = 11.2, P < 0.001, and F(2, 63) = 16.1, P < 0.001, respectively] and a near significant effect in the third [F(2, 63) = 3.0, P = 0.058]. Post hoc analyses indicated that all three participants reported significantly lower hunger when tested during supplementation for 57 months than when not receiving leptin (all P values were <0.05). After only 2 weeks of supplementation, ratings of one participant (P < 0.001), but not the others (P = 0.09 and 0.21, respectively) were significantly lower. Hunger ratings after 57 months were significantly lower than after 2 weeks of supplementation for one participant (P < 0.01) but not for the other two (P values >0.5). The increase in hunger rating was accompanied by an increase in BMI (average from 27.7 to 29.6, P = 0.009); BMI did not change after 2 weeks of leptin reinstatement (Table 2).
Table 1.
Mean (standard deviation) rating of hunger in response to images of high-calorie food presented during fMRI scan (1 = not at all, 7 = very hungry)
| Patient | 57 months with leptin | 33 days without leptin | 14 days with leptin |
|---|---|---|---|
| A | 4.6 (1.1) | 6.6 (0.7) | 5.6 (1.5) |
| B | 4.8 (1.6) | 6.8 (0.5) | 5.5 (1.3) |
| C | 5.9 (1.4) | 7.0 (0.0) | 6.5 (0.5) |
Table 2.
BMI of subjects at time of fMRI scans
| Patient | 57 months with leptin | 33 days without leptin | 14 days with leptin |
|---|---|---|---|
| A | 27.54 | 29.28 | 29.62 |
| B | 31.60 | 33.27 | 33.44 |
| C | 23.99 | 26.24 | 26.06 |
Neuroimaging Data.
Compared with the neutral stimuli condition, high-calorie foods activated the middle, inferior, superior, and medial frontal gyri and regions of the occipital, temporal, limbic, and parietal lobes, insula, amygdala, putamen, thalamus, midbrain, and cerebellum (P < 0.05, corrected FDR, t > 2.75, z score 2.72, extent >10 voxels). These findings were consistent with those obtained in healthy volunteers, using a similar paradigm (11).
When patients were not receiving leptin (vs. during supplementation), activity related to the contrast of high-calorie > low-calorie was greater in the insula and other parietal regions (Fig. 1) as well as occipital, limbic, and temporal regions (Table 3). In comparison, leptin supplementation accompanied significantly greater activation in the medial, superior, and middle frontal gyri, cingulate gyrus, occipital cortex, midbrain, pons, and cerebellum (Fig. 2 and Table 4). In the region of interest analysis of a subset of clusters, each subject showed an activation pattern resembling the group results (Fig. 3).
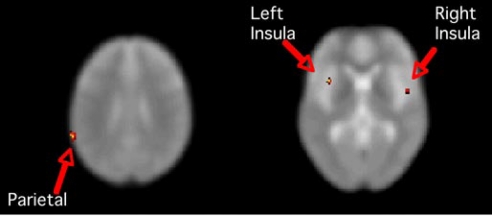
Fig. 1.
Greater activation in parietal cortex and insula in the absence of leptin supplementation. Images are SPM T maps of significant BOLD signal change greater when leptin supplementation was discontinued (vs. during supplementation) for the contrast high-calorie > low-calorie stimuli. Left image (z = 29) shows a significant cluster in the region of the left parietal supramarginal gyrus: extent = 52 voxels, t = 4.06, z score = 4.05, peak voxel x y z coordinates are −64 −48 26. Right image (z = 0) shows significant clusters bilaterally in the insula. Left insula: extent = 20 voxels, t = 3.9, z score = 3.89, peak voxel at −36 10 2. Right insula: extent = 26 voxels, t = 3.45, z score = 3.45, peak voxel at 48 0 0. Images are in neurologic orientation, displayed on the SPM5 EPI template. Coordinates are in MNI space, contrast comparisons at P < 0.001 uncorrected.
Table 3.
Greater activation while leptin-deficient in high-calorie > low-calorie contrast at t > 3.45 with P < 0.001 uncorrected and extent > 10 voxels
| Region | Voxel extent | z score | MNI coordinates (x y z) | ||
|---|---|---|---|---|---|
| Insula, left | 20 | 3.89 | −36 | 10 | 2 |
| 14 | 3.50 | −56 | −32 | 18 | |
| Insula, right | 26 | 3.45 | 48 | 0 | 0 |
| Limbic lobe, right | 19 | 3.65 | 22 | 6 | −32 |
| Parahippocampal gyrus, left | 17 | 3.54 | −26 | −24 | −24 |
| Parietal postcentral gyrus, right | 22 | 3.85 | 62 | −24 | 20 |
| Parietal supramarginal gyrus, left | 52 | 4.05 | −64 | −48 | 26 |
| Temporal fusiform gyrus, left | 29 | 3.95 | −40 | −52 | −24 |
| Occipital precuneus, right | 20 | 4.06 | 18 | −66 | 16 |
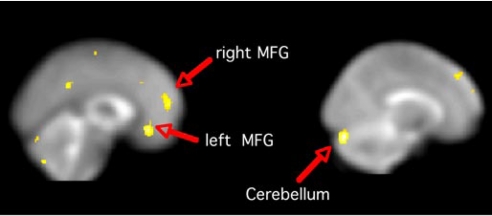
Fig. 2.
Leptin supplementation enhanced activation in PFC and cerebellum. Images are SPM T maps of significantly greater BOLD response during leptin supplementation (vs. 33 days after discontinuation) for the contrast of high-calorie > low-calorie stimuli. Left image (x = 0) shows largest significant clusters in the medial frontal gyrus (MFG): left MFG extent = 102, t = 4.70, z score = 4.70, peak voxel at −2 32 −12; right MFG extent = 202, t = 4.69, z score = 4.58, peak voxel at 10 56 12. Right image (x = 14) shows the largest significant cluster in the right cerebellum: extent = 379, t = 5.39, z score = 5.38, peak voxel at 16 −84 −24. Images are in neurologic orientation, displayed on the SPM5 EPI template. Coordinates are in MNI space, contrast comparisons at P < 0.001 uncorrected.
Table 4.
Greater activation with leptin supplement in high calorie > low calorie contrast at t > 3.45 with P < 0.001 uncorrected and extent > 10 voxels
| Region | Voxel extent | z score | MNI coordinates (x y z) | ||
|---|---|---|---|---|---|
| Medial frontal gyrus, left | 102 | 4.70 | −2 | 32 | −12 |
| 42 | 3.91 | −8 | 28 | 36 | |
| 14 | 4.03 | −14 | 14 | 48 | |
| Medial frontal gyrus, right | 202 | 4.58 | 10 | 56 | 12 |
| 72 | 3.83 | 10 | 44 | 46 | |
| Middle frontal gyrus, right | 11 | 3.70 | 24 | 54 | −8 |
| Superior frontal gyrus, left | 34 | 4.26 | −28 | 44 | 38 |
| 18 | 3.47 | −20 | 36 | 46 | |
| Frontal lobe, right | 13 | 3.65 | 40 | 22 | 56 |
| Cingulate gyrus, left | 29 | 3.80 | −2 | −46 | 34 |
| Occipital, left | 15 | 3.94 | −30 | −96 | 20 |
| Midbrain, left | 65 | 4.26 | −14 | −22 | −14 |
| Pons, right | 16 | 3.72 | 6 | −36 | −32 |
| Cerebellum, left | 14 | 3.61 | −50 | −52 | −40 |
| 21 | 3.75 | −32 | −60 | −38 | |
| 13 | 3.78 | −50 | −64 | −34 | |
| 20 | 4.31 | −2 | −74 | −46 | |
| 22 | 4.07 | −34 | −80 | −38 | |
| 14 | 3.86 | −20 | −82 | −32 | |
| 96 | 4.79 | −6 | −82 | −26 | |
| Cerebellum, right | 10 | 3.54 | 46 | −48 | −44 |
| 30 | 3.90 | 36 | −68 | −32 | |
| 37 | 3.92 | 44 | −76 | −42 | |
| 379 | 5.32 | 16 | −84 | −24 | |
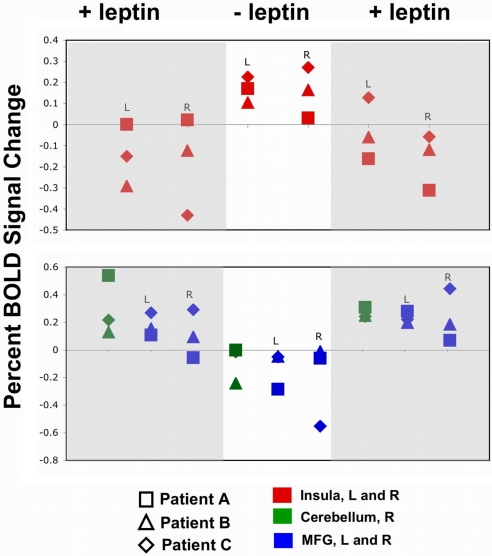
Fig. 3.
Region of interest analysis by subject by fMRI scan for the contrast high-calorie > low-calorie stimuli. Each subject showed greater activation in the insula but less activation in the right cerebellum and prefrontal cortex (medial frontal gyrus, MFG) when no leptin supplementation (center), compared with test sessions when supplemental leptin was administered (left and right).
Discussion
As expected, leptin supplementation reduced self-reports of cue-induced hunger, more consistently after 57 months than after 2 weeks. In addition, removal of leptin treatment increased BMI and altered the brain response to food-related cues. In the contrast high-calorie > low-calorie, leptin deficiency was associated with enhanced activation of areas in the parietal (especially insula), temporal, and occipital lobes.
The insula functions as the primary gustatory cortex (12) and is activated during the presentation of food (10), with increases during hunger and decreases after satiation (13–15). These findings may reflect the role of the insula in representing information about the internal bodily states as conscious emotional feelings, or interoception (16). Our results suggest that leptin deficiency may enhance insular interoception of cue-induced feelings of hunger.
Hunger also elicits activation in the temporal and parietal cortices of healthy adults (13), and there is clinical evidence of temporal involvement in the sensation of hunger (17), possibly accounting for the enhancement of temporal and parietal cortical activations when leptin supplementation is discontinued.
Along with reductions in self-reports of hunger, leptin treatment produced significantly greater brain activation in the middle, superior, and medial frontal gyri and cerebellum in the contrast high-calorie > low-calorie. The role of the PFC in the intentional control of behavior (18) and the inhibition of inappropriate behavioral responses (19) has been well established, thereby supporting the link between satiety (13–15, 20) and successful dieting (21) with increased activity in the PFC. Greater activation of the PFC during leptin supplementation may involve enhancement of satiety or activation of inhibitory processes, in line with the general belief that high calorie foods are unhealthy and should be avoided.
Although the cerebellum has not traditionally been linked with eating behavior, it is thought to play a role in reinforcement (22) and drug craving (23). The cerebellum contains leptin receptors (9), and participants studied here exhibited evidence of cerebellar plasticity in response to leptin replacement (6). With leptin supplementation, the cerebellum showed enhanced activation (in the contrast high-calorie > low-calorie stimuli). In healthy adults, high-calorie foods elicit greater activation of the cerebellum in an analogous contrast (11), but cerebellar perfusion reportedly decreases with satiety (13, 15). Further study is needed to clarify the role of leptin in modulating cerebellar function.
The results of this study are limited inasmuch as genetic leptin deficiency is extremely rare, and elevated plasma leptin usually accompanies obesity, indicating leptin resistance (24). However, in clinical trials, high-dose leptin administration can produce moderate weight loss in some obese patients (25). Because evolution pressured the development of redundant systems to prevent negative energy balance, the therapeutic use of leptin is likely limited to certain forms of obesity or combination therapies. Despite these limitations, elucidating the mechanisms by which leptin alters brain function in congenital leptin deficiency can provide understanding of normal leptin physiology and may ultimately help identify new targets for the treatment of obesity and related metabolic disorders. We show here that leptin reduces brain activation in regions linked to hunger (insula, parietal, and temporal cortex) and enhances activation in regions linked to inhibition and satiety (prefrontal cortex), suggesting possible therapeutic targets for human obesity.
Methods
Research Subjects.
Three adults from a Turkish family gave written informed consent and were admitted to the General Clinical Research Center at the University of California Los Angeles (UCLA) for study, under approval of the UCLA Institutional Review Board. There was a baseline adjustment period of 3 months after arriving in Los Angeles before initiation of leptin replacement therapy. The participants were allowed to eat ad libitum during their stay, and their level of physical activity was not restricted.
A detailed description of the treatment procedure (4) and a microanalysis of eating behavior for the first 15 weeks of treatment (5) have been published. Briefly, the subjects received daily (between 1800 and 2000 h) s.c. injections of recombinant methionyl human leptin (provided by Amgen, Thousand Oaks, CA) at low physiological doses (0.002–0.040 mg/kg) for the duration of the study. The dose was chosen to achieve physiological leptin concentrations and was administered in the evening to model the normal circadian variations in endogenous leptin. As subjects lost weight, the dose was reduced to avoid excessive weight loss. Along with BMI, leptin dose has remained stable, at low physiological replacement levels, for several years, indicating that there is no resistance to exogenous leptin supplementation in these patients.
Data Acquisition.
Each subject participated in three scanning sessions. The first session was conducted 57 months after the initial start of leptin replacement, which was then discontinued for 33 days before the second test session. To distinguish responses to leptin replacement from possible effects of task repetition, treatment was resumed for 14 days before the third test session. Participants received a standard breakfast that consisted of 20% of daily calories of an isocaloric weight-maintaining diet (according to the Mayo Clinic nomogram: 55% carbohydrates, 20% protein, 25% fat) 3 h before fMRI, with no intervening eating.
Neuroimaging data were collected on a 3 T MRI scanner (Allegra, Siemens). Using a T2*-weighted gradient-recalled echoplanar imager (EPI), with blood oxygen level-dependent (BOLD) contrast (repetition time, 1,500 ms; echo time, 30; flip angle, 70; slice thickness, 4 mm with a 1-mm interslice interval; matrix, 64 × 64; in-plane resolution, 3.12 mm2). A set of 240 images of each axial slice through the brain was acquired.
Using a block design, three sets of visual stimuli were presented once in each of two runs per test session. All stimuli were high-quality, full color photographs. Images of high-calorie foods (e.g., fried chicken, cheeseburgers, pizza), low-calorie foods (e.g., strawberries, salad), and brick walls (neutral condition) were presented. Each block lasted 30 sec, during which two photographs from the same set were presented consecutively for 9 s each, and a rating scale (“1” minimum to “7” maximum) was then presented for 12 s along with the expression “Aciktim?” (which means “I am hungry?” in Turkish). The participants were instructed to provide their ratings by pressing a button, with the number of presses indicating their score of how hungry the images made them feel.
Data Analysis.
Ratings of hunger were analyzed in separate analyses of variance for each of the three subjects, with stimulus type (high-calorie food, low-calorie food, neutral) and scan session [first (57-month supplementation), second (supplementation discontinued 33 days), third (supplementation resumed 2 weeks)] as independent variables. Data from the individual test sessions were compared by using Fisher's least significant differences post hoc tests. BMI was assessed by using paired samples t test.
Imaging data were analyzed by using Statistical Parametric Mapping (SPM5, Welcome Department of Cognitive Neurology, London, U.K.). Treating the data for each subject separately, all of the images from the functional scans were aligned to the first functional image collected in the first test session. They were then corrected for motion, coregistered, spatially normalized to the EPI template from SPM5, and then smoothed with a 6-mm full-width half-maximal Gaussian filter. A 128-s high-pass temporal filter was used, and individual movement parameters were applied as a multiple regressor. Data were analyzed by using the general linear model with model time courses constructed for each condition by convolving each block with the canonical hemodynamic response function.
A fixed effects group analysis included all of the functional scans in one general linear model. To determine whether the food-related stimuli were salient and our scanning paradigm valid for the population studied, SPM (T) maps were made of the contrast high-calorie > neutral, collapsing data across test sessions and subjects. Then SPM (T) maps were made of the contrast high-calorie > low-calorie in conditions of leptin supplementation and discontinuation. We selected this contrast because pictures of high-calorie foods elicit higher reward value, have greater motivational salience, and induce stronger emotional memories than pictures of low-calorie foods (11). We reasoned, therefore, that the possible differences observed would reflect leptin effects on conditioned responses to stimuli related to high-calorie food, rather than responses to complex visual stimuli in general. One-sample t tests were used to identify regions where BOLD signal change (with task condition) differed with leptin status. A voxel-level height threshold of P < 0.001 (uncorrected) was used to identify significant stimulus-related activity in a whole brain analysis.
Clusters of activation, significant at a voxel level threshold of t > 3.45 and having an extent ≥10 voxels, were extracted using the “volumes” option in SPM5. The Montreal Neurological Institute (MNI) coordinates of the peak voxels in each cluster were transformed to those of the atlas of Talairach and Tournoux (mni2tal.m matlab program by Matthew Brett), and the associated brain regions were labeled by using the Talairach Daemon Client (Peter Kochunov and Angela Uecker, http://ric.uthscsa.edu/projects/talairachdaemon.html)
To confirm that the findings represented those of the entire group and both long-term stabilization (57-month) and subacute supplementation (2 weeks), we analyzed data from significant activation clusters, selected on the basis of prior imaging studies (10, 11). These clusters were regions of interest from which mean % signal change was extracted (for each subject and test session) with the SPM5-compatible tool Marsbar (http://marsbar.sourceforge.net).
Acknowledgments
This work was supported in part by National Institutes of Health (NIH) Grants DA022539 and DA020726 (to E.D.L.), RR016996, DK058851, and GM061394 (to J.L.), and RR017365 and DK063240 (to M.-L.W.) and the University of California, Los Angeles, General Clinical Research Center (NIH Grant RR00865 to G. S. Levy). K.B. has been supported by NIH Training Grants GM08042 and DA021961. During the course of this study, Amgen, Inc., graciously provided leptin; Amylin, Inc., now provides leptin to these patients. Neither Amgen, Inc., nor Amylin, Inc., contributed to the design, analysis, or writing of this study.
Abbreviations
- fMRI
functional MRI
- PFC
prefrontal cortex
- BMI
body mass index
- EPI
echoplanar imaging
- BOLD
blood oxygen level-dependent.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Klok MD, Jakobsdottir S, Drent ML. Obes Rev. 2007;8:21–34. doi: 10.1111/j.1467-789X.2006.00270.x. [DOI] [PubMed] [Google Scholar]
- 2.Ozata M, Ozdemir IC, Licinio J. J Clin Endocrinol Metab. 1999;84:3686–3695. doi: 10.1210/jcem.84.10.5999. [DOI] [PubMed] [Google Scholar]
- 3.Gibson WT, Farooqi IS, Moreau M, DePaoli AM, Lawrence E, O'Rahilly S, Trussell RA. J Clin Endocrinol Metab. 2004;89:4821–4826. doi: 10.1210/jc.2004-0376. [DOI] [PubMed] [Google Scholar]
- 4.Licinio J, Caglayan S, Ozata M, Yildiz BO, de Miranda PB, O'Kirwan F, Whitby R, Liang L, Cohen P, Bhasin S, et al. Proc Natl Acad Sci USA. 2004;101:4531–4536. doi: 10.1073/pnas.0308767101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williamson DA, Ravussin E, Wong ML, Wagner A, Dipaoli A, Caglayan S, Ozata M, Martin C, Walden H, Arnett C, et al. Appetite. 2005;45:75–80. doi: 10.1016/j.appet.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Matochik JA, London ED, Yildiz BO, Ozata M, Caglayan S, DePaoli AM, Wong ML, Licinio J. J Clin Endocrinol Metab. 2005;90:2851–2854. doi: 10.1210/jc.2004-1979. [DOI] [PubMed] [Google Scholar]
- 7.Farooqi IS, Matarese G, Lord GM, Keogh JM, Lawrence E, Agwu C, Sanna V, Jebb SA, Perna F, Fontana S, et al. J Clin Invest. 2002;110:1093–1103. doi: 10.1172/JCI15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 9.Burguera B, Couce ME, Long J, Lamsam J, Laakso K, Jensen MD, Parisi JE, Lloyd RV. Neuroendocrinology. 2000;71:187–195. doi: 10.1159/000054536. [DOI] [PubMed] [Google Scholar]
- 10.Wang GJ, Volkow ND, Telang F, Jayne M, Ma J, Rao M, Zhu W, Wong CT, Pappas NR, Geliebter A, et al. NeuroImage. 2004;21:1790–1797. doi: 10.1016/j.neuroimage.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 11.Killgore WD, Young AD, Femia LA, Bogorodzki P, Rogowska J, Yurgelun-Todd DA. NeuroImage. 2003;19:1381–1394. doi: 10.1016/s1053-8119(03)00191-5. [DOI] [PubMed] [Google Scholar]
- 12.Simon SA, de Araujo IE, Gutierrez R, Nicolelis MA. Nat Rev Neurosci. 2006;7:890–901. doi: 10.1038/nrn2006. [DOI] [PubMed] [Google Scholar]
- 13.Tataranni PA, Gautier JF, Chen K, Uecker A, Bandy D, Salbe AD, Pratley RE, Lawson M, Reiman EM, Ravussin E. Proc Natl Acad Sci USA. 1999;96:4569–4574. doi: 10.1073/pnas.96.8.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Del Parigi A, Gautier JF, Chen K, Salbe AD, Ravussin E, Reiman E, Tataranni PA. Ann NY Acad Sci. 2002;967:389–397. [PubMed] [Google Scholar]
- 15.Gautier JF, Del Parigi A, Chen K, Salbe AD, Bandy D, Pratley RE, Ravussin E, Reiman EM, Tataranni PA. Obes Res. 2001;9:676–684. doi: 10.1038/oby.2001.92. [DOI] [PubMed] [Google Scholar]
- 16.Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Nat Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- 17.Fisher CM. Neurology. 1994;44:1577–1579. doi: 10.1212/wnl.44.9.1577. [DOI] [PubMed] [Google Scholar]
- 18.Miller EK, Cohen JD. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 19.Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- 20.Gautier JF, Chen K, Salbe AD, Bandy D, Pratley RE, Heiman M, Ravussin E, Reiman EM, Tataranni PA. Diabetes. 2000;49:838–846. doi: 10.2337/diabetes.49.5.838. [DOI] [PubMed] [Google Scholar]
- 21.DelParigi A, Chen K, Salbe AD, Hill JO, Wing RR, Reiman EM, Tataranni PA. Int J Obes (London) 2007;31:440–448. doi: 10.1038/sj.ijo.0803431. [DOI] [PubMed] [Google Scholar]
- 22.Martin-Solch C, Magyar S, Kunig G, Missimer J, Schultz W, Leenders KL. Exp Brain Res. 2001;139:278–286. doi: 10.1007/s002210100751. [DOI] [PubMed] [Google Scholar]
- 23.Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A. Proc Natl Acad Sci USA. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, et al. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 25.Heymsfield SB, Greenberg AS, Fujioka K, Dixon RM, Kushner R, Hunt T, Lubina JA, Patane J, Self B, Hunt P, et al. J Am Med Assoc. 1999;282:1568–1575. doi: 10.1001/jama.282.16.1568. [DOI] [PubMed] [Google Scholar]