Abstract
Microdamage formation is a critical determinant of bone fracture and the nature and type of damage formed in bone depends on the interaction of its extracellular matrix (ECM) with the applied loading. More importantly, because bone is a hierarchical composite with multiple length scales linked to each other, the nature and type of damage in bone could also be hierarchical. In this review article, based on new unpublished data and a reanalysis of literature reports on in vivo and in vitro observations of microdamage, three length scales including mineralized collagen fibrils, lamellar and osteonal levels have been identified as the key contributors to microdamage hierarchy and energy dissipation in bone. Inherent hierarchy in bone’s ECM therefore has specific microstructural features and energy dissipation mechanisms at different length scales that allow the bone to effectively resist the different components of the applied physiological loading. Furthermore, because human bones experience multiaxial cyclic loading and its ECM is subjected to variation with aging and disease, additional emphasis is placed on investigating how the nature of applied loading and the quality of ECM affect the hierarchy of microdamage formation with age.
Keywords: Microdamage, hierarchy, bone, fracture, age
1. Introduction
Age-related non-traumatic fractures are a major health problem in the United States and elsewhere resulting in morbidity, mortality and substantial economic costs (Melton [1], Ray et al., [2], Singer et al., [3]). Historically, only bone mass was considered to be a significant predictor of fracture risk but the current consensus is that loss of bone mass is not the only condition to cause fracture (Burr et al., [4]). Hui et al. [5] demonstrate that for a given bone mass an individual’s risk to fracture increases with age. Furthermore, using information on donors’ height, weight and sex in conjunction with the measurements of cross-section geometry, Stein et al. [6] show that once the effects of age-related variations in height, weight and sex are accounted for, the geometric variations in long bones including an increase in the outer diameter compensate for bone loss and maintain similar peak stress values across chronological age. In vivo strain recordings from young and old volunteers reported by Fyhrie et al. [7] seem to confirm Stein et al.’s [6] analyses as no age-related differences in strain magnitude, following muscle fatigue, were found. It may, therefore, be considered that factors other than bone mass including changes in material property of bone (Heaney [8], Ott [9], Burr et al. [4]) are of crucial importance to the understanding of age-related skeletal fragility.
A number of previous studies have reported age-related changes in the material property of bone including variations in strength and resistance of bone against initiation of a fracture crack or fracture toughness measured under the application of singly applied monotonic load (Evans [10], Burstein et al., [11], Bonfield et al., [12], McCalden et al., [13], Norman et al., [14], Zioupos and Currey [15], Wang et al., [16]). In these studies, material properties were correlated to either tissue composition (e.g. mineral, collagen) or tissue arrangement (e.g. osteon morphology) to various degrees of success (Evans and Riolo [17], McCalden et al., [13], Norman et al., [14], Yeni et al., [18–19], Wang et al., [16], Zioupos et al., [20]). The idea behind such correlations is that the variations in tissue composition and arrangement affect some aspect of the material behavior of bone that partially or wholly determines the measured property.
Until recently, the material behavior determining the strength and toughness of bone was not well established. Work done by the author (Vashishth et al., [21–25]) and others (Schaffler et al., [26], Zioupos and Currey [27], Burr et al., [28], Jepsen et al., [29]) have demonstrated that bone is a brittle microcracking solid and that bone derives a part of its fracture properties including strength, toughness and fatigue life from its ability to form discrete microcracks that absorb the applied energy and do not coalesce to propagate into a fracture.
It can therefore be considered that microdamage formation is a critical determinant of bone fracture and that the nature and type of damage formed in bone depends on the interaction of its extracellular matrix (ECM) with the applied loading. More importantly, because bone is a hierarchical composite with multiple length scales linked to each other (Figure 1), the nature and type of damage in bone could also be hierarchical. However, no information on the hierarchy of damage and its relationship to the established hierarchy of bone’s extracellular matrix is currently available.
Figure 1.
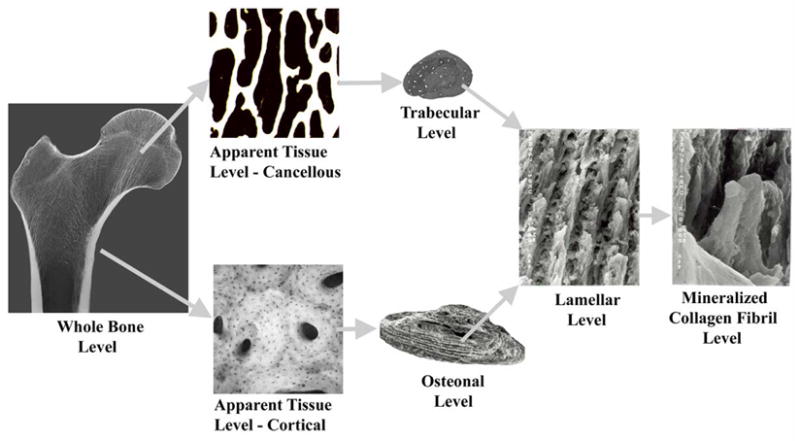
Hierarchical Scales of bone’s Extracellular Matrix. The individual scanning electron micrographs used above are adapted from Vashishth et al. [25; 77] and Ciarelli [65].
In this review article the occurrence of bone microdamage at ultrastructural, lamellar and tissular levels in bone is presented based on new unpublished data and a review or reanalysis of previously reported in vivo and in vitro observations of microdamage by the author (Vashishth et al., [24–25], 30–31], George & Vashishth [32], Diab and Vashishth [33], Diab et al., [34]) and others (Wenzel et al., [35], Schaffler et al., [36], Norman and Wang [37], Lee et al., [38], O’Brien et al., [39]). Furthermore, because human bones experience multiaxial cyclic loading and their extracellular matrix is subjected to variation with aging and disease, additional emphasis has been placed on investigating how the nature of applied loading and the quality of ECM affects the hierarchy of microdamage formation.
2. Identification and Nature of In vivo Damage
In vivo damage is generally identified using the method originally developed by Frost [40] and modified by Burr and Stafford [41]. The experimental technique involves en bloc staining of harvested sections of donor tissues in 1% basic fuchsin solution based in ascending series of ethanol (70, 80, 90 and 100%) in vacuum at room temperature for a period of five days. The en bloc stained cross-sections are then embedded in polymethlyl methacrylate (PMMA), serially sectioned to 200μm thickness and ground to 100μm thickness. With this method of preparation, only the microdamage present in bone at the time of donor death takes up basic fuchsin. The stained sections are then analyzed under a transmitted light microscope to determine the nature of induced microdamage.
Using the above method, the author [31] and others (Frost [40], Schaffler et al., [36], Wenzel et al., [35], Norman and Wang [37], Mori et al., [42]) have reported two distinct forms of in vivo microdamage in human bone i.e. linear microcracks and diffuse damage. Linear microcracks are intermediate in size (greater than a canaliculli but smaller than a vascular channel) and, under bright transmitted light, appear as a sharply defined line with edges that are more deeply stained than the intervening space (Burr and Stafford [41], Lee et al., [38], Diab and Vashishth [33]). In contrast, diffuse damage appears as a focal but diffused area of pooled staining not corresponding to an entire microstructural feature (osteon or trabecular packet) (Vashishth et al., [31]). The examination of the patches of diffuse areas of pooled staining under a laser confocal microscope reveals a meshwork of fine submicroscopic cracks (Vashishth et al., [31]).
A comparison of previously reported linear microcracks and diffuse damage observations in human vertebral cancellous bone tissue (Wenzel et al., [35], Vashishth et al., [31]) with new observations in the author’s laboratory on human cortical bone indicates for the first time that both cortical and cancellous tissues form similar morphologies of in vivo microdamage (Figure 2 (a to d)). It is noteworthy that the unloading and reloading of in vitro test specimens in conjunction with the measurement of the loss of apparent mechanical properties, and the characterization of microdamage have demonstrated that the in vitro damage behavior of cancellous bone is indeed similar to that of cortical bone (Fondrk et al., [43], Keaveny et al. [44], Zysset and Curnier, [45]). For example, when loaded in the post-yield region, both cortical and cancellous bones form microcracks (Zioupos et al., [46], Vashishth et al., [21], Wachtel and Keaveny [47]) and display a similar profile of modulus loss (Fondrk et al., [43], Keaveny et al., [44]). More significantly, by simulating the effects of trabecular microfracture and microdamage on apparent modulus reduction, Yeh and Keaveny [48] found that “extensive microdamage” was the primary reason for the loss of apparent modulus in the post-yield region. This analysis is strikingly similar to cortical bone where crack propagation and fracture involve extensive microcracking characterized by a diffuse array of microdamage (Zioupos et al., [44], Vashishth et al., [21,24,25]; Zarrikalam et al., [59]).
Figure 2.
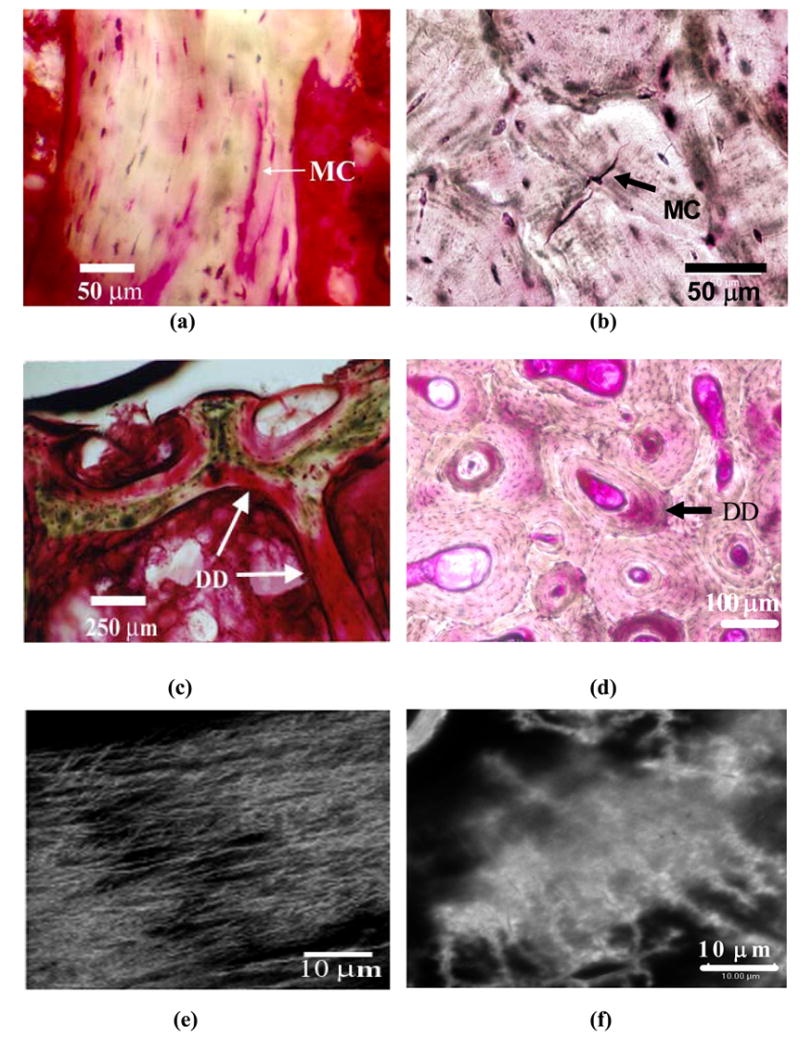
Forms of in vivo microdamage in human T12 vertebral cancellous and human tibial diaphyseal cortical bone. Linear microcrack (MC) in cancellous (a) and cortical (b) bones. Diffuse damage(DD) in cancellous (c) and cortical (d) bone. Images (e) and (f) demonstrate the occurrence of diffuse submicroscopic cracks under laser confocal microscope in areas of diffuse damage in cancellous and cortical bone images shown in (c) and (d), respectively.
Thus the observations of in vivo microdamage patterns as well as in vitro studies referred above indicate that, despite stark differences in failure patterns at the apparent tissue scale, both cortical and cancellous tissue fail similarly at smaller length scales. Linear microcrack and diffuse damage patterns may therefore be related to hierarchical length scales in bone that lie below the apparent tissue level.
3. Assessment of In vitro Microdamage and its Role in Bone Fracture
Using post-hoc histological staining of in vitro loaded and fractured specimens, Chamay [49] and Currey and Bear [50] were one of the first investigators to report the occurrence of microdamage formation under compressive and tensile loading of bone, respectively. Based on an earlier description on prefailure slip lines on minerals (Rine [51]) and polymerized crystals (Williams [52]), microdamage in bone was described as “stress lesions” containing oblique lines that stained positive with hematoxylin and eosin (H&E) (Chamay [49]) or as “fissures” and “intracortical microfractures” that were associated with stress concentrators in bone including osteocyte canaliculi, lacunae and Haversian canals (Currey [53]).
In a subsequent investigation, Currey [54] observed that, in a tensile test of wet bone, the specimen’s gage length became opaque in the post-yield region. He suggested that opacity was caused by innumerable interfaces that appeared in the form of very small cracks in bone. Carter and Caler [55] presented a cumulative damage model of bone and, based on its comparison with experimental data, concluded that failure of bone results when a certain amount of damage has accumulated in the specimen.
Studies done by the author [21–25, 31] and Zioupos & Currey [15,28] established the role of microdamage in crack propagation and post-yield deformation of bone, respectively. Specifically, using a fracture mechanics approach Vashishth et al. [21–25, 31] demonstrated that the application of mode I (tensile) loading results in the formation of microdamage in the form of a diffuse array of microcracks that originates at the level of mineralized collagen fibrils (> 1 μm). More importantly discrete microcrack formation behind the tip of a propagating fracture crack dissipates energy and decelerates the advancing fracture (Vashishth et al. [22, 25]) (Figure 3).
Figure 3.
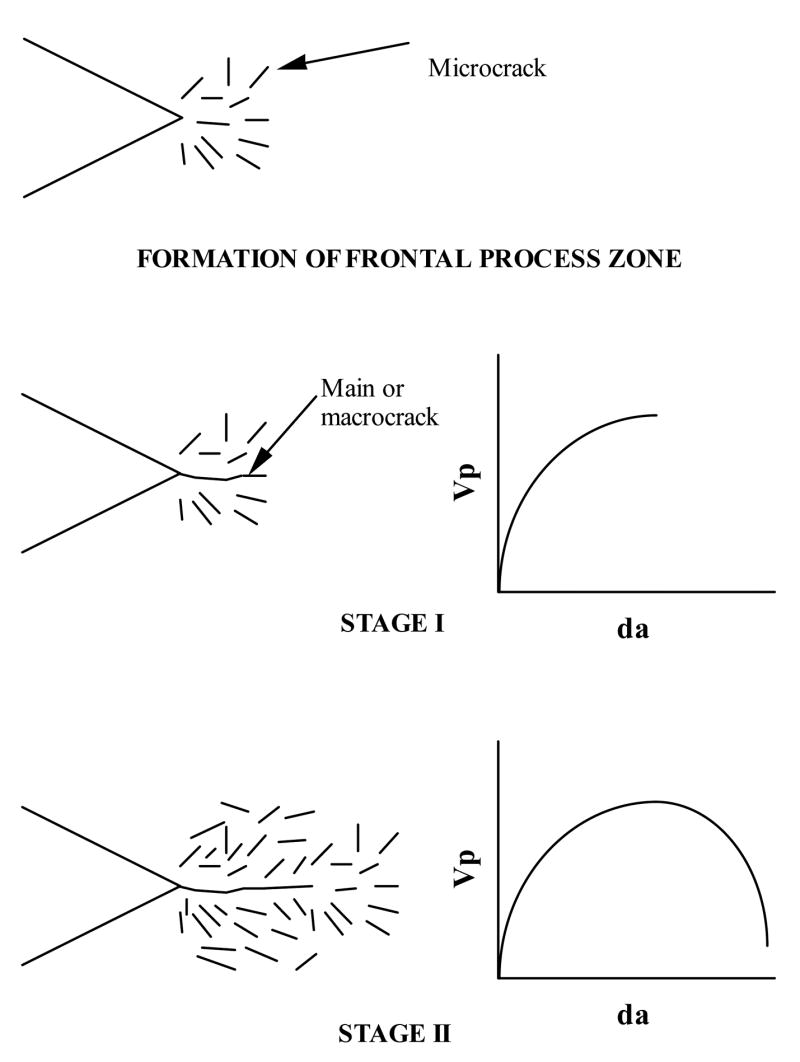
Crack propagation model showing the development of microcracking in cortical bone. The application of loading initially results in the formation of microcracks around the notch. With continued loading a main crack originates from the notch and accelerates through this zone (Stage I). Acceleration of the main crack brings it into uncracked material decelerating the crack. Microcrack formation occurs ahead and behind the tip of the main crack as it moves slowly under continued loading (Stage II). Reproduced from the Journal of Biomechanics 2000, 33:1169–1174. Copyright permission from Elsevier Inc.
Recent work by Ritche and coworkers has questioned the role of microcracking in toughening and have proposed the occurrence of uncracked ligaments as a major toughening mechanism in bone (Nalla et al., [56–58]). Their calculations of the magnitude of microcrack induced toughening in bone are based on the occurrence of limited microcracking [Estimated Volume fraction of microdamage = 3% (Nalla et al., [57])] in the vicinity of the crack tip. However, in the experience of the author (Figure 4) and others (Zarrikalam et al., [59]), microcracking in the vicinity of the crack tip is extensive and is likely to have a more significant role than proposed by Nalla et al. [57]. Moreover, it is likely that the formation of ‘uncracked ligament’ involves microdamage that arrests a propagating crack and initiates a new crack The arrest of a propagating crack and initiation of a new crack results in the formation of an ‘uncracked ligament’ (Hazenberg et al. [60]) and causes occurrence of a ‘stop and start’ type of crack growth pattern reported by Norman et al. [61].
Figure 4.

Micrographs showing transmitted light (a) and laser confocal (b) images of extensive microcrack formation in the crack tip region of a tested human cortical bone compact tension specimen
The discrepancies in the perceived role of microdamage in the fracture of bone highlight the need to better characterize damage and, more importantly, to relate microdamage to the established hierarchy in bone microstructure. For example, it is likely that diffuse array of microcracks shown in Figures 3 and 4 and larger microcracks resulting in the formation of uncracked ligaments represent microdamage in bone at two different scales respectively. More importantly under this scheme the lower scale phenomena will feed into the higher scale and explain its occurrence.
4. Microdamage Hierarchy, Applied Loading and Extracellular Bone Matrix
Although there are no reports of in vitro tests specifically designed to investigate the hierarchical aspects of bone microdamage, data from several previous in vitro studies can be analyzed to establish the hierarchy of microdamage and determine its relationship to the established hierarchy of bone’s ECM. Specifically, unlike the microdamage patterns resulting from the variable forms of in vivo loading, microdamage patterns observed in vitro can be related to specific components of physiological loading (tension, compression or torsion) and then reproduced and tested for correlation with hierarchical measures of bone’s ECM.
A review of the damage mechanisms and failure modes in bone under different components of physiological loading, reported recently by the author’s laboratory (Figure 5), indicates that, consistent with fracture mechanics tests under mode I (tensile) (Norman et al., [61] Vashishth et al., [21]) and creep-fatigue damage model (Caler and Carter [62]), cyclic tensile loading produces primarily time dependent damage in the form of diffuse damage, and microcracks of the order of 100 μm subsequently initiate from these areas in the transverse plane to cause fracture (Figure 6) (George and Vashishth [32]). Similarly a unique association between tensile deformation and diffuse damage has also been reported by Boyce et al. [63] and Diab and Vashishth [33]. More importantly revisiting the results reported by Diab et al. [34], in which no association between lamellar orientation and diffuse damage was found, indicates that diffuse damage originates below the lamellar length scale (< 1–3 μm). This observation is consistent with an earlier fractography study by Braidotti et al. [64] and Vashishth et al. [25] in which initiation of fracture in bone was shown to occur at the level of mineralized collagen fibrils (≤ 1 μm). The separation of mineralized collagen fibrils is thought to be governed by constituents of the organic matrix (Nicolella et al. [65]) that bridge the microcrack resulting from the separation and provide bone with additional resistance against fracture (Yeni and Fyhrie [66]). Furthermore according to several recent studies by Hansma and co-workers [67–68] the microcrack bridging mechanism involves an unidentified ‘glue’ that allows bone to resists the separation of the mineralized collagen fibrils through rupture of sacrificial bonds and consequent stretching of molecules within the glue. However, because sacrificial bonds involve ionic bridges formed by diavalent calcium ions (Thompson et al. [69]) and long-term treatment of bone in Ca++ solution does not alter the strength and toughness of bone (Yeni et al. [70]), the contribution of sacrificial bonds to fracture properties of bone at the macroscale remains controversial.
Figure 5.
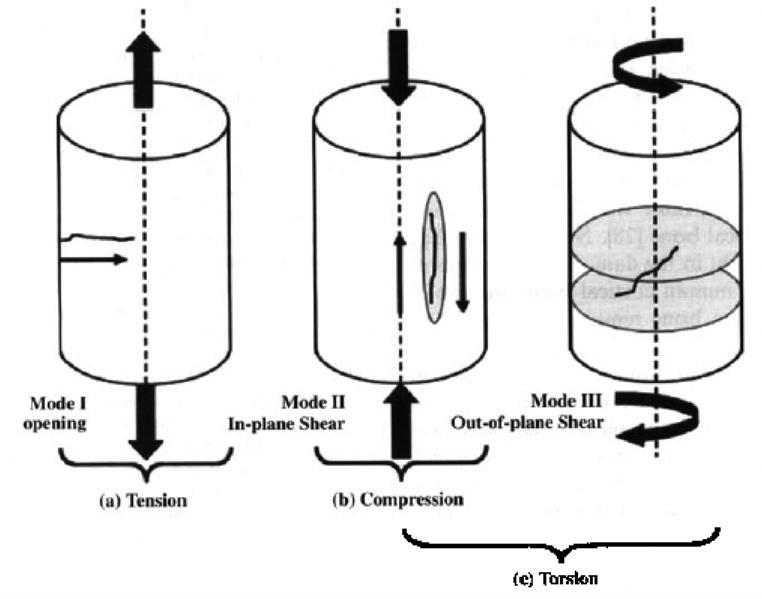
Schematic representation of microcrack development in specimens subjected to Zero-Tension (Mode I) (a), Zero-Compression (Mode II) (b), and Zero-Torsion (Modes II & III) (c) loading. Mode I fracture creates and propagates microcracks in the transverse direction. Mode II fracture occurs when crack surfaces slide over one another; damage is on a single plane. Mode III fracture is similar to a tearing motion where the crack surfaces move relative to each other on multiple planes. Reproduced from the Journal of Orthopaedic Research 2005, 23:1047–1053. Copyright permission from Elsevier Inc.
Figure 6.
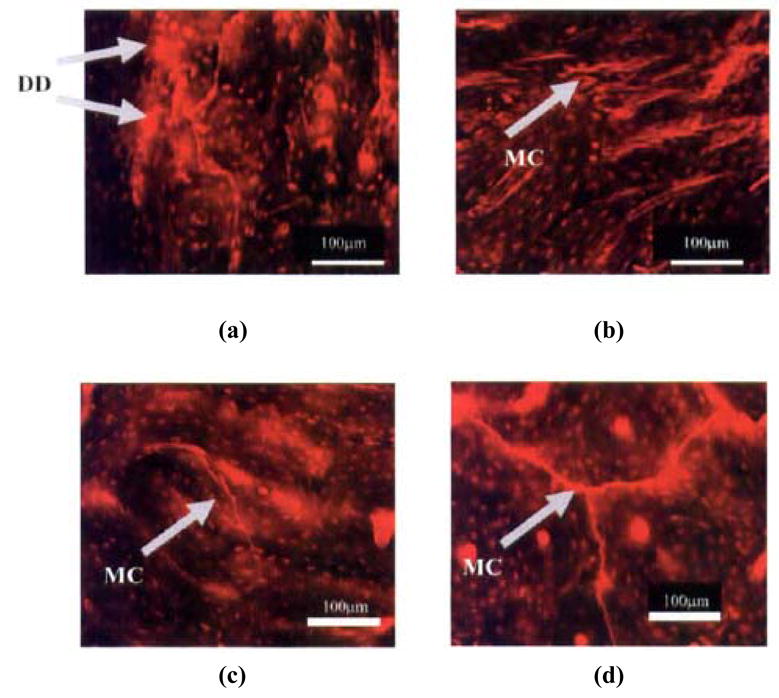
Typical micrographs showing damage in zero-tensile (OT) (a: transverse; b: longitudinal section), zero-compressive (OC) (c: transverse section) and zero-torsion (OR) (d: transverse section). Letters DD and MC in the micrographs indicate diffuse damage and linear micrographs, respectively. Scale bar in the figures represents 100 μm. Reproduced from the Journal of Orthopaedic Research 2005, 23:1047–1053. Copyright permission from Elsevier Inc.
In contrast to diffuse damage produced by tensile deformation, cyclic compressive loading produced predominantly cycle dependent damage in the form of short (100–200 μm) microcracks while torsional loading produced predominantly time dependent damage in the form of long (> 300 μm) microcracks with no or little diffuse damage formation (Caler and Carter [62]; George and Vashishth [32]) (Figure 6). A unique association between compressive loading and short microcracks of the order of 100–250 μm is similar to previous and recent reports (Boyce et al., [63], Diab and Vashishth [33], Diab et al., [34]). Additionally, shorter linear microcracks produced by mode II failure under compressive loading (Figure 5) show a strong association with the lamellar orientation of bone (Diab et al., [34]) while longer microcracks (> 300 μm), produced by mixed mode failure under torsional loading (Figure 5), are deflected by the larger microstructural features in bone i.e. osteons (Figure 6). An increase in the magnitude of mixed mode loading by the addition of axial component to torsional loading caused larger cracks (> 500 μm) that initially were deflected by osteons but later penetrated them (Figure 7) (George and Vashishth [32]). This observation is consistent with the recent results by O’Brien et al. [39] and Moschin et al. [71] that provide a more systematic association between the size of a crack and its ability to penetrate an osteon.
Figure 7.
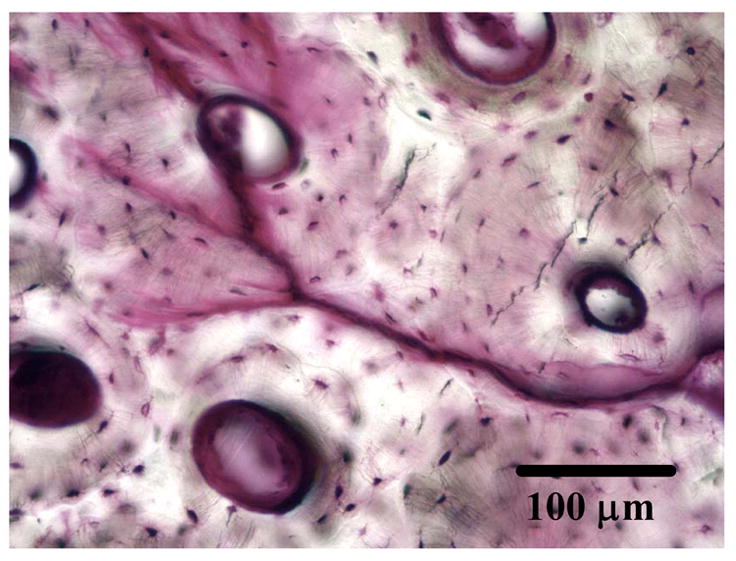
Transverse section of a axial-torsional specimens showing microcrack interaction with osteons (deflection on the right and penetration on the left). Note that the crack initiated from the right of the micrograph.
In summary, the applied loading interacts with the hierarchy in bone ECM and produces microdamage at various length scales. Tensile loading interacts with sublamellar structures including the mineralized collagen fibril matrix to produce primarily time dependent diffuse damage containing submicron cracks < 1 μm. Compressive loading interacts with lamellae and produces primarily cycle dependent short linear microcracks of the order of 100–200 μm. These microcracks can localize within lamellae or interact with the osteonal boundary. Torsional and other forms of mixed mode loadings interact with osteons and produce primarily time dependent microcracks of the order of 300 μm or greater that get deflected by the osteonal structure when short and penetrate the osteons when long (>500 μm). Inherent hierarchy in bone’s ECM therefore has specific microstructural features and energy dissipation mechanisms at different length scales that allow the bone to effectively resist the different components of the applied physiological loading.
5. Age Effects on Microdamage Hierarchy
Despite several studies on the characterization of bone’s extracellular matrix with age, the changes in its ECM that alter the microdamage hierarchy are unknown. Consequently a mechanistic understanding of the altered fracture behavior of aging bone is lacking.
Microdamage produced at the three length scales including mineralized collagen fibrils, lamellar and osteonal levels allows the bone to effectively resist the different components of applied physiological loading. Changes with aging and disease at each of the three length scales in the bone’s ECM should therefore affect microdamage formation at the scale where the modification takes place as well as other higher levels, and consequently alter the propensity of bone to fracture. Diab and Vashishth [33] have recently demonstrated that, under physiologically relevant four-point bending loading, diffuse damage formation occurs first in the tensile cortex and the more detrimental linear microcracks form in the compressive cortex only after the tensile cortex is saturated with diffuse damage. More importantly this mechanism of damage formation allows bone to dissipate energy and delay the development of a catastrophic fracture crack.
At the length scale of a mineralized collagen fibril, age-related changes may occur in its key constituents including the collagen, mineral and the constituents of the matrix (e.g ‘glue’) that hold the mineralized fibrils together. Because the specific contents of the non-fibrillar glue are yet unknown (Fantner et al., [69]), emphasis at this scale needs to be placed on the changes in mineral and collagen that may reduce the ability of the mineralized fibril in bone to stretch and deform decreasing the diffuse damage formation recently reported by Diab et al. [34]. Specifically it has been demonstrated that bone’s capability to form diffuse damage as well as the size of diffuse damage area are reduced with age but the reasons remain unclear (Diab et al.[34]).
Consequent to the decreased ability to form diffuse damage, aging bone also demonstrates significant changes in microdamage formation at the next hierarchical level, i.e. the lamellar level. A recent study by Ciarelli et al. [72] found differences in the distribution of mineralization between the age-matched control and fracture group but further work is necessary to determine if the heterogeneity of the mineral distribution occurs with age and explains the propensity of aging bone to form more numerous and longer interlamellar microcracks reported by several investigators (Courtney et al. [73]; Knott et al. [74]; and Diab et al. [34])
At the third and final level of interest to microdamage hierarchy is the osteon or the Haversian system. With age, the average size of an osteon decreases but the number of osteons and the amount of interstitial bone increase (Currey [75]; Kerley [76]; Ortner [77]; Evans [78]; Nyssen-Behtets [79]). Because interstitial bone is harder, stiffer and hence more brittle than osteonal bone (Rho [80]), mixed mode loading of aging bone could result in the formation of fewer and longer catastrophic cracks, however, investigations employing torsional or axial-torsional loading have been few (Vashishth et al. [81]; George & Vashishth [32, 82–83]) and the effects of changes in osteonal sizes and their distribution on microdamage hierarchy remain undetermined.
6. Summary
In this article the hierarchical nature of bone microdamage and its relationships with applied loading and the established hierarchy of bone’s ECM occurrence of bone microdamage is presented based on new data as well as a review and reanalysis of previously reported in vivo and in vitro observations of microdamage by the author and others. It was found that the applied loading interacts with the hierarchy in bone’s ECM and produces microdamage at various length scales. Tensile loading interacts with sublamellar structures including the mineralized collagen fibril matrix to produce primarily time dependent diffuse damage containing submicron cracks < 1 μm. Compressive loading interacts with lamellae and produces primarily cycle dependent short linear microcracks of the order of 100–200 μm. Torsional and other forms of mixed mode loadings interact with osteons and produce primarily time dependent microcracks of the order of 300 μm or greater that either get deflected by the osteonal structure and penetrate the osteons. Inherent hierarchy in bone’s ECM therefore has specific microstructural features and energy dissipation mechanisms at different length scales that allow the bone to effectively resist the different components of the applied physiological loading. However, despite several studies on the characterization of bone’s extracellular matrix with age, the changes in its ECM that may potentially alter the microdamage hierarchy at each of the three levels identified are still unknown.
Acknowledgments
NIH Grants AR49635 (NIAMS) and Tamim Diab MS and Winson George, PhD for histology
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Melton JL. In: Epidemiology of Fractures in Osteoporosis: Etiology, Diagnosis and Management. Lawrence Riggs B, Joseph Melton L, editors. Raven Press; NY: 1988. pp. 133–154. [Google Scholar]
- 2.Ray NF, Chan JK, Thamer M, Melton LJ. Medical expenditures for the treatment of osteoporotic fractures in the United States in 1995: report from the National Osteoporosis Foundation. J Bone Miner Res. 1997;12:24–35. doi: 10.1359/jbmr.1997.12.1.24. [DOI] [PubMed] [Google Scholar]
- 3.Singer BR, McLauchlan GJ, Robinson CM, Christe J. Epidemiology of fractures in 15000 adults: The influence of age and gender. J Bone Joint Surg. 1998;80-B:243–248. doi: 10.1302/0301-620x.80b2.7762. [DOI] [PubMed] [Google Scholar]
- 4.Burr DB, Forwood MR, Fyhrie DP, Martin RB, Schaffler MB, Turner CH. Bone microdamage and skeletal fragility in osteoporosis and stress fracture. J Bone Miner Res. 1997;12:6–15. doi: 10.1359/jbmr.1997.12.1.6. [DOI] [PubMed] [Google Scholar]
- 5.Hui S, Slemenda CW, Johnston CC. Age and bone mass as predictors of fracture in a prospective study. J Clin Invest. 1988;81:1804–1809. doi: 10.1172/JCI113523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stein MS, Thomas CDL, Feik SA, Wark JD, Clement JG. Bone size and mechanics at the femoral diaphysis across age and sex. J Biomechanics. 1110;1998:31. doi: 10.1016/s0021-9290(98)00127-4. [DOI] [PubMed] [Google Scholar]
- 7.Fyhrie DP, Milgrom C, Hoshaw SJ, Dar S, Drumb D, Burr DB. Effect of fatiguing exercise on longitudinal bone strain as related to stress fracture in humans. Ann Biomed Eng. 1998;26:660–665. doi: 10.1114/1.103. [DOI] [PubMed] [Google Scholar]
- 8.Heaney RP. Is there a role for bone quality in fragility fractures? Calcif Tiss Int. 1993;53 (suppl):S3–S6. doi: 10.1007/BF01673394. [DOI] [PubMed] [Google Scholar]
- 9.Ott SM. When bone mass fails to predict bone failure. Calcif Tiss Int. 1993;53 (suppl):S7–S13. doi: 10.1007/BF01673395. [DOI] [PubMed] [Google Scholar]
- 10.Evans FG. Mechanical properties and histology of cortical bone from younger and older men. Anat Rec. 1976;185:1–11. doi: 10.1002/ar.1091850102. [DOI] [PubMed] [Google Scholar]
- 11.Burstein AH, Reilly DT, Martens M. Aging of bone tissue: mechanical properties. J Bone Joint Surg Am. 1976;58:82–86. [PubMed] [Google Scholar]
- 12.Bonfield W, Behiri JC, Charalambides JC. Orientation and age-related dependence of the fracture toughness of cortical bone. In: Perrin SM, Schneider E, editors. Biomechanics: Current Interdisciplinary Research. Martinus Nijhoff; Dordrecht, Netherlands: 1985. pp. 185–189. [Google Scholar]
- 13.McCalden RW, McGeough JA, Barker MB, Court-Brown CM. Age-related changes in the tensile properties of cortical bone. The relative importance of changes in porosity, mineralization, and microstructure. J Bone Joint Surg Am. 1993;75:1193–1205. doi: 10.2106/00004623-199308000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Norman TL, Yeni YN, Brown CU, Wang Z. Influence of microdamage on fracture toughness of human femur and tibia. Bone. 1998;23:303–306. doi: 10.1016/s8756-3282(98)00103-3. [DOI] [PubMed] [Google Scholar]
- 15.Zioupos P, Currey JD. Changes in Stiffness, Strength, and Toughness of Human Cortical Bone with Age. Bone. 1998;22:57–66. doi: 10.1016/s8756-3282(97)00228-7. [DOI] [PubMed] [Google Scholar]
- 16.Wang XD, Masilamani NS, Mabrey JD, Alder ME, Agrawal CM. Changes in the fracture toughness of bone may not be reflected in its mineral density, porosity and tensile properties. Bone. 1998;23:67–72. doi: 10.1016/s8756-3282(98)00071-4. [DOI] [PubMed] [Google Scholar]
- 17.Evans FG, Riolo ML. Relations between the fatigue life and histology of adult human cortical bone. J Bone Joint Surg. 1970;52-A:1579–1586. [PubMed] [Google Scholar]
- 18.Yeni YN, Brown CU, Wang Z, Norman TL. The influence of bone morphology on fracture toughness of the human femur and tibia. Bone. 1997;21:453–459. doi: 10.1016/s8756-3282(97)00173-7. [DOI] [PubMed] [Google Scholar]
- 19.Yeni YN, Brown CU, Norman TL. The influence of bone composition and apparent density on fracture toughness of human femur and tibia. Bone. 1998;22:79–84. doi: 10.1016/s8756-3282(97)00227-5. [DOI] [PubMed] [Google Scholar]
- 20.Zioupos P, Currey JD, Hamer AJ. The role of collagen in the declining mechanical properties of aging human cortical bone. J Biomed Mater Res. 1999;45:108–116. doi: 10.1002/(sici)1097-4636(199905)45:2<108::aid-jbm5>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 21.Vashishth D, Trifonas J, Behiri JC, Bonfield W. Secondary crack propagation in cortical bone. ASME. 1994d;64–4:24–27. [Google Scholar]
- 22.Vashishth D, Tanner KE, Bonfield W. Experimental validation of a microcracking-based toughening mechanism for cortical bone. J Biomechanics. 2003;36:121–124. doi: 10.1016/s0021-9290(02)00319-6. [DOI] [PubMed] [Google Scholar]
- 23.Vashishth D, Bonfield W. Aetiology of cortical bone fracture. Proceedings of the 10th Conference of the European Society of Biomechanics; Leuven, Belgium. 1996b. p. 55. [Google Scholar]
- 24.Vashishth D, Behiri JC, Bonfield W. Crack growth resistance in cortical bone: Concept of microcrack toughening. J Biomechanics. 1997b;30:763–769. doi: 10.1016/s0021-9290(97)00029-8. [DOI] [PubMed] [Google Scholar]
- 25.Vashishth D, Tanner KE, Bonfield W. Contribution, Development and morphology of microcracking in cortical bone during crack propagation. J Biomechanics. 2000c;33:1169–1174. doi: 10.1016/s0021-9290(00)00010-5. [DOI] [PubMed] [Google Scholar]
- 26.Schaffler MB, Pitchford WC, Choi K, Riddle JM. Examination of compact bone microdamage using back-scattered electron microscopy. Bone. 1994;15:483–488. doi: 10.1016/8756-3282(94)90271-2. [DOI] [PubMed] [Google Scholar]
- 27.Zioupos P, Currey JD. The extent of microcracking and the morphology of microcracks in damaged bone. J Mater Sci. 1994;29:978–986. [Google Scholar]
- 28.Burr DB, Turner CH, Naick P, Forwood MR, Ambrosius W, Hasan MS, Pidaparti R. Does microdamage accumulation affect the mechanical properties of bone? J Biomechanics. 1998;31:337–345. doi: 10.1016/s0021-9290(98)00016-5. [DOI] [PubMed] [Google Scholar]
- 29.Japsen KJ, Davy DT, Krzypow DJ. The role of the lamellar interface during torsional yielding of human cortical bone. J Biomechanics. 1999;32:303–310. doi: 10.1016/s0021-9290(98)00179-1. [DOI] [PubMed] [Google Scholar]
- 30.Vashishth D, Tanner KE, Bonfield W. Experimental Validation of a Crack Propagation Mechanism in Cortical Bone. J Biomech. 2003b Jan;36(1):121–4. doi: 10.1016/s0021-9290(02)00319-6. [DOI] [PubMed] [Google Scholar]
- 31.Vashishth DJ, Koontz Qiu S, Cannon-Lundin D, Yeni YN, Schaffler MB, Fyhrie DP. In vivo diffuse damage in human trabecular bone. Bone. 2000a;26(No 2):147–152. doi: 10.1016/s8756-3282(99)00253-7. [DOI] [PubMed] [Google Scholar]
- 32.George and Vashishth 2005. Damage mechanisms and failure modes of cortical bone under components of physiological loading. J Orthop Res. 2005 Sep;23(5):1047–53. doi: 10.1016/j.orthres.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 33.Diab T, Vashishth D. Effects of Damage Morphology on Cortical Bone Fragility. Bone. 2005;37(1):96–102. doi: 10.1016/j.bone.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 34.Diab T, Condon KW, Burr DB, Vashishth D. Age-related change in the damage morphology in human cortical bone and its role in bone fragility. Bone. 2006 Mar;38(3):427–31. doi: 10.1016/j.bone.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 35.Wenzel TE, Schaffler MB, Fyhrie DP. In vivo trabecular microcracks in human vertebral bone. Bone. 1996;19:89–95. doi: 10.1016/8756-3282(96)88871-5. [DOI] [PubMed] [Google Scholar]
- 36.Schaffler MB, Choi K, Milgrom C. Aging and matrix microdamage accumulation in human compact bone. Bone. 1995;17:521–525. doi: 10.1016/8756-3282(95)00370-3. [DOI] [PubMed] [Google Scholar]
- 37.Norman TL, Wang Z. Microdamage of human cortical bone: incidence and morphology in long bones. Bone. 1997 Apr;20(4):375–9. doi: 10.1016/s8756-3282(97)00004-5. [DOI] [PubMed] [Google Scholar]
- 38.Lee TC, Mohsin S, Taylor D, Parkesh T, Gunnlaugsson T, O’Brien FJ, Giehl M, Gowin W. Detecting microdamage in bone. J Anat. 2003;203:161–172. doi: 10.1046/j.1469-7580.2003.00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Brien FJ, Taylor D, Lee TC. The effect of bone microstructure on the initiation and growth of microcracks. Journal of Orthopaedic Research. 2005;(23):475–480. doi: 10.1016/j.orthres.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 40.Frost HM. Presence of microscope cracks in vivo in bone. Henry Ford Hosp Bull. 1960;8:25–35. [Google Scholar]
- 41.Burr DB, Stafford T. Validity of the bulk-staining technique to separate artifactual from in vivo bone microdamage. Clin Orthop Relat Res. 1994;260:305–308. [PubMed] [Google Scholar]
- 42.Mori S, Harruff R, Ambrosius W, Burr DB. Trabecular bone volume and microdamage accumulation in femoral heads of women with and without femoral neck fractures. Bone. 1997;21:521–526. doi: 10.1016/s8756-3282(97)00200-7. [DOI] [PubMed] [Google Scholar]
- 43.Fondrk M, Bahniuk E, Davy DT, Michaels C. Some viscoplastic charactercteristics of bovine and human cortical bone. J Biomechanics. 1988;21:623–630. doi: 10.1016/0021-9290(88)90200-x. [DOI] [PubMed] [Google Scholar]
- 44.Keaveny TM, Wachtel EF, Guo XE, Hayes WC. Mechanical Behavior of Damaged Trabecular Bone. J Biomechanics. 1994;27 (11):1309–18. doi: 10.1016/0021-9290(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 45.Zysset PK, Curnier A. A 3D damage model for trabecular bone based on fabric tensors. J Biomechanics. 1996;29(12):1549–1558. [PubMed] [Google Scholar]
- 46.Zioupos P, Currey JD, Sedman AJ. An Examination of the micromechanics of failure of bone and antler by acoustic emission tests and lasers scanning confocal microscopy. Med Eng Phys. 1994;16:203–212. doi: 10.1016/1350-4533(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 47.Wachtel EF, Keaveny TM. Dependence of trabecular damage on mechanical strain. J Orthop Res. 1997 Sep;15(5):781–7. doi: 10.1002/jor.1100150522. [DOI] [PubMed] [Google Scholar]
- 48.Yeh OC, Keaveny TM. Relative roles of microdamage and microfracture in the mechanical behavior of trabecular bone. J Orthopaedic Res. 2001;19:1001–1007. doi: 10.1016/S0736-0266(01)00053-5. [DOI] [PubMed] [Google Scholar]
- 49.Chamay A. Mechanical and morphological aspects of experimental overload and fatigue in bone. J Biomechanics. 1970;(3):263–270. doi: 10.1016/0021-9290(70)90028-x. [DOI] [PubMed] [Google Scholar]
- 50.Currey JD, Brear K. Tensile yield in bone. Calc Tiss Res. 1974;(15):173–179. doi: 10.1007/BF02059054. [DOI] [PubMed] [Google Scholar]
- 51.Rinne F. La Science des Roches. 3. Lamarre; Paris: 1928. [Google Scholar]
- 52.Williams ML. The mechanical properties of crystalline polymers interpreted in terms of dislocations. Ann N Y Acad Sci. 1969;155(Art 2):539–560. [Google Scholar]
- 53.Currey JD. Stress concentrations in bone. J Micros Sci. 1962;(103):111–133. [Google Scholar]
- 54.Currey JD. The mechanical adaptations of bones. University Press; Princeton, NJ: 1984. [Google Scholar]
- 55.Carter DR, Caler W. A cumulative damage model for bone fracture. J Orthop Res. 1985;3(1):84–90. doi: 10.1002/jor.1100030110. [DOI] [PubMed] [Google Scholar]
- 56.Nalla RK, Kruzic JJ, Kinney JH, Ritchie RO. Mechanistic aspects of fracture and R-curve behavior in human cortical bone. Biomaterials. 2005 Jan;26(2):217–31. doi: 10.1016/j.biomaterials.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 57.Nalla RK, Kruzic JJ, Ritchie RO. On the origin of the toughness of mineralized tissue: microcracking or crack bridging? Bone. 2004 May;34(5):790–8. doi: 10.1016/j.bone.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 58.Nalla RK, Kinney JH, Ritchie RO. Mechanistic fracture criteria for the failure of human cortical bone. Nat Mater. 2003 Mar;2(3):164–8. doi: 10.1038/nmat832. [DOI] [PubMed] [Google Scholar]
- 59.Zarrinkalam KH, Kuliwaba JS, Martin RB, Wallwork MA, Fazzalari NL. New insights into the propagation of fatigue damage in cortical bone using confocal microscopy and chelating fluorochromes. Eur J Morphol. 2005 Feb–Apr;42(1–2):81–90. doi: 10.1080/09243860500096206. [DOI] [PubMed] [Google Scholar]
- 60.Hazenberg JG, Taylor D, Clive Lee T. Mechanisms of short crack growth at constant stress in bone. Biomaterials. 2006 Mar;27(9):2114–22. doi: 10.1016/j.biomaterials.2005.09.039. [DOI] [PubMed] [Google Scholar]
- 61.Norman TL, Vashishth D, Burr D. Fracture toughness of human bone under tension. J Biomechanics. 1995;28(No 3) doi: 10.1016/0021-9290(94)00069-g. [DOI] [PubMed] [Google Scholar]
- 62.Caler WE, Carter DR. Bone creep-fatigue damage accumulation. J Biomechanics. 1989;22:625–635. doi: 10.1016/0021-9290(89)90013-4. [DOI] [PubMed] [Google Scholar]
- 63.Boyce TM, Fyhrie DP, Glotkowski MC, Radin EL, Schaffler MB. Damage type and strain mode associations in human compact bone bending fatigue. J Orthop Res. 1998;16:322–329. doi: 10.1002/jor.1100160308. [DOI] [PubMed] [Google Scholar]
- 64.Braidotti P, Branca SP, Stagni L. Scanning electron microscopy of human cortical bone failure surfaces. J Biomechanics. 1997;30:155–162. doi: 10.1016/s0021-9290(96)00102-9. [DOI] [PubMed] [Google Scholar]
- 65.Nicolella DP, Moravits DE, Siller-Jackson AJ, Railsback RJ, Timmons SF, Jepsen KJ, Davy DT, Lankford J. Ultrastructural characterization of damaged cortical bone using atomic force microscopy. ASME-BED. 1999;42 [Google Scholar]
- 66.Yeni YN, Fyhrie DP. A rate-dependent microcrack-bridging model that can explain the strain rate dependency of cortical bone yield strength. J Biomechanics. 2003;36:1343–1353. doi: 10.1016/s0021-9290(03)00122-2. [DOI] [PubMed] [Google Scholar]
- 67.Hassenkam T, Fantner GE, Cutroni JA, Weaver JC, Morse DE, Hansma PK. High-resolution AFM imaging of intact and fractured trabecular bone. Bone. 2004;35:4–10. doi: 10.1016/j.bone.2004.02.024. [DOI] [PubMed] [Google Scholar]
- 68.Fantner GE, Hassenkam T, Kindt JH, Weaver JC, Birkedal H, Pechenik L, Cutroni JA, Cidade GA, Stucky GD, Morse DE, Hansma PK. Sacrificial bonds and hidden length dissipate energy as mineralized fibrils separate during bone fracture. Nat Mater. 2005 Aug;4(8):612–6. doi: 10.1038/nmat1428. [DOI] [PubMed] [Google Scholar]
- 69.Thompson JB, Kindt JH, Drake B, Hansma HG, Morse DE, Hansma PK. Bone indentation recovery time correlates with bond reforming time. Nature. 2001;414(6865):773–776. doi: 10.1038/414773a. [DOI] [PubMed] [Google Scholar]
- 70.Yeni YN, Kim DG, Dong XN, Turner AS, Les CM, Fyhrie DP. Do sacrificial bonds affect the viscoelastic and fracture properties of bone? Clin Orthop Relat Res. 2006;443:101–108. doi: 10.1097/01.blo.0000200239.29931.56. [DOI] [PubMed] [Google Scholar]
- 71.Mohsin S, O’Brien FJ, Lee TC. Osteonal crack barriers in ovine compact bone. J Anat. 2006 Jan;208(1):81–9. doi: 10.1111/j.1469-7580.2006.00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ciarelli TE, Fyhrie DP, Parfitt AM. Effects of vertebral bone fragility and bone formation rate on the mineralization levels of cancellous bone from white females. Bone. 2003;32:311–315. doi: 10.1016/s8756-3282(02)00975-4. [DOI] [PubMed] [Google Scholar]
- 73.Courtney AM, Hayes WC, Gibson LJ. Age-related differences in post-yield damage in human cortical bone. Experiment and model. J Biomechanics. 1996;29:1463–1471. doi: 10.1016/0021-9290(96)84542-8. [DOI] [PubMed] [Google Scholar]
- 74.Knott DF, Jepsen KJ, Davy DT. Age-related changes in tensile damage accumulation behavior of human cortical bone. Trans Orthop Res Soc; 46th Annual Meeting; 2000. p. 10. [Google Scholar]
- 75.Currey JD. Some effects of ageing in human Haversian systems. J Anat. 1964;98:69–75. [PMC free article] [PubMed] [Google Scholar]
- 76.Kerley ER. The microscopic determination of age in human bone. Am J Phys Anthrop. 1965;23:149–164. doi: 10.1002/ajpa.1330230215. [DOI] [PubMed] [Google Scholar]
- 77.Ortner DJ. Aging effects on osteon remodeling. Calcif Tissue Res. 1975;18:27–36. doi: 10.1007/BF02546224. [DOI] [PubMed] [Google Scholar]
- 78.Evans FG. Mechanical properties and histology of cortical bone from younger and older men. Anat Rec. 1976;185:1–11. doi: 10.1002/ar.1091850102. [DOI] [PubMed] [Google Scholar]
- 79.Nyssen-Behets C, Duchesne PY. Dhem A Structural changes with aging in cortical bone of the human tibia. Gerontology. 1997;43:316–25. doi: 10.1159/000213871. [DOI] [PubMed] [Google Scholar]
- 80.Rho JY, Zioupos P, Currey JD, Pharr GM. Microstructural elasticity and regional heterogeneity in human femoral bone of various ages examined by nano-indentation. J Biomechanics. 2002;35:189–198. doi: 10.1016/s0021-9290(01)00199-3. [DOI] [PubMed] [Google Scholar]
- 81.Vashishth D, Tanner KE, Bonfield W. Fatigue of cortical bone under combined axial-torsional loading. J Orthop Res. 2001 May;19(3):414–20. doi: 10.1016/S0736-0266(00)00036-X. [DOI] [PubMed] [Google Scholar]
- 82.George WT, Vashishth D. Susceptibility of aging human bone to mixed-mode fracture increases bone fragility. Bone. 2006 Jan;38(1):105–11. doi: 10.1016/j.bone.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 83.George WT, Vashishth D. Influence of phase angle between axial and torsional loadings on fatigue fractures of bone. J Biomech. 2005 Apr;38(4):819–25. doi: 10.1016/j.jbiomech.2004.05.008. [DOI] [PubMed] [Google Scholar]


