Abstract
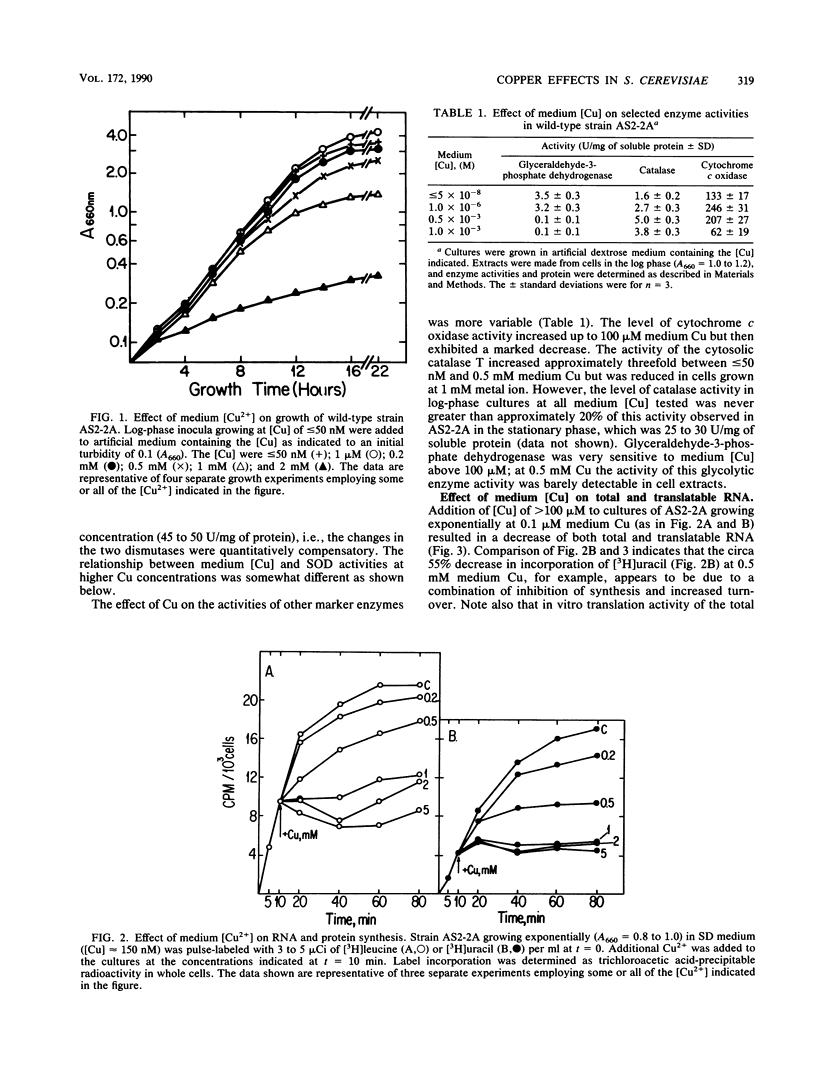
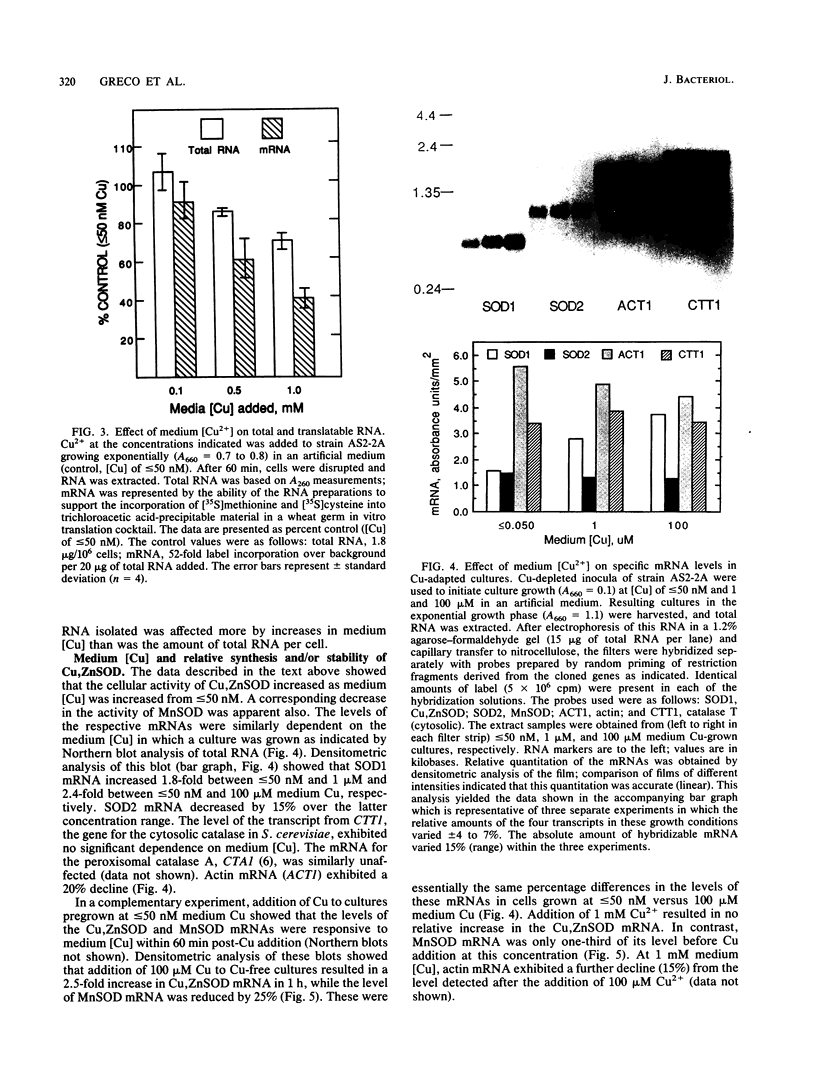
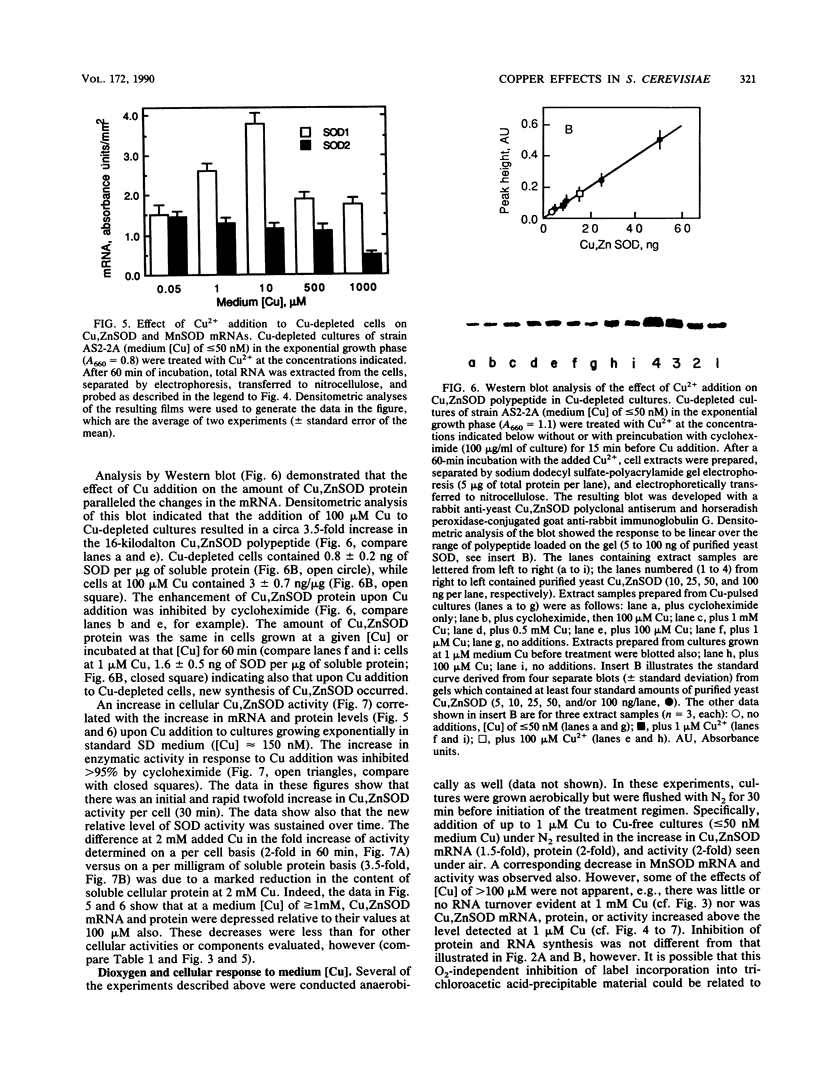
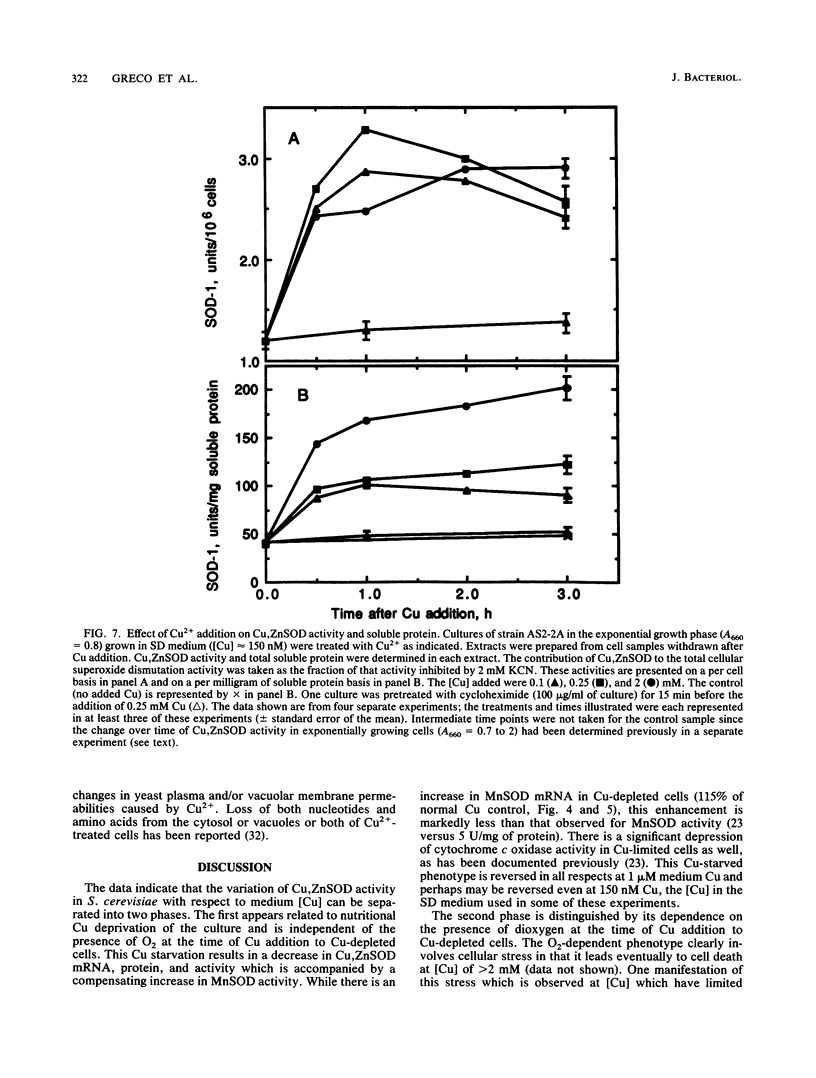
A wild-type strain of the yeast Saccharomyces cerevisiae grown at a medium [Cu] of less than or equal to 50 nM contained less Cu,Zn superoxide dismutase (SOD) mRNA (60%), protein (50%), and activity (50%) in comparison with control cultures grown in normal synthetic dextrose medium ([Cu] approximately 150 nM). A compensating increase in the activity of MnSOD was observed, as well as a smaller increase in MnSOD mRNA. These medium [Cu]-dependent differences were observed in cultures under N2 as well. Addition of Cu2+ (100 microM) to Cu-depleted cultures resulted in a rapid (30 min) increase in Cu,ZnSOD mRNA (2.5-fold), protein (3.5-fold), and activity (4-fold). Ethidium bromide (200 micrograms/ml of culture) inhibited by 50% the increase in Cu,ZnSOD mRNA, while cycloheximide (100 micrograms/ml of culture) inhibited completely the increase in protein and activity. Addition of Cu2+ to greater than or equal to 100 microM caused no further increase in these parameters but did result in a loss of total cellular RNA and translatable RNA, a decline in the population of specific mRNAs, a decrease in total soluble protein and the activity of specific enzymes, and an inhibition of incorporation of [3H]uracil and [3H]leucine into trichloroacetic acid-insoluble material. Cu,ZnSOD mRNA, protein, and activity appeared relatively more resistant to these effects of Cu toxicity than did the other cellular constituents examined. When evaluated in cultures under N2, the cellular response to [Cu] of greater than or equal to 100 microM was limited to the inhibition of radiolabel incorporation into trichloroacetic acid-insoluble material. All other effects were absent in the absence of O2. The data indicated that medium (cellular) Cu alters the steady-state level of Cu, ZnSOD. This regulation may be at the level of transcription. In addition, Cu,ZnSOD exhibits the characteristics of Cu-stress protein in that it and its mRNA are enhanced relative to other cellular species under conditions of Cu excess. This observation and the O2-dependence of some of the manifestations of Cu excess suggest that one mechanism of Cu toxicity involves the superoxide radical anion O2-.
Full text
PDF




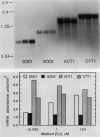
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Autor A. P. Biosynthesis of mitochondrial manganese superoxide dismutase in saccharomyces cerevisiae. Precursor form of mitochondrial superoxide dismutase made in the cytoplasm. J Biol Chem. 1982 Mar 10;257(5):2713–2718. [PubMed] [Google Scholar]
- Bermingham-McDonogh O., Gralla E. B., Valentine J. S. The copper, zinc-superoxide dismutase gene of Saccharomyces cerevisiae: cloning, sequencing, and biological activity. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4789–4793. doi: 10.1073/pnas.85.13.4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt T. R., Sternberg E. J., Gorman J. A., Clark P., Hamer D., Rosenberg M., Crooke S. T. Copper metallothionein of yeast, structure of the gene, and regulation of expression. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3332–3336. doi: 10.1073/pnas.81.11.3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung K., Romero N., Tinker D., Keen C. L., Amemiya K., Rucker R. Role of copper in the regulation and accumulation of superoxide dismutase and metallothionein in rat liver. J Nutr. 1988 Jul;118(7):859–864. doi: 10.1093/jn/118.7.859. [DOI] [PubMed] [Google Scholar]
- Cohen G., Fessl F., Traczyk A., Rytka J., Ruis H. Isolation of the catalase A gene of Saccharomyces cerevisiae by complementation of the cta1 mutation. Mol Gen Genet. 1985;200(1):74–79. doi: 10.1007/BF00383315. [DOI] [PubMed] [Google Scholar]
- Coruzzi G., Trembath M. K., Tzagoloff A. The isolation of mitochondrial and nuclear mutants of Saccharomyces cerevisiae with specific defects in mitochondrial functions. Methods Enzymol. 1979;56:95–106. doi: 10.1016/0076-6879(79)56012-1. [DOI] [PubMed] [Google Scholar]
- Crosti N., Bajer J., Serra A., Rigo A., Scarpa M., Viglino P. Coordinate expression of Mn-containing superoxide dismutase and Cu,Zn-containing superoxide dismutase in human fibroblasts with trisomy 21. J Cell Sci. 1985 Nov;79:95–103. doi: 10.1242/jcs.79.1.95. [DOI] [PubMed] [Google Scholar]
- Davies K. J. Protein damage and degradation by oxygen radicals. I. general aspects. J Biol Chem. 1987 Jul 15;262(20):9895–9901. [PubMed] [Google Scholar]
- Fridovich I. Biological effects of the superoxide radical. Arch Biochem Biophys. 1986 May 15;247(1):1–11. doi: 10.1016/0003-9861(86)90526-6. [DOI] [PubMed] [Google Scholar]
- Galiazzo F., Schiesser A., Rotilio G. Oxygen-independent induction of enzyme activities related to oxygen metabolism in yeast by copper. Biochim Biophys Acta. 1988 Apr 14;965(1):46–51. doi: 10.1016/0304-4165(88)90149-3. [DOI] [PubMed] [Google Scholar]
- Gregory E. M., Goscin S. A., Fridovich I. Superoxide dismutase and oxygen toxicity in a eukaryote. J Bacteriol. 1974 Feb;117(2):456–460. doi: 10.1128/jb.117.2.456-460.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984 Apr 1;219(1):1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer D. H., Thiele D. J., Lemontt J. E. Function and autoregulation of yeast copperthionein. Science. 1985 May 10;228(4700):685–690. doi: 10.1126/science.3887570. [DOI] [PubMed] [Google Scholar]
- Hartig A., Ruis H. Nucleotide sequence of the Saccharomyces cerevisiae CTT1 gene and deduced amino-acid sequence of yeast catalase T. Eur J Biochem. 1986 Nov 3;160(3):487–490. doi: 10.1111/j.1432-1033.1986.tb10065.x. [DOI] [PubMed] [Google Scholar]
- Hinshaw D. B., Armstrong B. C., Burger J. M., Beals T. F., Hyslop P. A. ATP and microfilaments in cellular oxidant injury. Am J Pathol. 1988 Sep;132(3):479–488. [PMC free article] [PubMed] [Google Scholar]
- Hyslop P. A., Hinshaw D. B., Halsey W. A., Jr, Schraufstätter I. U., Sauerheber R. D., Spragg R. G., Jackson J. H., Cochrane C. G. Mechanisms of oxidant-mediated cell injury. The glycolytic and mitochondrial pathways of ADP phosphorylation are major intracellular targets inactivated by hydrogen peroxide. J Biol Chem. 1988 Feb 5;263(4):1665–1675. [PubMed] [Google Scholar]
- Hörtner H., Ammerer G., Hartter E., Hamilton B., Rytka J., Bilinski T., Ruis H. Regulation of synthesis of catalases and iso-1-cytochrome c in Saccharomyces cerevisiae by glucose, oxygen and heme. Eur J Biochem. 1982 Nov;128(1):179–184. doi: 10.1111/j.1432-1033.1982.tb06949.x. [DOI] [PubMed] [Google Scholar]
- Iida H., Yahara I. Specific early-G1 blocks accompanied with stringent response in Saccharomyces cerevisiae lead to growth arrest in resting state similar to the G0 of higher eucaryotes. J Cell Biol. 1984 Apr;98(4):1185–1193. doi: 10.1083/jcb.98.4.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M., Najarian R., Haslinger A., Valenzuela P., Welch J., Fogel S. Primary structure and transcription of an amplified genetic locus: the CUP1 locus of yeast. Proc Natl Acad Sci U S A. 1984 Jan;81(2):337–341. doi: 10.1073/pnas.81.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyhani E., Keyhani J. Cytochrome c oxidase biosynthesis and assembly in Candida utilis yeast cells. Function of copper in the assembly of active cytochrome c oxidase. Arch Biochem Biophys. 1975 Apr;167(2):596–602. doi: 10.1016/0003-9861(75)90503-2. [DOI] [PubMed] [Google Scholar]
- Kulbe K. D., Foellmer H., Fuchs J. Simultaneous purification of glyceraldehyde-3-phosphate dehydrogenase, 3-phosphoglycerate kinase, and phosphoglycerate mutase from pig liver and muscle. Methods Enzymol. 1982;90(Pt E):498–511. doi: 10.1016/s0076-6879(82)90177-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee F. J., Hassan H. M. Biosynthesis of superoxide dismutase in Saccharomyces cerevisiae: effects of paraquat and copper. J Free Radic Biol Med. 1985;1(4):319–325. doi: 10.1016/0748-5514(85)90138-2. [DOI] [PubMed] [Google Scholar]
- Marres C. A., Van Loon A. P., Oudshoorn P., Van Steeg H., Grivell L. A., Slater E. C. Nucleotide sequence analysis of the nuclear gene coding for manganese superoxide dismutase of yeast mitochondria, a gene previously assumed to code for the Rieske iron-sulphur protein. Eur J Biochem. 1985 Feb 15;147(1):153–161. doi: 10.1111/j.1432-1033.1985.tb08731.x. [DOI] [PubMed] [Google Scholar]
- Ng R., Abelson J. Isolation and sequence of the gene for actin in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3912–3916. doi: 10.1073/pnas.77.7.3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsumi Y., Kitamoto K., Anraku Y. Changes induced in the permeability barrier of the yeast plasma membrane by cupric ion. J Bacteriol. 1988 Jun;170(6):2676–2682. doi: 10.1128/jb.170.6.2676-2682.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips P. G., Higgins P. J., Malik A. B., Tsan M. F. Effect of hyperoxia on the cytoarchitecture of cultured endothelial cells. Am J Pathol. 1988 Jul;132(1):59–72. [PMC free article] [PubMed] [Google Scholar]
- Salin M. L., McCord J. M. Superoxide dismutases in polymorphonuclear leukocytes. J Clin Invest. 1974 Oct;54(4):1005–1009. doi: 10.1172/JCI107816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatzman A. R., Kosman D. J. Biosynthesis and cellular distribution of the two superoxide dismutases of Dactylium dendroides. J Bacteriol. 1979 Jan;137(1):313–320. doi: 10.1128/jb.137.1.313-320.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A., Sherman F. Genetic and physiological characterization of met15 mutants of Saccharomyces cerevisiae: a selective system for forward and reverse mutations. Genetics. 1975 Sep;81(1):75–97. doi: 10.1093/genetics/81.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart P. R. Analytical methods for yeasts. Methods Cell Biol. 1975;12:111–147. doi: 10.1016/s0091-679x(08)60955-3. [DOI] [PubMed] [Google Scholar]
- Tonnesen T., Friesen J. D. Inhibitors of ribonucleic acid synthesis in Saccharomyces cerevisiae: decay rate of messenger ribonucleic acid. J Bacteriol. 1973 Sep;115(3):889–896. doi: 10.1128/jb.115.3.889-896.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdivia E., Martinez J., Ortega J. M., Montoya E. Control of catalase and peroxidase biosynthesis by carbon source and oxygen in the yeast Saccharomyces cerevisiae. Can J Microbiol. 1983 Sep;29(9):1200–1204. doi: 10.1139/m83-183. [DOI] [PubMed] [Google Scholar]
- Welch J. W., Fogel S., Cathala G., Karin M. Industrial yeasts display tandem gene iteration at the CUP1 region. Mol Cell Biol. 1983 Aug;3(8):1353–1361. doi: 10.1128/mcb.3.8.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerbeek-Marres C. A., Moore M. M., Autor A. P. Regulation of manganese superoxide dismutase in Saccharomyces cerevisiae. The role of respiratory chain activity. Eur J Biochem. 1988 Jul 1;174(4):611–620. doi: 10.1111/j.1432-1033.1988.tb14142.x. [DOI] [PubMed] [Google Scholar]
- Wolff S. P., Dean R. T. Fragmentation of proteins by free radicals and its effect on their susceptibility to enzymic hydrolysis. Biochem J. 1986 Mar 1;234(2):399–403. doi: 10.1042/bj2340399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimniak P., Hartter E., Woloszczuk W., Ruis H. Catalase biosynthesis in yeast: formation of catalase A and catalase T during oxygen adaptation of Saccharomyces cerevisiae. Eur J Biochem. 1976 Dec 11;71(2):393–398. doi: 10.1111/j.1432-1033.1976.tb11126.x. [DOI] [PubMed] [Google Scholar]